Abstract
Study design:
Prospective experimental.
Objectives:
The aim of this study was to develop a quantitative sensory test (QST) that could be used for assessing the level and the density (degree of impairment) of spinal cord injury (SCI) and for monitoring neurological changes in patients with SCI.
Setting:
National Spinal Injuries Centre, Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust, UK.
Methods:
Perceptual threshold to 3 Hz cutaneous electrical stimulation was measured in 30 control subjects and in 45 patients with SCI at American Spinal Injuries Association (ASIA) sensory key points for selected dermatomes between C3 and S2 bilaterally. Electrical perceptual threshold (EPT) was recorded as the lowest ascending stimulus intensity out of three tests at which the subject reported sensation. The level of SCI according to EPT results was established for right and left sides as the most caudal spinal segment at which patient's EPT was within the control range (mean±2 standard deviation (SD)). The level of SCI, according to EPT, was then compared with clinical sensory level derived according to ASIA classification.
Results:
In the control group, EPT depended on the dermatome tested and was lowest for T1 (1.01±0.23 mA, mean±SD) and highest for L5 (3.32±1.14 mA). There was strong correlation between corresponding right and left dermatomes and between repeated assessments. In the SCI group, the level of lesion according to EPT and clinical testing was the same in 43 of the 90 tests (48%). In 37 cases (41%), the EPT level was higher than the clinical level, and in 10 cases (11%), it was lower. Below the level of lesion in incomplete SCI and in the zone of partial preservation in complete SCI, the EPT values in most dermatomes were raised compared with the control group.
Conclusions:
EPT is a simple, reproducible QST that can assess both the level and the density of SCI. It seems to add sensitivity and resolution to the standard clinical testing and could be a useful adjunct in longitudinal monitoring of patients with SCI for research purposes during natural recovery and therapeutic interventions.
Sponsorship:
International Spinal Research Trust (ISRT), UK, Grant CLI001.
Similar content being viewed by others
Introduction
The work presented in this paper was part of the International Spinal Research Trust (ISRT) Clinical Initiative study. It was commissioned by ISRT with the aim of developing a battery of tests for assessing the level and the density (degree of impairment below the lesion level) of spinal cord injury (SCI), which could be used in clinical trials for monitoring efficacy of new therapeutic interventions. Because of the anticipated nature of the first ISRT-sponsored clinical trials, specific requirements of the study were that newly developed tests be able to detect minor neurological changes over a few spinal segments and be applicable in the thoracic region. The technique presented here was developed keeping these requirements in mind, but it can be used in patients with any level and type of SCI.
The need for quantitative sensory tests (QST) as a supplement to standard clinical neurological examination in patients with SCI has been emphasised in the past. Vibration and thermal sensation thresholds1, 2, 3 and somatosensory and motor-evoked potentials4 have been suggested as useful additions to clinical testing in evaluating SCI. Techniques utilising electrical current perception threshold have been used for assessing sensory function in different peripheral neuropathies,5 particularly diabetic,6, 7, 8 in radiculopathies9, 10 and post-transplant surgery,11 but they have not been reported in patients with SCI so far.
For the purpose of this study, the technique of perceptual threshold to cutaneous electrical stimulation, originally developed in control subjects by Davey et al12 in 2001, was adapted to suit patients with SCI of any level, including thoracic, and of any impairment grade.
Preliminary findings of this study were presented as part of an overall progress review of the ISRT Clinical Initiative study at the International Spinal Cord Society (ISCoS) annual scientific meeting in 2003.13
Materials and methods
The study was approved by Aylesbury Vale Local Research Ethics Committee. All volunteers were given a written information sheet and a verbal explanation of the procedure before signing a consent form.
Subjects
Perceptual thresholds to cutaneous electrical stimulation were measured in 30 control subjects with no known neurological deficit and in 45 patients with SCI of different level and impairment grade. Subjects with any known neurological disorder (other than SCI in the patient group) were excluded, as were subjects with diabetes mellitus even in the absence of diagnosed peripheral neuropathy.
The measurements were repeated within 3 months of the original test in eight control subjects.
