Abstract
In plants, non-cell autonomous RNA silencing spreads between cells and over long distances. Recent work has revealed insight on the genetic and molecular components essential for cell-to-cell movement of RNA silencing in Arabidopsis. Using a local RNA silencing assay, we report on a distinct mechanism that may govern the short-range (6–10 cell) trafficking of virus-induced RNA silencing from epidermal to neighbouring palisade and spongy parenchyma cells in Nicotiana benthamiana. This process involves a previously unrecognised function of the RNA-dependent RNA polymerase 6 (RDR6) gene. Our data suggest that plants may have evolved distinct genetic controls in intercellular RNA silencing among different types of cells.
Similar content being viewed by others
Introduction
RNA silencing is a potent surveillance system to protect against virus infections in plants, fungi and animals. Viruses have evolved both passive and active strategies to escape and effectively suppress RNA silencing. In particular, viruses encode suppressors of RNA silencing (VSRs) that can directly or indirectly target various steps in intra-, intercellular and systemic RNA silencing1. Indeed, VSRs can subvert antiviral silencing by inhibiting the biosynthesis of small interfering (si)RNAs, by preventing the assembly of RNA-induced silencing complexes (RISC), by repressing the activity of the Argonaute (AGO) proteins, by impeding silencing amplification, and/or by blocking the spread of silencing signals. For example, the P38 coat protein (CP) of Turnip crinkle virus (TCV) is an effective VSR2,3. It binds to dsRNA and siRNA and specifically inhibits DCL4 activity to prevent siRNA production. TCV P38 also interacts with (and subsequently inactivates) AGO1 to impede RISC-mediated targeting and the degradation of viral RNAs4,5,6. The silencing suppression function of TCV P38 also requires the ethylene-inducible host transcription factor RAV2a7.
In plants, RNA silencing is non-cell autonomous8. The 21-nt siRNA produced by DCL4 from inverted-repeat transgenes, acts as the primary silencing signal and spreads short-range over 10 – 15 cells. This type of intercellular trafficking of RNA silencing does not require RNA-dependent RNA polymerase 6 (RDR6). However, DCL4 also generates an RDR6-dependent 21-nt secondary siRNA for long-range (i.e., beyond 10 – 15 cells) signalling in systemic RNA silencing9,10. Genetic analysis in Arabidopsis has revealed three recessive silencing-movement-deficient (smd1-3) mutations which are compromised in short-range cell-to-cell movement of RNA silencing9. SMD1 and SMD2 are allelic to RDR2 and to NRPD1a, respectively. Both genes are required for intercellular but not intracellular RNA silencing11. More recently, through cell-specific rescue of the DCL4 function and cell-specific suppression of the movement of RNA silencing, it has been shown that 21-nt siRNA duplexes probably represent a component of the mobile silencing signal that spreads between plant cells12. Interestingly, mobile silencing signals also include a range of small RNA (sRNA) species. Indeed, transgene-derived and endogenous 22-, 23- and 24-nt siRNAs are able to move between plant cells through plasmodesmata and traffic long distances via phloem transportation13. These findings have led to an elegant model that explains the genetic and molecular basis for limited (10 – 15 cell) spread of RNA silencing from companion cells to neighbouring parenchyma and mesophyll cells outside the vasculature in Arabidopsis8,9,10,11,12. It should be noted that in this model RNA silencing is triggered by a long dsRNA-producing transgene or a synthetic 21-nt siRNA. Interestingly, plants have also been shown to be capable of hijacking viral RNA movement proteins in order to facilitate trafficking of virus-induced and transgene-mediated RNA silencing14,15.
On the other hand, RNA silencing can be initiated by transgenes, high molecular weight RNAs, siRNA or plant RNA and DNA viruses in various cell types. In these scenarios, the initiation of silencing and the subsequent movement of silencing signals between cells may have different requirements. Using a local RNA silencing assay coupled with molecular and cellular analyses, we have uncovered a mechanism associated with short-range (6 – 10 cell) spread of virus-induced RNA silencing from individual epidermal cells to adjacent cells. This process requires a functional RDR6 gene in Nicotiana benthamiana.
