Abstract
The basis for communication between nerve cells lies in the process of exocytosis, the fusion of neurotransmitter filled vesicles with the cell membrane resulting in release of the signaling molecules. Even though much is known about this process, the extent that the vesicles are emptied upon fusion is a topic that is being debated. We have analyzed amperometric peaks corresponding to release at PC12 cells and find stable plateau currents during the decay of peaks, indicating closing of the vesicle after incomplete release of its content. Using lipid incubations to alter the amount of transmitter released we were able to estimate the initial vesicular content and from that, the fraction of release. We propose a process for most exocytosis events where the vesicle partially opens to release transmitter and then closes directly again, leaving the possibility for regulation of transmission within events.
Similar content being viewed by others
Introduction
Traditionally, exocytosis has been thought of as an all-or-nothing process where the entire content of the vesicle is released upon fusion and the vesicle completely collapses into the cellular membrane. However, transient vesicle fusion with partial release of the vesicular transmitter content has been demonstrated for small synaptic vesicles1 as well as for large dense core vesicles2,3,4. This transient fusion is often referred to as simple kiss-and-run exocytosis if the vesicle fuses once or complex kiss-and-run or flickering fusion if the vesicle fuses repeatedly releasing a fraction of its content in each fusion5. We would like to introduce the concept of extended kiss-and-run for release events where the vesicle closes up again following massive release (see Supplementary Fig. S3 online). Our evidence suggests that the large majority of exocytosis events observed with amperometry represent partial opening of the vesicle and thus operate via extended kiss-and-run. We have previously reported that the amount of catecholamine released from PC12 cells during exocytosis only represents a fraction of the amount contained in the vesicle prior to release6, thus supporting the idea of partial release as an important mode of exocytosis. In that study the catecholamine content of isolated vesicles was analyzed and compared to the amount released during exocytosis. It was concluded that the average PC12 cell vesicle releases approximately 40% of its content upon fusion, indicating that what has previously been thought of as full fusion is in fact extended kiss-and-run fusion where the vesicle closes up again following release. Supporting the idea of extended kiss-and-run as a major mode of exocytosis, Orwar and coworkers demonstrated using a quartz crystal microbalance with dissipation that exocytosis from PC12 cells to a great extent is immediately followed by rapid endocytosis7. Furthermore, the Amatore group has shown recently through modeling and statistical analysis of exocytosis from chromaffin cells, that full fusion is highly unlikely to occur during the time course of exocytosis8. Also supporting the theory of partial release, Borges and coworkers reported that chromaffin cells release about three times more catecholamine during the average exocytosis event measured by patch amperometry compared to when measured by traditional amperometry, indicating that the entire vesicular content is not expelled when the cell membrane is under normal tension9.
Amperometry at microelectrodes positioned on top of secreting cells is a powerful method in the study of exocytosis10. The amperometric trace provides quantitative as well as kinetic information about the exocytotic event with millisecond time resolution. In a fraction of exocytotic events recorded with amperometric detection a small increase in current is observed directly preceding the current peak. This feature has been termed the foot of the peak and it represents leakage of neurotransmitter through a fusion pore formed as an early stage of vesicle fusion11. In this paper we examine amperometric recordings of exocytosis at PC12 cells focusing on the occurrence of feet on the descending part of the amperometric peak (post spike feet), as should be predicted if the vesicle closes again following release. Post spike feet were indeed found to be almost as prevalent as traditional feet (pre spike feet) in PC12 cells, providing further evidence for partial release as a major form of exocytosis in these cells. We also investigated the effect of short-term incubation with lipids on pre and post spike feet. Lipids have been shown to affect exocytosis and might have some mechanistic bearing on short-term memory12,13. The amount of catecholamine released during exocytosis was found to decrease following incubation with phosphatidylethanolamine (PE) and increase following incubation with lysophosphatidylcholine (LPC). This was interpreted as changes in the fraction of transmitter released. Consistent with this interpretation, the ratio of the post spike foot current to the pre spike foot current was found to increase following incubation with PE and decrease following incubation with LPC compared to control cells. The calculated foot current ratios were then correlated with the amount of messenger released to estimate the vesicular content prior to release. The result was used to calculate the fraction of transmitter released as 39%, a value in apparent agreement with the results from the previous cytometry experiments6. These data suggest a model for exocytosis where the vesicles normally open and close and the transmitter released during individual events can be regulated.
