Abstract
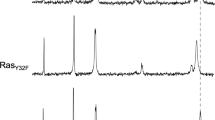
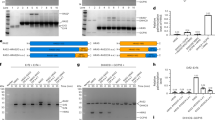
Raf-1 activation is a complex process which involves plasma membrane recruitment, phosphorylation, protein-protein and lipid-protein interactions. We now show that PP1 and PP2A serine-threonine phosphatases also have a positive role in Ras dependent Raf-1 activation. General serine-threonine phosphatase inhibitors such sodium fluoride, or ß-glycerophosphate and sodium pyrophosphate, or specific PP1 and PP2A inhibitors including microcystin-LR, protein phosphatase 2A inhibitor I1 or protein phosphatase inhibitor 2 all abrogate H-Ras and K-Ras dependent Raf-1 activation in vitro. A critical Raf-1 target residue for PP1 and PP2A is S259. Serine phosphatase inhibitors block the dephosphorylation of S259, which accompanies Raf-1 activation, and Ras dependent activation of mutant Raf259A is relatively resistant to serine phosphatase inhibitors. Sucrose gradient analysis demonstrates that serine phosphatase inhibition increases the total amount of 14-3-3 and Raf-1 associated with the plasma membrane and significantly alters the distribution of 14-3-3 and Raf-1 across different plasma membrane microdomains. These observations suggest that dephosphorylation of S259 is a critical early step in Ras dependent Raf-1 activation which facilitates 14-3-3 displacement. Inhibition of PP1 and PP2A therefore causes plasma membrane accumulation of Raf-1/14-3-3 complexes which cannot be activated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M . 2000 J. Biol. Chem. 275: 22300–22304
Carroll MP, May WS . 1994 J. Biol. Chem. 269: 1249–1256
Cary LA, Cooper JA . 2000 Nature 404: 945–947
Ceresa BP, Schmid SL . 2000 Curr. Opin. Cell. Biol. 12: 204–210
Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, Cobb MH, Marshall MS, Brugge JS . 2000 Curr. Biol. 10: 551–554
Cissel DS, Beaven MA . 2000 J. Biol. Chem. 275: 7066–7070
Clark GJ, Drugan JK, Rossman KL, Carpenter JW, Rogers-Graham K, Fu H, Der CJ, Campbell SL . 1997 J. Biol. Chem. 272: 20990–20993
Couet J, Sargiacomo M, Lisanti MP . 1997 J. Biol. Chem. 272: 30429–30438
Daub M, Jockel J, Quack T, Weber CK, Schmitz F, Rapp UR, Wittinghofer A, Block C . 1998 Mol. Cell. Biol. 18: 6698–6710
Dent P, Jelinek T, Morrison DK, Weber MJ, Sturgill TW . 1995 Science 268: 1902–1906
Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ . 1994 Embo J 13: 4269–4277
Drugan JK, Khosravi Far R, White MA, Der CJ, Sung YJ, Hwang YW, Campbell SL . 1996 J. Biol. Chem. 271: 233–237
Fabian JR, Daar IO, Morrison DK . 1993 Mol. Cell. Biol. 13: 7170–7179
Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM . 1996 J. Biol. Chem. 271: 8472–8480
Hafner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W . 1994 Mol. Cell. Biol. 14: 6696–6703
Huang DC, Marshall CJ, Hancock JF . 1993 Mol. Cell. Biol. 13: 2420–2431
Improta-Brears T, Ghosh S, Bell RM . 1999 Mol. Cell. Biochem. 198: 171–178
King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS . 1998 Nature 396: 180–183
Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR . 1993 Nature 364: 249–252
Kurzchalia TV, Parton RG . 1999 Curr. Opin. Cell. Biol. 11: 424–431
Leevers SJ, Paterson HF, Marshall CJ . 1994 Nature 369: 411–414
Liu P, Ying Y, Anderson RG . 1997 Proc. Natl. Acad. Sci. USA 94: 13666–13670
Liu P, Ying Y, Ko YG, Anderson RG . 1996 J. Biol. Chem. 271: 10299–10303
Luo Z, Diaz B, Marshall M, Avruch J . 1997 Mol. Cell. Biol. 17: 46–53
Marais R, Light Y, Paterson HF, Marshall CJ . 1995 EMBO J 14: 3136–3145
Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ . 1997 J. Biol. Chem. 272: 4378–4383
Marais R, Marshall CJ . 1996 Cancer Surv. 27: 101–125
Marshall CJ . 1995a Cell 80: 179–185
Marshall M . 1995b Mol. Reprod. Dev. 42: 493–499
Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R . 1999 Embo J 18: 2137–2148
McPherson RA, Harding A, Roy S, Lane A, Hancock JF . 1999 Oncogene 18: 3862–3869
Michaud NR, Fabian JR, Mathes KD, Morrison DK . 1995 Mol. Cell. Biol. 15: 3390–3397
Mineo C, Anderson RG, White MA . 1997 J. Biol. Chem. 272: 10345–10348
Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, Philipp A, Kolch W . 1996 Mol. Cell. Biol. 16: 5409–5418
Morrison DK, Cutler RE . 1997 Curr. Opin. Cell. Biol. 9: 174–179
Morrison DK, Heidecker G, Rapp UR, Copeland TD . 1993 J. Biol. Chem. 268: 17309–17316