Results
RDR6 is essential for cell-to-cell trafficking of local virus induced RNA silencing
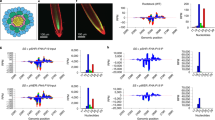
We exploited the Turnip crinkle virus (TCV)-based local RNA silencing assay devised to dissect the cell-to-cell spread of antiviral RNA silencing in plants3,14. The movement-deficient TCV-GFPΔCP was constructed by replacing the P38 CP gene with the green fluorescent protein (GFP) coding sequence3. Thus, it lacks the potent silencing suppressor CP and is restricted to single epidermal cells of N. benthamiana2,3,4,5,7,12. When mechanically applied to the upper epidermis of young leaves of GFP-expressing N. benthamiana GFP16c transgenic plants16, TCV-GFPΔCP was able to initiate gfp RNA silencing in single epidermal cells. Then, silencing spread from cell-to-cell to form gfp-silenced foci, that were visible under long-wavelength UV light (Fig. 1a, b, e, f, i, j, m, n, q, r). Efficient and multi-dimensional spread of gfp silencing from a single epidermal cell to other cell types was evident (Table 1; Fig. 2a). Large numbers of silencing foci per inoculated leaf were counted on both the upper (161 ± 6) and lower (146 ± 12) epidermises of four leaves. Silencing foci, selected at random, appeared to have similar sizes (0.98 ± 0.39 mm, 0.97 ± 0.27 mm; n = 12) on upper and lower epidermis, respectively. Interestingly, we noticed that the number of silencing foci was always higher in the 2nd inoculated leaf than in the 1st inoculated leaf (Fig. 1), suggesting that the age of leaf tissues might have an effect of local RNA silencing. These data were consistent with previous results3,14,17. However, in GFP16c/RDR6i plants in which RDR6 was silenced16, we found a marked reduction in the numbers of visible gfp silencing foci, on the upper (25 ± 25) and, particularly the lower (4 ± 4) epidermis of four treated leaves (Table 1; Fig. 1c, d, g, h, k, l, o, p, s, t; Fig. 2a). There was also a significant decrease in the average size (diameter) of the gfp silencing foci on the upper (0.50 ± 0.35 mm) and lower (0.51 ± 0.39 mm) epidermises (Table 1; Fig. 2b). This phenomenon was observed in all repeated (more than three separate) experiments. Thus, in RDR6-“knockdown” plants, the silencing initiated by TCV-GFPΔCP in individual upper epidermal cell was incompetent to spread between epidermal cells and to penetrate to palisade and spongy parenchyma cells in order to reach lower epidermis. These findings suggest that RDR6 was required for the cell-to-cell communication of RNA silencing in N. benthamiana. It should be noted that sizes of individual silencing foci could vary because the initiation of intracellular silencing may not be synchronous and the development of each silencing focus is probably a dynamic process.
Local induction and cell-to-cell spread of virus-induced RNA silencing.
The first and second young leaves of N. benthamiana GFP16c and GFP16c/RDR6i at the six-leaf stage were mechanically inoculated with an equal amount of TCV-GFPΔCP RNA transcripts. Control plants were mock-inoculated with 10 mM Tris-HCl (pH 8.0) containing 10 mM EDTA. Both the upper and lower epidermises of inoculated leaves were photographed 8 days post-inoculation (dpi) (e–l) or 12 dpi (a–d, m–t) using a Nikon Coolpix995 digital camera under long-wavelength UV illumination through a yellow Kodak No. 58 filter. Gfp RNA-silenced tissue (foci) showed red chlorophyll fluorescence and gfp-expressing tissue showed green fluorescence. Colour-coded arrows in Panels (g), (h), (o) and (p) show the same gfp silencing foci observed from the upper or lower epidermis. A diagram showing the genome of TCV-GFPΔCP is included. A cartoon of a plant shows the positions of the first and second inoculated leaves (I. L.).