Results
Observation of exocytotic post spike feet
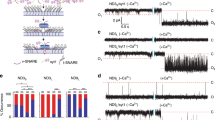
Exocytosis from PC12 cells was studied using amperometry at carbon fiber microelectrodes. The peaks were investigated for feet prior to the peak but also immediately following the peak. In addition to the traditional feet, stemming from the initial opening of the fusion pore, we observed feet during the descending part of the spike. We refer to the feet on the descending part of the peak as post spike feet while the traditional feet are referred to as pre spike feet. Post spike feet were recognized as having a stable current transient of more than three times the RMS noise for at least 2 ms during the decay of the peak followed by a quick decline to the base line (Fig. 1A). The same limits were set for the analysis of pre spike feet. The post spike feet are hypothesized to be the result of a formation of a stable pore during closing of the vesicle following partial release through extended kiss-and-run fusion. Two categories of pre spike feet have previously been described14,15. The two categories differ from each other in the shape of the foot. The first category of feet displays a slowly increasing current prior to the sharp rise of the peak. These feet are referred to as “ramp” feet. The second category constitutes an increase in current followed by a stable plateau current leading up to the peak. This type of foot is referred to as “ramp + plateau”15. Since it would be difficult to identify ramp type feet during the decay part of the peak, we only considered plateau shaped feet for both pre and post spike feet in this paper (see Fig. 1B and Supplementary Fig. S2 online). To assess the frequency of these features, we adjusted the number of peaks for each cell by removing peaks that were too close to each other, making the detection of a foot impossible. Pre spike feet were detected for 5.1 ± 1.5% of the peaks while post spike feet were detected for 2.3 ± 0.5% of amperometric peaks for control cells (n = 1,127 peaks from 5 cells).
Observation and analysis of plateau shaped pre and post spike feet.
(a) Three example peaks from control cells. The first peak displays the traditional plateau foot, here termed pre spike foot. The second peak shows what we refer to as a post spike foot, while the third peak displays both types of feet. (b) Definition of the foot features that were used in the analysis. The lifetime of the foot (tfoot), the current of the foot (Ifoot) and the charge of the foot (Qfoot) for both pre and post spike feet. (c) Current, lifetime and charge are shown for pre and post spike feet of control cells. Results are given as mean ± SEM. Data was recorded from 5 cells, for pre spike feet n = 50 and for post spike feet n = 24. * p<0.05 and *** p<0.001 by single factor one-way ANOVA.
Foot current is reduced after exocytosis
When analyzing feet, there are three features of the foot that are traditionally considered. The lifetime of the foot (tfoot) is indicative of the stability of the fusion pore, the current of the foot (Ifoot) is dependent on the flux of neurotransmitter through the pore and the charge of the foot (Qfoot), which represents the charge released through the pore during its lifetime (Fig. 1B). We have only considered feet (both pre and post spike) with lifetimes longer than 2 ms. The average foot current for the pre spike feet of control cells was found to be 2.5±0.1 pA. The post spike foot current was lower than the pre spike foot current for control cells with a post foot current of 1.9±0.3 pA (p<0.05) resulting in a ratio of 0.76 between post spike foot current and pre spike foot current. The foot time of the post spike feet was found to be significantly (p<0.001) longer than the foot time of the pre spike feet. This is most likely due to a difficulty in detecting really short post spike feet since they are masked by the diffusional decay of the peak. This problem is also reflected in the lower apparent percentage of post versus pre spike feet. The charge released in the foot (which is simply calculated by multiplying the foot current by the foot time) was not different between pre and post spike feet (Fig. 1C).
Peaks with feet release more transmitter and release more quickly than the average peak
The overall amperometric peaks were analyzed for maximum current (Imax), half time (t1/2), fall time, which was defined as the time from 75% to 25% of the peak height and amount of catecholamine released. The number of moles (N) of neurotransmitter released during vesicle fusion was calculated using Faraday's law (Q = nNF) where Q is the charge integrated under the amperometric peak, n is the number of electrons transferred per molecule of analyte oxidized (2 e− for catecholamine) and F is the Faraday constant (96,485 C/mol). Three groups of peaks were compared regarding peak characteristics: all peaks, peaks with pre spike feet and peaks with post spike feet (Fig. 2A). Peaks with pre spike feet were significantly faster, i.e. shorter t1/2 and fall time, than the average amperometric event (p<0.01). Peaks with post spike feet also showed a tendency to faster kinetics than the average peak, although not significantly different. Furthermore, there is a discrepancy in the longest fall times between all peaks and peaks with post spike feet as can be seen in the histogram in figure 2B. Peaks with pre spike feet and peaks with post spike feet were also found to release a larger amount of catecholamine compared to the average event (p<0.001), while peaks with pre spike feet were found to have a higher Imax than the average peak (p<0.05).