All patient volunteers also had a clinical neurological examination and classification carried out according to American Spinal Injuries Association (ASIA) standards,14, 15 so that the perceptual threshold results could be compared with clinical sensory examination results. The same examiner performed both tests. This ensured the consistency in electrode placement over clinically tested ASIA sensory key points.
Technique
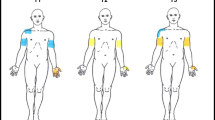
An electrical stimulator (Digitimer DS7 connected through Digitimer D4030) was used to produce constant current square wave electrical pulses of 0.5 ms duration, 3 Hz stimulation frequency and variable amplitude. Stimuli were delivered via a self-adhesive circular cathode 20 mm in diameter (Arbo Neonatal ECG Blue) to the skin over ASIA sensory key points in dermatomes between C3 and S2 (Figure 1). The inactive anode was a metal plate electrode measuring 40 × 60 mm, strapped around the forearm. Electrodes were placed only on intact skin, cleaned with alcohol wipes (preinjection swabs) and allowed to dry. Every subject had a trial run in a dermatome with normal innervation and with stimulus intensity above expected sensory threshold in order for them to recognise the sensation. It was described as a gentle pulsating sensation and was not reported as painful by any of the volunteers. For every dermatome tested, stimulus intensity was manually turned up and down three times, until the subject first reported the sensation (ascending) and its disappearance (descending) under the cathode. Electrical perceptual threshold (EPT) was recorded as the lowest ascending stimulus intensity out of the three trials at which the subject reported sensation, expressed in mA, and measured to 0.1 mA precision. The maximum stimulus intensity was preset at 10 mA.
ASIA sensory key points by spinal dermatomes. Reproduced (with permission) from American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2002. Chicago, IL: American Spinal Injury Association; 200215
Subjects were lying comfortably on a bed in a warm, quiet room throughout the test. Informed consent procedure and detailed explanation of the test before testing allowed for a standard temperature adaptation time. In the control group, all dermatomes between C3 and S2 were tested bilaterally at ASIA sensory key points. Dermatome C2 was excluded because of the hair and dermatomes S3 and S4,5 because they were unacceptable to most control subjects. In the patient group, in order to reduce testing time, EPT was measured only in selected dermatomes: in several dermatomes, above the clinically determined level of injury; in several dermatomes, below the level of incomplete SCI injury; and in all dermatomes, in the zone of partial preservation in complete SCI. This way, total testing time in control subjects was 45–50 min, in patients with incomplete SCI 15–20 min and in patients with complete SCI 10–15 min.
Each patient's EPT results were then compared with the normative EPT values for every dermatome tested. The level of SCI according to EPT results was established for the right and left side in each patient as the most caudal spinal segment in which the patient's EPT was within the control group EPT range (mean±2 standard deviation (SD)).
The level of SCI according to EPT testing was compared with clinical sensory levels derived according to ASIA standards: first with overall clinical sensory level (the most caudal spinal segment with normal sensory function for both sensory modalities tested) and then separately with pin prick and light touch sensory level.
Statistical analysis
In the control group, EPT values for each dermatome were calculated as mean±SD. Paired sample t-test and Pearson correlation coefficient were used for comparison between the left- and right-side EPT results and t-test for comparing results between genders. Spearman's rank correlation coefficient was used for testing the correlation with age and between repeated measurements. Coefficient of variation (expressed as the percentage of the mean) was used to calculate the degree of variation between repeated measurements.
In the patient group, comparison between EPT and clinical results was expressed in absolute numbers and as percentages. χ2 test was used for comparing frequency of occurrence between patient subgroups.
Results
Control group
Demographic characteristics
The control group consisted of 30 volunteers, 14 male and 16 female participants, mean age of 32 years (range 20–55), with no known neurological disorder.
EPT results
The mean EPT varied depending on the dermatome tested and was lowest for dermatomes T1 (1.01±0.23 mA, mean±SD) and L1 (1.07±0.15) and highest for dermatomes L4 (3.25±1.16 mA) and L5 (3.32±1.14 mA). Control group results were used to construct a normative EPT template, by plotting mean+2 SD threshold values for each ASIA dermatome (Figure 2). The normative template was used for comparing individual patient's threshold results with the control group range.