Influence of RDR6 on intercellular RNA silencing.
(a–b) The RDR6 gene affects the intercellular spread of TCV-GFPΔCP-induced gfp silencing. The average numbers of silencing foci per inoculated leaf for four leaves in a typical experiment using recombinant RNA transcripts produced from 2.5 µg of TCV-GFPΔCP DNA template are shown (a). Numbers of foci were counted 8 days post-inoculation (dpi). The average sizes of 12 randomly selected silencing foci from the upper and lower epidermises were analysed (b). (c–l) Dark gfp RNA silencing foci from the upper and lower epidermises of N. benthamiana GFP16c and GFP16c/RDR6i were examined under a fluorescence microscope using a green filter (c–f) or through bright field illumination (g–l). The boxed areas were used to estimate the numbers of epidermal cells in which gfp-silencing occurred. Individual epidermal cells are outlined and marked with asterisks. Scale bars are indicated.
RDR6 is required for the short-range (6 – 10 cell) intercellular spread of virus-induced RNA silencing
We examined 12 randomly selected silencing foci by fluorescent microscopy and estimated the numbers of epidermal cells in which gfp RNA silencing had occurred (Fig. 2c–l). A typical silencing focus that formed in GFP16c leaves inoculated with TCV-GFPΔCP consisted of 100–300 epidermal cells, equivalent to a circular zone with a radius of 6 – 10 epidermal cells (Fig. 2c, d, g, h, k). These data demonstrate that virus-induced RNA silencing can move from a single upper epidermal cell over a short-range of 6 – 10 upper epidermal cells. However, intracellular RNA silencing originating from a single upper epidermal cell could move, simultaneously, in three-dimensions to and through many more cell types, including palisade, mesophyll and lower epidermal cells, to form a visible focus of silencing (Fig. 1). Such RNA silencing was unlikely to move into the vasculatures as systemic RNA silencing was not observed in distal leaf tissues3,14,17. Consistent with their reduced size (Fig. 2b), the gfp RNA silencing foci that formed on GFP16c/RDR6i leaves encompassed only 30 – 100 epidermal cells, equivalent to a circular zone with a radius of 3–6 epidermal cells (Fig. 2e, f, i, j, l). These data provide additional evidence that a functional RDR6 gene plays an important role in short-range (6 – 10 cell) trafficking of virus-induced RNA silencing.
RDR6 does not influence the replication and defective movement of TCV-GFPΔCP in N. benthamiana
To address whether RDR6 gene expression could affect replication and cell-to-cell spread of TCV-GFPΔCP, we first performed quantitative RT-PCR (qRT-PCR) assays to detect and compare the levels of RDR6 mRNA in wild-type and RDR6-silenced lines NbRDR6i and GFP16c/RDR6i16 using two housekeeping gene GAPDH and EF1α transcripts as internal controls. Our data showed that RDR6 gene expression was statistically significantly reduced in NbRDR6i and GFP16c/RDR6i plants when compared to that in the wild-type RDR6 (Nb and GFP16c) plants (Fig. 3a, b; Table S1). Moreover, qRT-PCR analyses showed that TCV-GFPΔCP RNA accumulation reached equivalent levels with no statistical significance in wild type N. benthamiana (Nb) and RDR6-silenced NbRDR6i (Fig. 3a, b; Table S1). We also showed that the levels of TCV-GFPΔCP RNA in the inoculated leaves of GFP16c and GFP16c/RDR6i were similar, but decreased substantially when compared to the viral RNA levels in wild-type Nb or RDR6-silenced NbRDR6i plants, respectively (Fig. 3a, b; Table S1). This is suggesting a partial resistance to TCV-GFPΔCP in the GFP16c lines. Such resistance may be derived from homologous sequence-dependent specific targeting to the GFP marker gene, which then resulted in degradation of the recombinant virus by RNA silencing. It is worthwhile noting that the transgenic GFP16c line and the wild-type N. benthamiana plants are susceptible to infection with TCV, Tobacco mosaic virus and Potato virus X3,17,18, suggesting the GFP16c line should not have abnormalities in viral immunity at least for these viruses. Furthermore, TCV-GFPΔCP was always found to be restricted to single epidermal cells with a wild-type or RDR6-silenced genetic background (Fig. 3c, d). These data indicate that TCV-GFPΔCP was an effective inducer and the target of intracellular RNA silencing and that RDR6 was not required for the induction of antiviral RNA silencing in individual epidermal cells.
