Abstract
The co-abuse of marijuana with cocaine is widespread, but it has not been until recently that the relationship between the behavioral effects of cannabinoids and cocaine has begun to be unveiled in animal models. Male Wistar rats were trained to intravenously self-administer cocaine until a stable baseline was reached. Rats then were subjected to a 5-day cocaine deprivation period during which they were treated daily with the cannabinoid receptor agonist WIN 55,212-2 (R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) (0, 0.3, 1, and 3 mg/kg; i.p.). Following this subchronic treatment, rats were tested, in counterbalanced order, in a test of anxiety (elevated plus-maze), as well as extinction and cue-induced reinstatement tests, the latter conducted according to a between-within procedure. Subchronic administration of WIN 55,212-2 was found to produce dose-dependent alterations of performance in the extinction, reinstatement, and anxiety tests with the lowest dose of WIN 55,212-2 producing the highest resistance to extinction and reinstatement, and the highest dose of WIN 55,212-2 producing the highest anxiolytic activity. Subchronic treatment with WIN 55,212-2 in rats without a history of cocaine self-administration did not affect anxiety levels. The results suggest an important role of the cannabinoid system in neuronal processes underlying cocaine seeking behavior. However, further studies will be necessary to understand possible implications of these findings for a role of the cannabinoid system as a treatment target for human cocaine abuse.
Similar content being viewed by others
INTRODUCTION
Cannabis represents the most widely used illicit drug and its co-abuse with cocaine has been identified as highly popular among drug users. It is thought that cannabis not only potentiates the positive effects of cocaine (by increasing its duration), but also diminishes the dysphoria associated with cocaine's unpleasant effects, particularly those experienced during the cocaine ‘crash’ syndrome (Lukas et al, 1994). Indeed, it is known that cannabis-induced vasodilation of the nasal mucosa attenuates the vasoconstrictive effects of cocaine increasing its absorption (Foltin et al, 1993). At the same time, cocaine produces a number of negative symptoms including anxiety, depression, anergia, insomnia, drug craving, and dysphoria (Koob and Le Moal, 2006), conditions that may be ameliorated by cannabis.
The endocannabinoid system, which mediates the effects of exogenous cannabionoids, plays an important role in multiple physiological and behavioral processes (Freund et al, 2003; Marx, 2006), including the regulation of emotional states (Witkin et al, 2005) such as anxiety (Navarro et al, 1997), the mediation of the reinforcing effects of depressants (ie, heroin, morphine and alcohol) and nicotine (Maldonado et al, 2006) and the modulation of drug-seeking in animal models of relapse (Lopez-Moreno et al, 2004; De Vries and Schoffelmeer, 2005).
The role of the endocannabinoid system in the neurobehavioral effects of cocaine appears to be different compared to its role on other drugs of abuse such as depressants and nicotine (González et al, 2002a, 2002b; Maldonado et al, 2006). Studies utilizing pharmacological antagonism of CB1 receptors or CB1 deletion in knockout mice have failed to implicate endogenous cannabinoids in either the primary reinforcing actions of cocaine or cocaine-induced behavioral sensitization (Arnold et al, 1998; Ferrari et al, 1999; Martin et al, 2000; Cossu et al, 2001; Lesscher et al, 2004; Soria et al, 2005). However, a CB1 cannabinoid receptor agonist, WIN 55,212-2, has been shown to decrease intravenous cocaine self-administration in rats at low doses (Fattore et al, 1999). Furthermore, WIN 55,212-2 is self-administered by mice at low doses (Martellotta et al, 1998). According to the unit dose-response model (Caine et al, 1993), low doses of WIN 55,212-2 may decrease cocaine self-administration in a manner similar to increasing the unit dose of cocaine. Although the role of the endocannabinoid system in the acute reinforcing effects remains unclear, there is growing evidence for a participation of CB1 receptor in the motivation to cocaine-seeking in a progressive ratio schedule (Soria et al, 2005) and in the reinstatement of cocaine-seeking by exposure to cocaine-associated environmental stimuli or cocaine itself (De Vries and Schoffelmeer, 2005; Arnold, 2005). Although the potent cannabinoid receptor agonist HU-210 produced reinstatement of cocaine-seeking following long-term extinction, blockade of CB1 receptors by the antagonist SR141716A attenuated reinstatement induced by cocaine cues or cocaine priming, but not reinstatement induced by stress (De Vries et al, 2001).