Men had higher mean EPT than women for most dermatomes, but the difference was statistically significant only for the left L3 dermatome (t-test, P=0.02) and approached significance for the right L3 dermatome (t-test, P=0.054).
There was no correlation between EPT values and control subjects' age, except for the dermatome L5, where weak but significant positive correlation was found (Spearman's rank correlation coefficient r=0.27, P<0.05).
When the EPT results for corresponding dermatomes on the two sides of the body were compared, there was a significant, strong to very strong correlation (Pearson correlation coefficient r=0.57–0.95, P<0.01 depending on the dermatome) and the difference was not statistically significant (paired sample t-test, P>0.01). For this reason, the normative template (Figure 2) was constructed using the mean value of EPT for the two sides for each dermatome.
The results of the repeated measurements in eight control subjects correlated well with the first test (Spearman's correlation coefficient for pooled data r=0.88, P<0.01).
The coefficient of variation (CV) for repeated measurements differed by the dermatome and was lowest for C3 dermatome (CV=12.2%) and highest for L5 dermatome (CV=18.7%).
Patient group
Demographic and injury characteristics
The group consisted of 45 patient volunteers, 31 male and 14 female patients, mean age 43 years (range 19–70), with SCI of different levels (between C1 and T12) and different impairment grades (ASIA A, B, C and D). Twenty-nine patients had a thoracic injury and 16 cervical, 29 had a complete SCI (ASIA grade A), one sensory incomplete (ASIA grade B) and 15 motor incomplete (nine with ASIA grade C and six with ASIA grade D). All patients were more than 3-month post injury, in active rehabilitation or post initial discharge.
EPT results
As explained in Materials and methods, the level of SCI according to EPT results was established for the right and left sides in each patient as the lowest segment in which the patient's EPT was within the control group EPT range (mean±2 SD). An example of a patient's results superimposed on the normative template is shown in Figure 3.
Because the level of SCI can differ on the two sides of the body, when determining the EPT, the right and left sides were treated separately. Therefore, there was a total of 90 sides tested (45 patients).
The level of injury according to EPT testing was first compared with the overall clinical sensory level (the most caudal spinal segment with normal sensory function for both sensory modalities – pin prick and light touch). Levels of injury according to EPT and clinical testing were the same in 43 of the 90 sides tested (48%). In the remaining 47 tested sides, the levels differed and the EPT level was usually higher than the clinical level (37 sides, 41%) and usually by one to two segments. In 10 tested sides (11%), the level according to EPT was lower than the clinically determined level (Figure 4).
The level of injury according to EPT was then compared with the pin prick and the light touch sensory levels separately (Figure 5).
The level of injury according to EPT was the same as the clinical pin prick level in 43 of the 90 sides tested (48%) and same as the clinical light touch level in 34 of the 90 sides tested (38%). The EPT level was higher than the clinical pin prick level in 37 cases (41%) and higher than the clinical light touch level in 45 cases (50%). In 10 cases (11%), the level according to EPT was lower than the clinical pin prick level, and in 11 cases (12%), lower than the clinical light touch level.
When patients with cervical and thoracic injuries were compared, there was no statistically significant difference in the frequency of agreement between the clinical and EPT level of injury in the two patient subgroups (χ2 test, P>0.05). The same was true when patients with complete and incomplete SCI were compared (χ2 test, P>0.05).
Below the level of incomplete SCI and in the zone of partial preservation in patients with complete SCI, the EPT was usually raised compared with control values (mean+2 SD) for the given dermatome. In four patients with incomplete SCI, an EPT could not be measured in all dermatomes tested below the level of injury, particularly lower lumbar.
An EPT could sometimes be detected in dermatomes with none or only one clinical sensory modality partially preserved. This was observed in 11 patients (on 13 sides).