Whole peak characteristics.
(a) Molecules released, Imax, t1/2 and fall time for all peaks, peaks with pre spike feet and peaks with post spike feet. (b) Histogram of fall time for all peaks and peaks with post spike feet. In addition to the peaks identified by the analysis program and used in the rest of this paper we have manually detected large, broad peaks, which have been added to this histogram. Data was collected from 5 cells. Peaks were pooled for averages where n = 1498 for all spikes, n = 50 for peaks with pre spike feet, n = 24 for peaks with post spike feet. Groups were tested for significant differences using single factor one-way ANOVA. * P<0.05, ** p<0.01, *** p<0.001 versus control and # p<0.05 versus peaks with pre spike feet.
Phospholipid shape changes foot characteristics
In order to study the relationship between post spike foot current and vesicular catecholamine concentration we incubated cells with phospholipids prior to measurement. This was found to alter the fraction of neurotransmitter released and is consistent with work showing that incubation with high curvature lipids quickly alters the amount of catechol released from chromaffin cells13. The average amount of catecholamine released per event from control cells was found to be 113,400 ± 2,100 molecules. Following 30-min incubation with 100 µM LPC the amount released was increased to 132,900 ± 3,000 molecules (p<0.001), whereas incubation with 100 µM PE decreased the amount released to 94,000 ± 2,400 molecules (p<0.001). When the analysis was done using only spikes with feet (both pre and post) the average amount released for control cells was 162,700 ± 11,500, for LPC treated cells 196,100 ± 7,100 and for PE treated cells 126,400 ± 12,900 molecules (Fig. 3A). In agreement with the altered amount of catecholamine released following lipid incubations, the post spike foot to pre spike foot current ratio was found to decrease to 0.67 following incubation with LPC with a pre spike foot current of 2.6 ± 0.2 pA and a post spike foot current of 1.7 ± 0.2 pA and increase to 0.88 following incubation with PE with a pre spike foot current of 1.9 ± 0.1 and a post foot current of 1.7 ± 0.2 compared to the 0.76 found for control cells (Fig. 3B).
Amount released and pre and post spike foot currents with and without short-time incubation of the cells with lipids.
(a) Average number of molecules released during single exocytotic events for control cells, cells incubated with LPC and cells incubated with PE. For all peaks n = 1,498 peaks from 5 cells, 819 peaks from 5 cells and 1,013 peaks from 6 cells for control, LPC treated and PE treated cells, respectively (for more detailed information on the number of peaks per cell please refer to the Supplementary information). For peaks with feet n = 71 peaks from 5 cells, 27 peaks from 5 cells and 18 peaks from 6 cells for control, LPC treated and PE treated cells, respectively *** p<0.001 versus all peaks from control cells by single factor one-way ANOVA. (b) Pre and post spike foot currents for control cells, cells incubated with LPC and cells incubated with PE. For the analysis of feet, n = 50 for control pre spike feet, 24 for control post spike feet, 17 for LPC pre spike feet, 11 for LPC post spike feet, 13 for PE pre spike feet and 5 for PE post spike feet. Data was recorded from 5 control cells, 5 LPC treated cells and 6 PE treated cells. * p<0.05 and ** p<0.01 vs. pre spike feet in the same treatment group by single factor one-way ANOVA. All results are presented as mean ± SEM.