Mott HR, Carpenter JW, Zhong S, Ghosh S, Bell RM, Campbell SL . 1996 Proc. Natl. Acad. Sci. USA 93: 8312–8317
Muslin AJ, Tanner JW, Allen PM, Shaw AS . 1996 Cell 84: 889–897
Okada T, Hu CD, Jin TG, Kariya K, Yamawaki-Kataoka Y, Kataoka T . 1999 Mol. Cell. Biol. 19: 6057–6064
Okamoto T, Schlegel A, Scherer PE, Lisanti MP . 1998 J. Biol. Chem. 273: 5419–5422
Plyte S, Majolini MB, Pacini S, Scarpini F, Bianchini C, Lanfrancone L, Pelicci P, Baldari CT . 2000 Oncogene 19: 1529–1537
Pol A, Calvo M, Enrich C . 1998 FEBS Lett. 441: 34–38
Pol A, Lu A, Pons M, Peiro S, Enrich C . 2000 J. Biol. Chem. 275: 30566–30572
Prior I, Harding A, Yan J, Sluimer J, Parton R, Hancock JF . 2001 Nature Cell Biol. 3: 368–375
Rizzo MA, Shome K, Watkins SC, Romero G . 2000 J. Biol. Chem. 275: 23911–23918
Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ . 1999 Science 286: 1738–1741
Rommel C, Radziwill G, Lovric J, Noeldeke J, Heinicke T, Jones D, Aitken A, Moelling K . 1996 Oncogene 12: 609–619
Roy S, Lane A, Yan J, McPherson R, Hancock JF . 1997 J. Biol. Chem. 272: 20139–20145
Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF, Parton RG . 1999 Nature Cell Biol. 1: 98–105
Roy S, McPherson RA, Apolloni A, Yan J, Clyde-Smith J, Hancock JF . 1998 Mol. Cell. Biol. 18: 3947–3955
Schonwasser DC, Marais RM, Marshall CJ, Parker PJ . 1998 Mol. Cell. Biol. 18: 790–798
Sidovar MF, Kozlowski P, Lee JW, Collins MA, He Y, Graves LM . 2000 J. Biol. Chem. 275: 28688–28694
Sieburth DS, Sundaram M, Howard RM, Han M . 1999 Genes Dev. 13: 2562–2569
Simons K, Ikonen E . 1997 Nature 387: 569–572
Sithanandam G, Kolch W, Duh FM, Rapp UR . 1990 Oncogene 5: 1775–1780
Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF . 1994 Science 264: 1463–1467
Sun H, King AJ, Diaz HB, Marshall MS . 2000 Curr. Biol. 10: 281–284
Thorson JA, Yu LWK, Hsu AL, Shih N-Y, Graves PR, Tanner JW, Allen PM, Piwnica-Worms H, Shaw AS . 1998 Mol. Cell. Biol. 18: 5229–5238
Traverse S, Cohen P, Paterson H, Marshall C, Rapp U, Grand RJ . 1993 Oncogene 8: 3175–3181
Tzivion G, Luo Z, Avruch J . 1998 Nature 394: 88–92
Wartmann M, Davis RJ . 1994 J. Biol. Chem. 269: 6695–6701
Wassarman DA, Solomon NM, Chang HC, Karim FD, Therrien M, Rubin GM . 1996 Genes Dev. 10: 272–278
Williams JG, Drugan JK, Yi GS, Clark GJ, Der CJ, Campbell SL . 2000 J. Biol. Chem. 275: 22172–22179
Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M . 1997 Mol. Cell. Biol. 17: 5598–5611
Xia K, Mukhopadhyay NK, Inhorn RC, Barber DL, Rose PE, Lee RS, Narsimhan RP, D'Andrea AD, Griffin JD, Roberts TM . 1996 Proc. Natl. Acad. Sci. USA 93: 11681–11686
Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R . 1995 Nature 378: 307–310
Yip-Schneider MT, Miao W, Lin A, Barnard DS, Tzivion G, Marshall MS . 2000 Biochem. J 351: 151–159
Zhu J, Woods D, McMahon M, Bishop JM . 1998 Genes Dev. 12: 2997–3007
Zimmermann S, Moelling K . 1999 Science 286: 1741–1744
Acknowledgements
We thank Andrey Shaw for supplying the phosphospecific Raf antisera and the Raf259A expression plasmid and Brian Gabrielli, Albert Pol and other members of the Oncology Laboratory for helpful comments on the manuscript. This work was supported by a grant from the National Health and Medical Research Council of Australia. JF Hancock is also supported by the Royal Children's Hospital Foundation, Queensland, Australia.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jaumot, M., Hancock, J. Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions. Oncogene 20, 3949–3958 (2001). https://doi.org/10.1038/sj.onc.1204526
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204526
Keywords
This article is cited by
-
A pigmentary manifestation associated with PPP2R5D-related neurodevelopmental disorder: a case report and review of literature
Bulletin of the National Research Centre (2023)
-
Coat proteins of necroviruses target 14-3-3a to subvert MAPKKKα-mediated antiviral immunity in plants
Nature Communications (2022)
-
Structural keys unlock RAS–MAPK cellular signalling pathway
Nature (2022)
-
The phosphatase/kinase balance affects phytochrome A and its native pools, phyA′ and phyA″, in etiolated maize roots: evidence from the induction of phyA′ destruction by a protein phosphatase inhibitor sodium fluoride
Photochemical & Photobiological Sciences (2021)
-
Dissecting the sequence determinants for dephosphorylation by the catalytic subunits of phosphatases PP1 and PP2A
Nature Communications (2020)