In two patients with incomplete SCI, an EPT could be determined in dermatomes with light touch grade ‘1=impaired’ and pin prick grade ‘0=absent’. Similarly, in five patients with complete SCI, in the zone of partial preservation, an EPT could be determined in dermatomes with only one sensory modality partially preserved (light touch in four patients and pin prick in one patient). In four patients with complete SCI, an EPT was determined in one segment below the zone of partial preservation, that is, in dermatomes that had absent sensation to both sensory modalities tested clinically (grade ‘0’ for light touch and pin prick). The EPT values in all the above cases were raised compared with the control values.
Discussion
In this study, a QST using electrical stimulation of ASIA sensory key points was developed for the first time for use in patients with SCI.
Development of the technique in the control group
The technique was based on the original work by Davey et al,12 in which perceptual threshold to cutaneous electrical stimulation and two-point discrimination were tested in seven spinal dermatomes and three trigeminal nerve fields in eight control subjects. The two tests did not show good correlation with each other, but both had good correlation between the corresponding stimulation sites on the left and right sides. EPT was found to be easier to measure and to have better repeatability of the two tests, and was suggested as a possible useful assessment tool to monitor recovery or deterioration in neuropathies or neurotrauma, or after surgery.
In our study, Davey's original technique was reproduced, but the stimulation sites were extended to include all 24 spinal dermatomes from C3 to S2. Similar to the results of Davey et al,12 our control group results showed expected variations between different dermatomes, strong correlation between the corresponding right and left dermatomes, and good repeatability. Somatotopic variations were most likely due to local differences in skin quality and distribution of somatosensory afferents at the stimulation sites. Interestingly, the only dermatome that showed statistically significant difference between men and women (L3) in our control group was also found to differ between genders in the study by Davey et al.12 The only dermatome that showed increased threshold values with age in our control group was dermatome L5 on the foot. Age-related differences in our study may have been minimised by the use of the low stimulation frequency, which has also been observed by others7, 16 albeit with the use of sinusoidally varying current waveforms rather than repetitive pulses. Additionally, however, it is worth mentioning that our control group had no subjects over the age of 55 years.
The L5 dermatome, when tested on the ASIA key point on the foot, had the highest perceptual threshold, the greatest variation between subjects and between repeated measurements and by age. When an alternative testing site within the L5 dermatome on the shin was tried, it significantly reduced those variations. However, for consistency, in this study we kept to the original ASIA sensory key points for all dermatomes.
Application of the technique in the patient group
Inclusion of all 24 spinal dermatomes between C3 and S2 allowed construction of a normative EPT template with which patients' results were compared (Figures 2 and 3). As gender- and age-related differences in our control group were minimal, the same template was used for all the patients.
When levels of SCI according to clinical sensory testing were compared with levels of injury according to EPT results in our patient group, both levels were the same in 48% of all the cases. In 41% of the cases, the EPT level of injury was higher than the clinical sensory level, in most cases by one or two segments. This suggests that the EPT test might be sensitive to small sensory impairments, which are not detectable by standard clinical testing. Similar observations were made in the past with other QSTs. Previous applications of QST measures2, 3 of perceptual threshold for temperature and vibration provided more sensitive detection of preserved sensory function than standard clinical examination in patients with SCI. However, in the remaining 10% of cases in our patient group, clinical testing seemed to be more sensitive, as the EPT level of injury was lower than the clinical level.
These observations would support the generally agreed assumption that a combination of two or more diagnostic tests improves accuracy of the diagnosis. This is especially important when only minor changes of improvement or deterioration are expected, as may be the case in anticipated therapeutic interventions in patients with SCI.
The main purpose of the study was to develop a QST that could be used for accurately determining the level of SCI, especially in the thoracic region, which currently relies mainly on clinical sensory testing. The EPT test can be equally applied to any level of SCI. It may also be useful for assessing the degree of impairment below the level of incomplete SCI and in the zone of partial preservation in complete SCI. Our results showed that several patients had a measurable EPT (above the control group reference range) in dermatomes graded ‘0’ for one or both clinical sensory testing modalities. The EPT thus adds sensitivity to clinical testing both at and below the level of SCI.