Discussion
The observation of post spike feet reveals that exocytosis is often transient with the vesicle closing again after releasing some content, even during large amounts of release. The classic view of exocytosis as an all-or-nothing process has during recent years come to be challenged by a model of transient vesicle fusion with partial release of the vesicular transmitter content. Our data provide an observable link during the exocytosis process to incomplete release of neurotransmitter as we show that amperometric peaks from PC12 cells display plateau feet on the descending part of the spike. We interpret this as the fusion pore never opening all the way to completely collapse into the cell membrane, but instead forms a wider pore followed by its restriction to form a stable smaller pore during closing of the vesicle at the end of exocytotic release. We term this form of exocytosis extended kiss-and-run. The frequency of post spike feet observed is almost the same as for pre spike feet. The discrepancy between the two can be explained by the difficulty in distinguishing a post spike foot from the diffusional decay of the peak. The observation that post spike feet are nearly as common as pre spike feet in PC12 cells suggests by correlation that nearly all exocytosis events undergo extended kiss-and run-fusion in this cell type.
We then hypothesized that if most events open and close, then the amount lost during release should be reflected as a difference in the pre and post foot current. Indeed, the current of the post spike foot was found to be lower than the current of the pre spike foot. To be more precise, the magnitude of the foot current is proportional to the neurotransmitter concentration gradient along the length of the pore. In a 1st order approximation, this gradient is proportional to the concentration of neurotransmitter inside the vesicle since the neurotransmitter concentration at the electrode will be 0 at any time because of the immediate oxidation of the transmitter at the surface of the electrode placed just outside the cell. The current is also proportional to the square of the radius of the pore and inversely proportional to the length of the pore16,17. The concentration of transmitter inside the vesicle is lower during closing of the vesicle than during opening and this decrease in concentration results in the observed decrease in foot current between pre and post spike feet. In addition, this conclusion is supported by our finding that when altering the fraction of neurotransmitter released, through incubation with high curvature lipids, the ratio between post and pre spike foot current changes accordingly (Fig. 3). The changes in pre spike foot current following lipid treatment are consistent with previous results by Amatore and coworkers where the inverted cone shaped lipid LPC was shown to increase the foot current and the cone shaped arachidonic acid to decrease it13. These changes were attributed to the curvature of the lipids affecting the size of the fusion pore. The post spike foot current in our study did not change between the three treatment groups in contrast to the pre spike foot current. However, the post spike foot current will be dependent both on the size of the pore and on the amount of transmitter remaining in the vesicle following fusion making the evaluation of the pore size less straight forward than for the pre spike foot. The time of the pre spike foot was the same for all lipid treatments while the post spike foot time was shorter for PE-treated cells than for control cells (p = 0.13) (see Supplementary Fig. 4 online). This observation could be explained by a lowered stability in the closing pore rendering shorter feet. Also, the fraction of peaks with post spike feet was as low as 0.8 ± 0.3% for PE-treated cells compared to 2.3 ± 0.5% and 2.5 ± 0.5% for control and LPC-treated cells, respectively. This lower percentage could result from difficulty in detecting the shorter post spike feet, or it might indicate that we are only detecting the post pike feet with the highest currents since we are close to the limit of detection. We do observe post spike feet in all treatment groups that do not meet our criteria for detection of 3 times the RMS current and if the noise level was lower, these results might be different.
Peaks displaying pre spike feet as well as peaks with post spike feet had a higher overall spike charge than the average peak (p<0.001). The higher charge released for the peaks with feet in this study can only in part be explained by release that occurs in the foot portion of the spike. The fact that events with post spike feet are larger than average events again suggests that most events are extended kiss-and-run exocytosis and that this mode of release does not represent a less complete form of exocytosis since this should result in smaller amounts of released transmitter.
In addition to a higher charge, the events with pre spike feet as well as post spike feet were found to have somewhat faster release dynamics than the average amperometric peak. Peaks with pre spike feet showed both significantly shorter half widths and fall times (p<0.01) than the average peaks, whereas peaks with post spike feet also had slower half widths and fall times although not statistically different from the average event (p = 0.3 and 0.4, respectively). There are some previous studies linking the kinetics of exocytosis to the properties of the fusion pore. Lindau and coworkers studied the effect of a less tight zipping of the SNARE complex on the formation of the fusion pore18. This was accomplished through deletion of the last nine amino acids of the C-terminal of the SNAP-25 protein. They found that cells expressing this mutant displayed smaller foot currents. This was attributed to a less tight zipping of the fusion pore, leading to a longer pore with a lower conductance. Furthermore, the mutant displayed significantly longer half-widths for the amperometric peaks than control cells. This is consistent with the data shown here where shorter half-widths are observed for amperometric spikes displaying feet suggesting a tighter zipping of the vesicle when both pre and post spike feet are observed. The number of SNARE complexes anchoring the vesicle to the membrane has also been shown to influence the kinetics of exocytosis as well as the opening of the fusion pore, where a lower number of SNARE complexes was shown to slow down the rate of exocytosis and to lower the foot charge and foot time in chromaffin cells19, again consistent with the faster rates observed here for spikes with both pre and post spike feet.