RDR6-independent intracellular RNA silencing.
(a and b) The RDR6 gene does not affect replication of TCV-GFPΔCP. The RDR6 mRNA were analysed 12 days post-inoculation (dpi) by quantitative (q) RT-PCR, in triplicate, using total RNAs extracted from mock- or TCV-GFPΔCP-inoculated leaves of wild-type N. benthamiana (Nb) and NbRDR6i (RDR6i), transgenic GFP 16c (GFP16c) and GFP16c/RDR6i plants. Virus-induced intracellular RNA silencing targets TCV-GFPΔCP. TCV-GFP RNA was analysed by qRT-PCR in triplicate, using total RNAs extracted from mock- or TCV-GFPΔCP-inoculated GFP16c and GFP16c/RDR6i leaves at 12 dpi. N. benthamiana housekeeping GAPDH (a) and EF1α (b) transcripts were used as internal controls. Relative RNA levels of RDR6 or TCV were obtained by normalising against the baseline expression levels of GAPDH (a) and EF1α (b) mRNA, respectively and showed similar tendencies between the two internal controls. Student's t-tests were carried out to evaluate whether there would be any statistical significance in RNA levels between different biological samples (Table S1). (c and d) Fluorescence microscopic examination of gfp expression in a single epidermal cell in TCV-GFPΔCP-inoculated Nb (c) or NbRDR6i (d) leaves 6 dpi using a Zeiss Axiophot microscope through a green filter. Bar = 100 µm.
Taken together, our findings support the hypothesis that RDR6 is probably involved in promoting the cell-to-cell spread of TCV-GFPΔCP induced RNA silencing and the significant decrease in the intercellular RNA silencing in GFP16c/RDR6i plants is not due to any impact from RDR6 on viral RNA replication and intercellular virus trafficking.
Discussion
Plants have multiple RDR genes that function in different biological processes. In Arabidopsis, there are six RDR genes (RDR1-6) that encode potential RNA dependent RNA polymerases (RDRs), of which RDR6 is the major RDR acting in post-transcriptional gene silencing and virus induced RNA silencing11,19,20, while RDR2 plays an important role in transcriptional gene silencing21. RDR6-dependent RNA silencing forms an important antiviral defence. It defends both differentiated and meristematic tissues from viral invasion. RDR6 silencing causes plants to be hyper-susceptible to virus infection16,22,23. RDR1 is also involved in antiviral RNA silencing, but its effects on plant responses to viral invasion tend to be virus specific24,25,26,27. Recently, small RNA deep sequencing revealed a role for RDR1 and RDR6 in the biogenesis of viral siRNA24,28 and showed RDR6 was essential for the maintenance and transitivity of RNA silencing19,29,30,31. In Arabidopsis, RDR6 is also thought to play a role in long-range (> 10 – 15 cell) intercellular and systemic RNA silencing, but not in short-range (10 – 15 cell) cell-to-cell signalling of RNA silencing that was initiated in companion and phloem cells10,16,32.