When the EPT results were compared with the two clinical sensory modalities separately, the agreement was better with the pin prick than with the light touch sensory level. It is possible that this could be partly owing to the choice of a low stimulation frequency,7, 12 but this is on the assumption that repetitive pulses are equivalent to sinusoidally varying current intensities, which is unlikely. Low-frequency sinusoidal stimulation allows preferential recruitment of small diameter fibres projecting predominantly via spinothalamic tract. Alternatively, the agreement could be related to the fact that the pin prick level was usually the higher of the two clinical levels and the EPT level was often higher than the overall clinical level. However, in the absence of pin prick sensation below the injury level, the EPT generally agreed with the light touch sensation. In the dermatomes with partially preserved light touch sensation (ASIA grade ‘1’) and absent pin prick sensation (ASIA grade ‘0’), the preserved sensation was often measurable by EPT testing, but with very high threshold values. It seems that the chosen 3 Hz frequency, notwithstanding the possibility of some relative neuroselectivity, was able to detect any type of sensory sparing at higher stimulus intensities. However, it is worth noting that within the preset test limits (10 mA maximum stimulus intensity), an EPT could not always be measured in dermatomes with present clinical sensory sparing, especially in lower lumbar dermatomes, which had high control threshold values.
The EPT also adds resolution to clinical sensory testing. By giving a continuous numeric threshold value in mA, the EPT can quantify the sensory impairment within the ASIA sensory grade ‘1=impaired’ and, in some cases, even within grade ‘0=absent’. This could be particularly useful in monitoring any changes in the degree of sensory impairment, not quantifiable by standard clinical testing, results of which are expressed on an ordinal scale (0=absent, 1=impaired or 2=normal). This would also make the statistical analysis of the results easier by giving a choice of more robust statistical tests.
As a QST, the EPT test greatly reduces examiner's subjectivity in sensory testing, which is further reduced by clearly defining the stimulation points as ASIA sensory key points and by using self-adhesive electrodes and thus eliminating the pressure that hand-held electrodes exert on the skin.
Patient's subjectivity in reporting the sensation remains however, and this inevitably affects reproducibility of the test. Repeated tests in our control group showed good reproducibility and an acceptable degree of variation, but all QSTs are known to show greater test-to-test variation in patient groups than in control groups.1, 7
Repeatability in SCI patients was not in the domain of this paper and will be addressed in future studies. In the current study, repeated measurements were performed only in six patients: in four patients with chronic stable SCI, the level of injury remained unchanged as judged both by clinical and EPT testing. In two patients with incomplete SCI, which clinically improved between the two measurements, the degree of impairment below the level of injury also improved judged by the EPT (the threshold values went down). However, the number of patients was too small to perform any statistical analysis. While the technique was being developed, two authors from this group tried the new test as one of the outcome measures in a pilot study of an experimental therapeutic intervention in SCI.17 The EPT showed reduction in threshold values in patients receiving the non-invasive experimental treatment (repetitive transcranial magnetic stimulation). Again, the number of patients was very small, as this was only a pilot study. It has since been approved as a full study and the patient recruitment is underway.
In order to test the reproducibility of the EPT technique in patients with SCI, and its sensitivity to neurological changes over time, longitudinal monitoring in greater number of patients with stable and changing neurological deficit is needed.
Conclusions
EPT is a simple, reproducible QST that can assess both the level and the density of SCI. It adds sensitivity to the neurological testing by detecting minor subclinical sensory impairments and improves resolution by quantifying the degree of sensory deficit.
EPT could be a useful adjunct to standard clinical testing in longitudinal monitoring of SCI for research purposes, both during natural recovery and in therapeutic clinical trials.
References
Krassioukov A, Wolfe DL, Hsieh JT, Hayes KC, Durham CE . Quantitative sensory testing in patients with incomplete spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1258–1263.
Hayes KC, Wolfe DL, Hsieth JT, Potter PJ, Krassioukov A, Durham CE . Clinical and electrophysiologic correlates of quantitative sensory testing in patients with incomplete spinal cord injury. Arch Phys Med Rehabil 2002; 83: 1612–1619.