The slightly faster dynamics of spikes with post spike feet might result from some small fraction of release events in the overall group undergoing full exocytosis and opening. Full opening of the vesicle with complete release should be reflected in larger, perhaps slower events that would not display post spike feet, thus skewing the average of all events towards slower dynamics. We found that the events with the longest fall times do not display post spike feet. The distributions of fall time for all spikes and spikes with post spike feet are shown in Fig. 2B. The fraction of all spikes with slower fall times than the slowest peak with a post spike foot was found to be 17%. Some of these slow events might represent vesicle fusion where the fast endocytosis machinery for some reason is not employed, but instead the vesicle collapses completely into the cell membrane releasing its entire contents. One can speculate that this happens when a vesicle has lost a key component of its protein machinery and needs to be degraded or recycled. Indeed, we specifically examined spike trains for slow and large events and found that approximately 2% of all events released more than 200,000 molecules in addition to having base widths longer than 50 ms. We thus argue that most events are extended kiss-and-run and only a small fraction of exocytosis events involve full distension of the vesicle and complete release.
An important question that arises if exocytosis is open and closed is what fraction is released? This is a complicated question. First, from the average amount of catecholamine released in spikes with feet and the magnitude of the decrease in foot current between pre spike foot and post spike foot we have estimated the vesicular content prior to fusion. Then, we compare this to the amount detected during release.
During exocytosis, the vesicular content is released through the fusion pore, inducing the characteristic shape of an exocytotic spike. A 1st order linearization model was used to simplify Fick's laws describing diffusion, as detailed in the Supplementary Information. As a result, it can be shown that the vesicular efflux at instant t, J(t) (see Supplementary Fig. S1 online), is proportional to an equivalent diffusional resistance of the pore R(t), defined in equation (1)17,

where l(t) and r(t) are the length and the radius of the pore at instant t and D is the diffusion coefficient of the neurotransmitter. In this model, the geometry of the pore is the main parameter controlling exocytosis, consistent with the pore not opening to full distention. During a plateau foot, the stable exocytotic current indicates the establishment of a short, ephemeral steady state. The steady state current can be explained by our model, where the current is assumed to be dependent solely on i) the pore diffusional resistance and ii) the vesicular concentration at that instant. We have assumed that the dimensions of the pore before and after the peak are different. The ratio of the pore resistance corresponding to the pre and post spike pores, α (see equation (2)), is however assumed to be the same for all three treatment groups.

The fact that lipid incubation alters the amount released was then exploited. By assuming that the vesicular content is not altered during the 30-min lipid incubation but that the integration of exogenous lipids changes the pore dimensions and thereby the fraction released, we have solved the model and estimate the initial vesicle content Q0 (see Supplementary information). Solving this system for α and minimizing the variances between the three calculated Q0, an initial total vesicular content of 412,700 ± 18,700 molecules was calculated for peaks with feet. This corresponds to a released fraction of 39% for control cells, 48% for LPC treated cells and 31% for PE treated cells. α was found to be 0.75, by assuming that lpost ≈ lpre as α shows a quadratic dependence on r, this corresponds to a ratio of rpost/rpre of 1.12 thus suggesting a post spike pore radius that is 12% larger than the pre spike pore radius. Assuming that all events undergo partial release of approximately 39% of the vesicular content we can estimate the initial content of the average vesicle from the average amount released from control cells to 290,800 ± 5400 molecules.
Methods
Cell culture
PC12 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as previously described (Kozminski et al., 1998). PC12 cells were grown on collagen type IV coated cell culture flasks (BD BioCoatTM) and subcultured every 7 days. The cells were maintained in RPMI-1640 medium supplemented with 10% equine, 5% fetal bovine serum and 0.4% penicillin streptomycin solution (PAA laboratories) in 7% CO2 atmosphere at 37°C, 100% humidity. The medium was replaced every 2–3 days. For experiments, cells were subcultured sparsely on collagen type IV coated dishes (BD BioCoatTM) 4–8 days before experiments and the cell medium was replaced every day.