However, considering the fact that TCV-GFPΔCP is restricted to single epidermal cell of mechanically inoculated leaves, our data suggest that the short-range (6 – 10 cell) intercellular trafficking of virus-induced RNA silencing from a single epidermal cell to adjacent palisade and spongy parenchyma cells in N. benthamiana may require a functional RDR6 gene (Table 1; Fig. 1; Fig. 2). It is possible that spread of this type of RNA silencing may be associated with a small RNA signal8. The RDR6 protein is known to bind ssRNA, dsRNA and even dsDNA in plants33. Thus, RDR6 and small RNAs could form an RNA-protein complex that may directly promote 6 – 10 cell-to-cell movement of silencing signal. This model is consistent with the localization of the RDR6 protein in the cytoplasm, the nucleus and the cell membrane34,35. Alternatively, RDR6 may be required to produce a signal for limited intercellular silencing. Single cells with a normal RDR6 function in GFP16c plants could generate sufficient quantities of mobile signal molecules to enable the formation of silencing foci. In contrast, reduced RDR6 activity in GFP16c/RDR6i plants could lead to decreased synthesis of such a signal. Thus, the level of the mobile signal may be an important factor for intercellular RNA silencing. In this scenario, RDR6 may play an indirect role in short-range (6 – 10 cell) trafficking of RNA silencing. Also supporting a basic role of RDR6, not only the size but also the number of foci is lower in the RDR6-deficient plants. Moreover, some silencing foci were detected in the lower epidermis of leaves inoculated in their upper faces (Fig. 1). However, this could be caused by that RDR6 was not completely knocked out and residual RDR6 activity might persist in the RDR6i plants. Indeed, low but quantifiable levels of RDR6 gene expression were readily detectable in all RDR6-silenced plants (Fig. 3a, b). A third possibility could be that indirect impacts of the knockdown of RDR6 on the plant defensive responses, or even on the physiological transport of macro-molecules might affect the cell-to-cell spread of virus induced RNA silencing. It should be noted that the cell-to-cell spread of RNA silencing in TCV-GFPΔCP/GFP16c-based local RNA silencing assay depends on the expression of functional P8 and P9 proteins of TCV14. This assay is also susceptible to different VSRs17. These factors may affect how RDR6 protein is recruited to promote 6 – 10 cell-to-cell spread of virus-induced RNA silencing, a process that may have different genetic and molecular requirements in Arabidopsis and other plants8,9,10,11,12. Regardless of the underpinning mechanism, our data have revealed a previously unknown function for the RDR6 gene in short-range (6 – 10 cell) intercellular RNA silencing in N. benthamiana.
Our unexpected finding contradicts previous reports that RDR6 was not involved in the limited 10 – 15 cell-to-cell spread of RNA silencing in Arabidopsis8,9,10,11,12. Such a discrepancy may be due to a combination of factors (Table S2). Compared with Arabidopsis, N. benthamiana is permissive and can be a non-native host for a wide range of plant viruses, supposedly due to a naturally occurring mutation in the N. bethamiana RDR1 gene26,27. Indeed, N. benthamiana and Arabidopsis plants respond differently to TCV-GFPΔCP. For instance, TCV CP is essential for cell-to-cell virus movement in N. benthamiana3,36, but not in Arabidopsis5. Consequently, TCV-GFPΔCP moved effectively from cell-to-cell to form multi-cellular lesions in inoculated Arabidopsis leaves (unpublished data). However, the same virus was restricted to single cell in wild-type and RDR6i N. benthamiana plants (Fig. 3c, d). Such distinctive virus-host responses and different genetic backgrounds may affect the biosynthesis and spread of the silencing signal, thus have a significant impact on TCV-GFPΔCP-mediated local RNA silencing in these plant species.
