Abstract
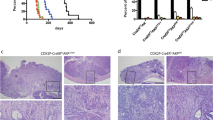
Flap endonuclease 1 (Fen1) and exonuclease 1 (Exo1) have sequence homology and similar nuclease capabilities. Both function in multiple pathways of DNA metabolism, but appear to have distinct in vivo nucleic acid substrates, and therefore distinct metabolic roles. When combined with Apc1638N, Fen1 promotes tumor progression. Because of functional similarity to Fen1, and because Exo1 is involved in DNA mismatch repair (MMR) by interaction with Msh2 and Mlh1, genes that cause hereditary nonpolyposis colorectal cancer (HNPCC), we investigated the possibility that Exo1 might also act as a modifier to Apc1638N. We present evidence that mice with combined mutations in Apc1638N and Exo1 and Apc1638N, Exo1 and Fen1 genes show moderate increased tumor incidence and multiplicity in comparison to Apc1638N siblings, implying a low penetrance role for Exo1 in early gastrointestinal (GI) tumorigenesis. Despite a decrease in median survival (10 months) in Apc1638N Exo1 mice, their tumors do not progress any more rapidly than those of Apc1638N. Instead these animals die from infections that are the result of impaired immune response. Apc1638N Exo1 Fen1 mice survive longer (18 months), and therefore appear relatively immune competent. They die of invasive GI tumors that display microsatellite instability (MSI). Our results show that Exo1 has a modest tumor suppressor function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alam NA, Gorman P, Jaeger EE, Kelsell D, Leigh IM, Ratnavel R et al. (2003). Germline deletions of EXO1 do not cause colorectal tumors and lesions which are null for EXO1 do not have microsatellite instability. Cancer Genet Cytogenet 147: 121–127.
Amin NS, Nguyen MN, Oh S, Kolodner RD . (2001). exo1-Dependent mutator mutations: model system for studying functional interactions in mismatch repair. Mol Cell Biol 21: 5142–5155.
Bardwell PD, Woo CJ, Wei K, Li Z, Martin A, Sack SZ et al. (2004). Altered somatic hypermutation and reduced class-switch recombination in exonuclease 1-mutant mice. Nat Immunol 5: 224–229.
Coletta PL, Muller AM, Jones EA, Muhl B, Holwell S, Clarke D et al. (2004). Lymphodepletion in the ApcMin/+ mouse model of intestinal tumorigenesis. Blood 103: 1050–1058.
Dzantiev L, Constantin N, Genschel J, Iyer RR, Burgers PM, Modrich P . (2004). A defined human system that supports bidirectional mismatch-provoked excision. Mol Cell 15: 31–41.
Edelmann L, Edelmann W . (2004). Loss of DNA mismatch repair function and cancer predisposition in the mouse: animal models for human hereditary nonpolyposis colorectal cancer. Am J Med Genet C Semin Med Genet 129: 91–99.
Edelmann W, Umar A, Yang K, Heyer J, Kucherlapati M, Lia M et al. (2000). The DNA mismatch repair genes Msh3 and Msh6 cooperate in intestinal tumor suppression. Cancer Res 60: 803–807.
Edelmann W, Yang K, Kuraguchi M, Heyer J, Lia M, Kneitz B et al. (1999). Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer Res 59: 1301–1307.
Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B et al. (1994). A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 91: 8969–8973.
Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E et al. (2005). Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol 6: 800–809.
Jagmohan-Changur S, Poikonen T, Vilkki S, Launonen V, Wikman F, Orntoft TF et al. (2003). EXO1 variants occur commonly in normal population: evidence against a role in hereditary nonpolyposis colorectal cancer. Cancer Res 63: 154–158.
Kucherlapati M, Yang K, Kuraguchi M, Zhao J, Lia M, Heyer J et al. (2002). Haploinsufficiency of Flap endonuclease (Fen1) leads to rapid tumor progression. Proc Natl Acad Sci USA 99: 9924–9929.
Lee Bi BI, Nguyen LH, Barsky D, Fernandes M, Wilson III DM . (2002). Molecular interactions of human Exo1 with DNA. Nucleic Acids Res 30: 942–949.
Lee BI, Wilson III DM . (1999). The RAD2 domain of human exonuclease 1 exhibits 5′ to 3′ exonuclease and flap structure-specific endonuclease activities. J Biol Chem 274: 37763–37769.
Liu Y, Kao HI, Bambara RA . (2004). Flap endonuclease 1: a central component of DNA metabolism. Annu Rev Biochem 73: 589–615.
Nielsen FC, Jager AC, Lutzen A, Bundgaard JR, Rasmussen LJ . (2004). Characterization of human exonuclease 1 in complex with mismatch repair proteins, subcellular localization and association with PCNA. Oncogene 23: 1457–1468.
Peltomaki P, Vasen HF . (1997). Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International collaborative group on hereditary nonpolyposis colorectal cancer. Gastroenterology 113: 1146–1158.
Rudolph C, Fleck O, Kohli J . (1998). Schizosaccharomyces pombe exo1 is involved in the same mismatch repair pathway as msh2 and pms1. Curr Genet 34: 343–350.
Schmutte C, Marinescu RC, Sadoff MM, Guerrette S, Overhauser J, Fishel R . (1998). Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Res 58: 4537–4542.
Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R . (2001). The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem 276: 33011–33018.
Sokolsky T, Alani E . (2000). EXO1 and MSH6 are high-copy suppressors of conditional mutations in the MSH2 mismatch repair gene of Saccharomyces cerevisiae. Genetics 155: 589–599.
Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF et al. (1997). Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci USA 94: 7487–7492.
Tran HT, Gordenin DA, Resnick MA . (1999). The 3′ → 5′ exonucleases of DNA polymerases delta and epsilon and the 5′ → 3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol Cell Biol 19: 2000–2007.
Tran PT, Erdeniz N, Dudley S, Liskay RM . (2002). Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst) 1: 895–912.
Tran PT, Erdeniz N, Symington LS, Liskay RM . (2004). EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 3: 1549–1559.
Tran PT, Simon JA, Liskay RM . (2001). Interactions of Exo1p with components of MutLalpha in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 98: 9760–9765.
Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T et al. (2003). Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev 17: 603–614.
Wu Y, Berends MJ, Post JG, Mensink RG, Verlind E, Van Der Sluis T et al. (2001). Germline mutations of EXO1 gene in patients with hereditary nonpolyposis colorectal cancer (HNPCC) and atypical HNPCC forms. Gastroenterology 120: 1580–1587.
Yamamoto H, Hanafusa H, Ouchida M, Yano M, Suzuki H, Murakami M et al. (2005). Single nucleotide polymorphisms in the EXO1 gene and risk of colorectal cancer in a Japanese population. Carcinogenesis 26: 411–416.
Acknowledgements
This work is supported by NIH grants CA-084301 and NIEHS grant ES-011040 (RS Kucherlapati).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kucherlapati, M., Nguyen, A., Kuraguchi, M. et al. Tumor progression in Apc1638N mice with Exo1 and Fen1 deficiencies. Oncogene 26, 6297–6306 (2007). https://doi.org/10.1038/sj.onc.1210453
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210453
Keywords
This article is cited by
-
Somatic APC mosaicism and oligogenic inheritance in genetically unsolved colorectal adenomatous polyposis patients
European Journal of Human Genetics (2018)
-
Variants and haplotypes in Flap endonuclease 1 and risk of gallbladder cancer and gallstones: a population-based study in China
Scientific Reports (2015)
-
Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer
Genome Biology (2011)
-
FEN1 contributes to telomere stability in ALT-positive tumor cells
Oncogene (2009)