Electrode preparation
Carbon fiber microelectrodes were constructed by aspirating a 5-µm carbon fiber into a glass capillary (o.d. 1.2 mm i.d. 0.69 mm, no filament; Sutter Instrument Co., Novato, CA). The capillary was pulled with a commercial micropipette puller (model PE-21, Narishige, Japan) to produce two narrowing, fiber-containing pipettes. The protruding fiber was cut near the glass tip with a scalpel. The cut electrode tip was dipped into freshly made epoxy (EpoTek 301, Billerica, MA) for 4 min to create a seal between the carbon fiber and the glass. The electrodes were polished at a 45° angle on a diamond dust-embedded micropipette beveller (Model BV-10; Sutter Instruments, Novato, CA) to produce a fine, angled tip and back filled with 3 M KCl. Prior to experiments, the electrodes were tested in a 100 µM dopamine solution resulting in an average steady state current of 170 pA. Only electrodes with stable I–E curves were used.
Experimental setup
Liposome solutions were prepared by rotation evaporation of phospholipid dissolved in chloroform to a thin film on the inside of a round bottom flask. The lipids used were soy PE #840024C and egg LPC #830071P (Avanti Polar Lipids, Alabaster, AL). Cell medium was added to a final concentration of 500 µM lipid and the film was left to hydrate over night at 4°C. Lipid mixture, or plain cell medium for control experiments, was sonicated (Vibra-Cell VCX 130; Sonics & Materials, Newtown, CT) for 15 min at 30% amplitude (no pulsing) on ice. Sonicated lipid mixture was then added to fresh cell medium to a final concentration of 100 µM lipid. For control experiment, sonicated cell medium was mixed with fresh cell medium at a ratio of 1:4. Cells were incubated for 30 min in 7% CO2 atmosphere at 37°C, 100% humidity. After incubation the cells were prepared by rinsing three times in warm (37°C) isotonic saline solution (150 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 5 mM glucose, 10 mM HEPES, pH 7.4), 10 mL of isotonic saline was then added to the cells and the dish was placed onto a heat stage (37°C) on an inverted microscope (IX71S1F-2, Olympus). The electrode was carefully positioned on top of a cell. Accurate placement was confirmed by a slight deformation of the edge of the cell. Cells were stimulated for 5 s at 20 p.s.i. (Picospritzer II; General Valve Instruments, Fairfield, NJ) with a high K+ saline solution (55 mM NaCl, 100 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 5 mM glucose, 10 mM HEPES, pH 7.4). The stimulation pipette was placed approximately 50 µm away from the cell and each cell was stimulated 5 times with 45 s between pulses. All cell experiments were performed under identical conditions and treatments were alternated on any given day.
Data acquisition and data analysis
The electrode was held at + 0.7 V vs. a silver/silver chloride reference electrode (World Precision Instruments, Inc., Sarasota, FL) using a commercial patch-clamp instrument (Axopatch 200B; Axon Instruments, Foster City, CA). The signal was digitized at 5 kHz and filtered with an internal low-pass Bessel filter at 2 kHz. The signal was displayed in real time (AxoScope 8.1; Axon Instruments) and stored digitally. The collected data was analyzed in Igor Pro 6 (Version 6.2.2.0; WaveMetrics, Lake Oswego, OR) using an Igor Procedure File designed for analysis of quantal release by the group of David Sulzer17. The signal was digitally smoothed using a binominal filter at 1 kHz and the 1st derivative was filtered at 300 Hz. Peaks were detected if exceeding a threshold of 3 times the RMS noise for a 1-s period preceding the first stimulation, typically between 0.3 and 0.5 pA. Feet were analyzed manually in a blind fashion and only feet with currents larger than 3 times the RMS noise and with semi-stable foot plateaus for more than 2 ms were considered.
Statistical analysis
Mean values for both peak and foot parameters were calculated by pooling all the data from different cells. Data were tested for significant differences by single factor one-way ANOVA. All results are presented as mean ± SEM.
References
Staal, R. G. W., Mosharov, E. V. & Sulzer, D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 7, 341–346 (2004).
de Toledo, G. A., Fernandez-Chacon, R. & Fernandez, J. M. Release of secretory products during transient vesicle fusion. Nature. 363, 554–558 (1993).