On the other hand, confinement of virus infection to individual epidermis cells was only demonstrated in plants not expressing GFP (Fig. 3c, d), but not directly in the centre of the silenced foci of the GFP16c and GFP16c/RDR6i plants or in single infected cells that are not surrounded of silenced foci in the GFP16c/RDR6i plants. However, we had checked hundreds of silencing foci under epi-fluorescent and confocal microscopes, no individual epidermal cells with viral transient GFP fluorescence in the centre of silencing foci were ever found, although GFP-expressing cells of Nb and NbRDR6i leaves inoculated with TCV-GFPΔCP were readily observed at 2 – 12 dpi. Virus can be a trigger and a target of virus-induced RNA silencing (Fig. 3a, b). Once a silencing focus became visible, virus-induced RNA silencing would be certainly well-established and it could target viral RNAs for degradation. Therefore, accumulation of viral RNAs and proteins in single epidermal cells could be reduced to an undetectable level. Nevertheless, our findings emphasise that the signalling of intercellular RNA silencing is a more complex process than previously proposed (Table S2). This process may have different molecular components in different cell types in same plants and exhibit distinct genetic requirements in different plant species.
Methods
Plasmid construction, inoculation and plant maintenance
Plasmid TCV-GFPΔCP has been described3. Wild-type (Nb) and RDR6-knocked down (NbRDR6i)16 N. benthamiana and Arabidopsis plants were inoculated with TCV-GFPΔCP as described3,37. Plants were maintained in an insect-free glasshouse at 25°C with supplementary lighting to give a 16-hour photoperiod. To measure the accumulation of viral RNA, total RNAs were extracted from tissues using the RNeasy Plant Minikit (Qiagen) as described17and assayed by Quantitative RT–PCR (qRT-PCR).
Local RNA silencing assay
Two-to-four seedlings of gfp-expressing transgenic lines of N. benthamiana (GFP16c and GFP16c/RDR6i)16 were mechanically inoculated with RNA produced by in vitro transcription from linearised TCV-GFPΔCP plasmid DNA in three separate experiments. The induction of intercellular gfp RNA silencing was examined under long-wavelength UV light and recorded photographically through a Kodak No. 58 filter using a Nikon Digital Camera Coolpix995 as described14. Regions of leaf lamina in which silencing of gfp RNA occurred show only red chlorophyll fluorescence, while tissues expressing GFP show green fluorescence.
qRT-PCR assay
Virus or mock-inoculated leaves of 3 plants (Nb, NbRDR6i, GFP16c and GFP16c/RDR6i) were separately taken at 12 days post inoculation in repeated experiments for RNA extraction17. The first-stranded cDNA was synthesized using total RNAs treated with RNase-free DNase I as templates by the M-MLV Reverse Transcriptase (Promega). The qRT–PCR analyses of viral and RDR6 mRNA levels were performed using specific primers (Table S3) and the iQTM SYBR Green Supermix. The amplification program for SYBR Green I was performed at 95°C for 10 seconds and 55°C for 30 seconds on the CFX96 machine (Bio-Rad), following the manufacturer's instructions. Triplicate quantitative assays were performed on cDNA of each biological sample. The relative quantification of viral or RDR6 mRNA was calculated using the formula  and normalized to the amount of GAPDH (Genbank accession number TC17509) or EF1α (Genbank accession number AY206006) mRNA detected in the same sample, respectively.
and normalized to the amount of GAPDH (Genbank accession number TC17509) or EF1α (Genbank accession number AY206006) mRNA detected in the same sample, respectively.
Fluorescence and laser confocal microscopy
Mock- or virus-inoculated leaves were collected, examined under a Zeiss Axiphot microscope through a green filter and photographed with a Nikon Coolpix995 digital camera38,39, or under an LSM 710 Laser Scanning Microscope using settings to visualise GFP green and chlorophyll red fluorescence40.
References
Shi, Y., Gu, M., Fan, Z. & Hong, Y. RNA silencing suppressors: how viruses fight back. Future Virol. 3, 125–133 (2008).
Qu, F., Ren, T. & Morris, T. J. The coat protein of turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J. Virol. 77, 511–522 (2003).
Ryabov, E. V., van Wezel, R., Walsh, J. & Hong, Y. Cell-to-Cell, but not long-distance spread of RNA silencing that is induced in individual epidermal cells. J. Virol. 78, 3149–3154 (2004).