Nicotra A, Ellaway PH . Thermal perception thresholds: assessing the level of human spinal cord injury. Spinal Cord advance online publication, 24 January 2006; doi:10.1038/sj.sc.3101877.
Curt A, Dietz V . Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord 1999; 37: 157–165.
Katims JJ, Naviasky EH, Rendell MS, Ng LK, Bleecker ML . Constant current sine wave transcutaneous nerve stimulation for the evaluation of peripheral neuropathy. Arch Phys Med Rehabil 1987; 68: 210–213.
Rendell MS, Dovgan DJ, Bergman TF, O'Donnell GP, Drobny EP, Katims JJ . Mapping diabetic sensory neuropathy by current perception threshold testing. Diabetes Care 1989; 12: 636–640.
Pitei DL, Watkins PJ, Stevens MJ, Edmonds ME . The value of the Neurometer in assessing diabetic neuropathy by measurement of the current perception threshold. Diabetic Med 1994; 11: 872–876.
Donaghue VM, Giurini JM, Rosenblum BI, Weissman PN, Veves A . Variability in function measurements of three sensory foot nerves in neuropathic diabetic patients. Diabetes Res Clin Pract 1995; 29: 37–42.
Yamashita T, Kanaka K, Sekine M, Takebayashi T, Kawaguchi S, Katahira G . A quantitative analysis of sensory function in lumbar radiculopathy using current perception threshold testing. Spine 2002; 27: 1567–1570.
Quraishi NA, Taherzadeh O, McGregor AH, Hughes SP, Anand P . Correlation of nerve root pain with dermatomal sensory threshold and back pain with spinal movement in single level lumbar spondylosis. J Bone Joint Surg Br 2004; 86: 74–80.
Chu NS . Current perception thresholds in toe-to-digit transplantation and digit-to-digit replantation. Muscle Nerve 1996; 19: 183–186.
Davey NJ, Nowicky AV, Zaman R . Somatotopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol 2001; 86: 127–130.
Ellaway PH et al. Towards improved clinical and physiological assessments of recovery in spinal cord injury: a clinical initiative. Spinal Cord 2004; 42: 325–337.
Maynard FM et al. International standards for neurological and functional classification of spinal cord injury. American spinal injury association. Spinal Cord 1997; 35: 266–274.
American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, Revised 2002. American Spinal Injury Association: Chicago, IL 2002.
Takekuma K, Ando F, Niino N, Shimokata H . Age and gender differences in skin sensory threshold assessed by current perception in community-dwelling Japanese. J Epidemiol 2000; 10: S33–S38.
Belci M, Catley M, Husain M, Frankel HL, Davey NJ . Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004; 42: 417–419.
Acknowledgements
The Clinical Initiative Study was funded by The International Spinal Research Trust, UK, Grant CLI001. We thank all the patient and control volunteers for their generous participation in the study, the members of the Clinical Initiative team for their contribution to the work and the staff at the National Spinal Injuries Centre, Stoke Mandeville Hospital for their help during the study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Savic, G., Bergström, E., Frankel, H. et al. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord 44, 560–566 (2006). https://doi.org/10.1038/sj.sc.3101921
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101921
Keywords
This article is cited by
-
Quantitative electrophysiological assessments as predictive markers of lower limb motor recovery after spinal cord injury: a pilot study with an adaptive trial design
Spinal Cord Series and Cases (2022)
-
Retraining walking over ground in a powered exoskeleton after spinal cord injury: a prospective cohort study to examine functional gains and neuroplasticity
Journal of NeuroEngineering and Rehabilitation (2019)
-
Application of electrophysiological measures in spinal cord injury clinical trials: a narrative review
Spinal Cord (2019)
-
Discrepancies between clinical assessments of sensory function and electrical perceptual thresholds after incomplete chronic cervical spinal cord injury
Spinal Cord (2016)
-
Reliability of the electrical perceptual threshold and Semmes-Weinstein monofilament tests of cutaneous sensibility
Spinal Cord (2013)