Elhamdani, A., Palfrey, H. C. & Artalejo, C. R. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 31, 819–830 (2001).
Burgoyne, R. D. & Barclay, J. W. Splitting the quantum: regulation of quantal release during vesicle fusion. Trends Neurosci. 25, 176–178 (2002).
Wightman, R. M. & Haynes, C. L. Synaptic vesicles really do kiss and run. Nat Neurosci. 7, 321–322 (2004).
Omiatek, D. M., Dong, Y., Heien, M. L. & Ewing, A. G. Only a fraction of quantal content is released during exocytosis as revealed by electrochemical cytometry of secretory vesicles. Acs Chem Neurosci. 1, 234–245 (2010).
Cans, A. S. et al. Measurement of the dynamics of exocytosis and vesicle retrieval at cell populations using a quartz crystal microbalance. Anal Chem. 73, 5805–5811 (2001).
Amatore, C., Oleinick, A. I. & Svir, I. Reconstruction of aperture functions during full fusion in vesicular exocytosis of neurotransmitters. Chemphyschem. 11, 159–174 (2010).
Montesinos, M. S. et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 28, 3350–3358 (2008).
Adams, K. L., Puchades, M. & Ewing, A. G. In vitro electrochemistry of biological systems. Annu Rev Anal Chem. 1, 329–355 (2008).
Chow, R. H., Vonruden, L. & Neher, E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 356, 60–63 (1992).
Uchiyama, Y., Maxson, M. M., Sawada, T., Nakano, A. & Ewing, A. G. Phospholipid mediated plasticity in exocytosis observed in PC12 cells. Brain Res. 1151, 46–54 (2007).
Amatore, C. et al. Regulation of exocytosis in chromaffin cells by trans-insertion of lysophosphatidylcholine and arachidonic acid into the outer leaflet of the cell membrane. Chembiochem. 7, 1998–2003 (2006).
Amatore, C. et al. Relationship between amperometric pre-spike feet and secretion granule composition in chromaffin cells: An overview. Biophys Chem. 129, 181–189 (2007).
Amatore, C., Arbault, S., Bonifas, I. & Guille, M. Quantitative investigations of amperometric spike feet suggest different controlling factors of the fusion pore in exocytosis at chromaffin cells. Biophys Chem. 143, 124–131 (2009).
Adams, K. L. et al. Steady-state electrochemical determination of lipidic nanotube diameter utilizing an artificial cell model. Anal Chem. 82, 1020–1026 (2010).
Mosharov, E. & Sulzer, D. Analysis of exocytotic events recorded by amperometry. Nat. Methods. 2, 651–658 (2005).
Fang, Q. H. et al. The role of the C terminus of the SNARE protein SNAP-25 in fusion pore opening and a model for fusion pore mechanics. Proc Natl Acad Sci USA. 105, 15388–15392 (2008).
Mohrmann, R., de Wit, H., Verhage, M., Neher, E. & Sorensen, J. B. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 330, 502–505 (2010).
Acknowledgements
We acknowledge the European Research Council (Advanced Grant), Knut and Alice Wallenberg Foundation, the Swedish Research Council (VR) for financial support.
Author information
Authors and Affiliations
Contributions
LJM collected and analyzed data, interpreted the data and models and wrote the manuscript. RT analyzed data and performed modeling and interpretation of the data and models. MIS carried out some experiments and participated in interpretation. AGE participated in design and interpretation of the experiments, writing of the manuscript and acquired the resources and environment for the work to be done.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion: Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mellander, L., Trouillon, R., Svensson, M. et al. Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion. Sci Rep 2, 907 (2012). https://doi.org/10.1038/srep00907
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00907
This article is cited by
-
Quantifying neurotransmitter secretion at single-vesicle resolution using high-density complementary metal–oxide–semiconductor electrode array
Nature Communications (2021)
-
Amperometry methods for monitoring vesicular quantal size and regulation of exocytosis release
Pflügers Archiv - European Journal of Physiology (2018)
-
Electrochemical measurement of quantal exocytosis using microchips
Pflügers Archiv - European Journal of Physiology (2018)
-
Interpretation of amperometric kinetics of content release during contacts of vesicles with a lipid membrane
European Biophysics Journal (2017)
-
Cholesterol Alters the Dynamics of Release in Protein Independent Cell Models for Exocytosis
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.