Azevedo, J. et al. Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev. 24, 904–915 (2010).
Deleris, A. et al. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313, 68–71 (2006).
Mérai, Z. et al. Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J. Virol. 80, 5747– 5756 (2006).
Endres, M. W. et al. Two plant viral suppressors of silencing require the ethylene-inducible host transcription factor RAV2 to block RNA silencing. PLoS Pathog. 6, e1000729 (2010).
Melnyk, C. W., Molnar, A. & Baulcombe, D. C. Intercellular and systemic movement of RNA silencing signals. EMBO J. 30, 3553–3563 (2011).
Dunoyer, P., Himber, C. & Voinnet, O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nature Genet. 37, 1356–1360 (2005).
Himber, C., Dunoyer, P., Moissiard, G., Ritzenthaler, C. & Voinnet, O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J. 22, 4523–4533 (2003).
Dunoyer, P., Himber, C., Ruiz-Ferrer, V., Alioua, A. & Voinnet, O. Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nature Genet. 39, 848–856 (2007).
Dunoyer, P. et al. Small RNA duplexes function as mobile silencing signals between plant cells. Science 328, 912–916 (2010).
Molnar, A. et al. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328, 872–875 (2010).
Zhou, Y., Ryabov, E., Zhang, X. & Hong, Y. Influence of viral genes on the cell-to-cell spread of RNA silencing. J. Exp. Bot. 59, 2803–2813 (2008).
Vogler, H. et al. Tobacco mosaic virus movement protein enhances the spread of RNA silencing. PloS Pathog. 4, e1000038 (2008).
Schwach, F., Vaistij, F. E., Jones, L. & Baulcombe, D. C. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 138, 1842–1852 (2005).
Shi, Y. et al. Suppression of local RNA silencing is not sufficient to promote cell-to-cell movement of Turnip crinkle virus in Nicotiana benthamiana. Plant Signal. Behav. 4, 15–22 (2009).
Van Wezel, R. & Hong, Y. Virus survival of RNA silencing without deploying protein-mediated suppression in Nicotiana benthamiana. FEBS Lett. 562, 65–70 (2004).
Dalmay, T., Hamilton, A., Rudd, S., Angell, S. & Baulcombe, D. C. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101, 543–553 (2000).
Xie, Z., Fan, B., Chen, C. & Chen, Z. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. USA. 98, 6516–6521 (2001).
Xie, Z. et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2, E104 (2004).
Muangsan, N., Beclin, C., Vaucheret, H. & Robertson, D. Geminivirus VIGS of endogenous genes requires SGS2/SDE1 and SGS3 and defines a new branch in the genetic pathway for silencing in plants. Plant J. 38, 1004–1014 (2004).
Qu, F. et al. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J. Virol. 79, 15209–15217 (2005).
Garcia-Ruiz, H. et al. Arabidopsis RNA-dependent RNA polymerases and Dicer-Like proteins in antiviral defense and small interfering RNA biogenesis during Turnip mosaic virus infection. Plant Cell 22, 481–496 (2010).
Rakhshandehroo, F., Takeshita, M., Squires, J. & Palukaitis, P. The Influence of RNA-Dependent RNA Polymerase 1 on Potato virus Y Infection and on Other Antiviral Response Genes. Mol. Plant-Microbe Interact. 22, 1312–1318 (2009).
Yang, S.-J., Carter, S. A., Cole, A. B., Cheng, N.-H. & Nelson, R. S. A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA. 101, 6297–6302 (2004).
Ying, X.-B. et al. RNA-dependent RNA polymerase 1 from Nicotiana tabacum suppresses RNA silencing and enhances viral infection in Nicotiana benthamiana. Plant Cell 22, 1358–1372 (2010).
Qi, X., Bao, F. S. & Xie, Z. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PloS ONE 4, e4971 (2009).
Dalmay, T., Horsefield, R., Braunstein, T. H. & Baulcombe, D. C. SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 20, 2069–2078 (2001).
Mourrain, P. et al. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101, 533–542 (2000).
Vaistij, P. E., Jones, L. & Baulcombe, D. C. Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell 14, 857–867 (2002).
Brosnan, C. A. et al. Nuclear gene silencing directs reception of long-distance mRNA silencing in Arabidopsis. Proc. Natl. Acad. Sci. USA. 104, 14741–14746 (2007).
Curaba, J. & Chen, X. Biochemical activities of Arabidopsis RNA-dependent RNA polymerase 6. J. Biol. Chem. 283, 3059–3066 (2008).
Hoffer, P. et al. Posttranscriptional gene silencing in nuclei. Proc. Natl. Acad. Sci. USA. 108, 409–414 (2011).
Luo, Z. & Chen, Z. Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell. 19, 943–958 (2007).
Cohen, Y., Gisel, A. & Zambryski, P. C. Cell-to-cell and systemic movement of recombinant green fluorescent protein-tagged turnip crinkle viruses. Virology 273, 258–266 (2000).
Li, C. et al. A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J. Virol. 83,3540–3548 (2009).
Hong, Y., Stanley, J. & Van Wezel, R. Novel system for the simultaneous analysis of geminivirus DNA replication and plant interactions in Nicotiana benthamiana. J. Virol. 77, 13315–13322 (2003).
Dong, X., Van Wezel, R., Stanley, J. & Hong. Y. Functional characterization of the nuclear localization signal for a suppressor of posttranscriptional gene silencing. J. Virol. 77, 7026–7033 (2003).
Li, C. et al. Mobile FT mRNA contributes to the systemic florigen signalling in floral induction. Sci. Rep. 1, 73; 10.1038/srep00073 (2011).
Acknowledgements
We thank David Baulcombe for providing seed of transgenic lines GFP16c, GFP16c/RDR6i and NbRDR6i and critical comments on the original manuscript, Andy Maule and Bryan Harrison for constructive suggestions and Michael Wilson for critical reading of the manuscript. We are grateful to John Carr and Jack Westwood for providing the qRT-PCR protocol. We thank the Special Foundation for Hangzhou Talents, the Science Foundation of the Key Laboratory of Hangzhou (20090232T05) and the China Natural Science Foundation (NSFC30770185, 30870180) for grants and a scholarship to N.S. and the Ministry of Higher Education of Egypt for a PhD studentship to A.M. H.Z. was supported by an R&D grant from Warwick Ventures to Y.H. The work is also in part supported by an innovative R&D grant from Hangzhou Normal University to Y.H.
Author information
Authors and Affiliations
Contributions
C.Q., N.S., M.G., H.Z., B.L., J.S. and A.M. designed and performed experiments, C.L. and E.R. performed researches, H.W., Y.L., T.O. and M.V. contributed through discussions and revised the paper. Y.H. initiated the project, designed the experiments and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Involvement of RDR6 in short-range intercellular RNA silencing in Nicotiana benthamiana
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Qin, C., Shi, N., Gu, M. et al. Involvement of RDR6 in short-range intercellular RNA silencing in Nicotiana benthamiana. Sci Rep 2, 467 (2012). https://doi.org/10.1038/srep00467
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00467
This article is cited by
-
Overview of plant RNA dependent RNA polymerases in antiviral defense and gene silencing
Indian Journal of Plant Physiology (2017)
-
Chilli leaf curl virus-based vector for phloem-specific silencing of endogenous genes and overexpression of foreign genes
Applied Microbiology and Biotechnology (2017)
-
Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana
BMC Plant Biology (2016)
-
Requirement of CHROMOMETHYLASE3 for somatic inheritance of the spontaneous tomato epimutation Colourless non-ripening
Scientific Reports (2015)
-
Differential response of diverse solanaceous hosts to tomato leaf curl New Delhi virus infection indicates coordinated action of NBS-LRR and RNAi-mediated host defense
Archives of Virology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.