Cocaine is not only euphorigenic, but also can produce anxiety in humans and anxiety-like behavioral effects in animals (Blanchard and Blanchard, 1999; Ettenberg, 2004) following both acute and chronic administration (Yang et al, 1992) as well as during cocaine withdrawal (Wood and Lal, 1987). In addition, cocaine-associated cues were shown to produce anxiety-like behavior in rats after systemic administration of cocaine (DeVries and Pert, 1998). Similarly, increases in subjective anxiety and negative affect were recently observed in association with stress-induced and drug cue-induced craving in cocaine addicts (Sinha et al, 2000, 2003). Interestingly, activation of CB1 receptors seems to attenuate the anxiogenic or stressful aspects of cocaine's actions. For example, anxiogenic effects associated with either single or repeated doses of cocaine in mice were attenuated by the CB1 agonists, CP 55,940, anandamide, 2-arachidonylglycerol, N-arachidonyldopamine, and noladin ether (Hayase et al, 2005). Moreover, recent cannabis use has been found to decrease stress-induced blood oxygen level-dependent (BOLD) signals in the frontal and cingulate cortices of cocaine dependent individuals (Li et al, 2005). Although it is well-documented that acute administration of CB1 agonists, at low doses, may induce anxiolysis, whereas higher doses result in anxiety-like behavior (Navarro et al, 1997; Valverde, 2005; Viveros et al, 2005), the direction in which emotional responses are modulated by repeated or chronic administration of cannabinoid agonists still remains to be elucidated.
The purpose of this study was to investigate the effects of subchronic treatment with the cannabinoid agonist WIN 55,212-2—conducted during abstinence from daily cocaine self-administration in rats—on subsequent motivational measures of cocaine-seeking, including resistance to extinction and conditioned reinstatement, as well as anxiety-like behavior in the elevated plus-maze. It was hypothesized that subchronic treatment with the cannabinoid agonist WIN 55,212-2 will alter cocaine seeking behaviour in extinction and conditioned reinstatement tests and that the direction of changes in cocaine-seeking behavior will be linked to the direction of changes in anxiety-like behaviour as measured on the elevated plus-maze.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River) weighing 200–250 g at the beginning of the experiments were housed in groups of three on a 12 h reverse light/dark cycle (lights off at 0800 h). Experimental sessions were carried out during the dark phase of the day/night cycle. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline. WIN 55,212-2 (Sigma) was dissolved in a solution consisting of ethanol, emulphor, and saline in a 1 : 1 : 18 ratio and injected i.p. at a volume of 1 ml/kg.
Surgery
Rats were anesthetized with isoflurane (1.0–1.5%), prepared surgically with chronic silastic catheters in the right jugular vein as described previously (Caine et al, 1993) and allowed at least 5 days to recover. Catheter patency was maintained by flushing with 0.1 ml of sterile heparine/saline (33.3 USP U/ml) solution before and after each self-administration session. Catheter patency was tested whenever responses differed from the baseline, by infusing 0.1 ml of Brevital® sodium through the catheter. Animals with intact catheters showed a rapid loss of muscle tone within 3 s after the infusion. Animals not showing this response (ie, with compromised catheters) were removed from the experiment.
Apparatus
Self-administration apparatus
Rats were trained and tested in standard operant conditioning chambers located inside ventilated sound attenuating cubicles (BRS/LVE Inc., Laurel, MD). The chambers were equipped with two retractable levers, a white cue light above each lever, and a house light located at the top of the chamber's front panel. The chambers were equipped also with a 80 Ω speaker located in the center of the chamber's front panel below the house light for presentation of a white noise (70 db) stimulus. Intravenous infusions were administered by a syringe pump (Razel Scientific Instruments, Stamford, CT) located outside the sound attenuating boxes. Testing equipment and data collection were controlled by an IBM-compatible microcomputer.
Elevated plus maze apparatus
The elevated plus maze apparatus consisted of four arms (50 cm long × 10 cm wide). The two enclosed arms had 40 cm high dark walls, whereas the two open arms had 0.5 cm high ledges. Lighting on the open arm was 1.5–2.0 lux. The maze was elevated to a height of 50 cm. White noise (70 dB) was present throughout habituation and testing.
Procedures
Operant self-administration
Rats were food restricted (for 24 h the first day and from the second day on, fed 15 g of standard rodent chow per day) and trained on a fixed-ratio (FR) schedule of food reinforcement. Responding on an FR1 schedule resulted in delivery of one food pellet (45 mg, Research Diets). Once rats had earned 100 pellets, they were returned to ad libitum feeding and catheters were implanted. Following post-operative recovery, rats were allowed to self-administer cocaine on the right lever at a dose of 0.25 mg/infusion (delivered at a volume of 0.1 ml infused over 4 s) under a FR1 schedule for 2 h. Each reinforced response was followed by a compound stimulus, consisting of illumination of the white cue light above the right lever for 20 s and presentation of white noise for 5 s. In order to avoid accidental overdosing the lever remained inactive for 20 s following every infusion. Responses at the inactive lever were recorded but had no programmed consequences.
Self-administration sessions continued until animals reached an acquisition criterion (±10% of mean active lever presses over three consecutive sessions). Once rats developed stable cocaine intake, daily self-administration sessions were discontinued (ie, rats were cocaine deprived) and the animals were treated with the cannabinoid agonist WIN 55,212-2 (veh, 0.3, 1, and 3 mg/kg; i.p.; n=7–8 per group) for 5 days. Following this subchronic treatment, rats were tested in extinction/reinstatement tests and a test of anxiety-like behavior (ie, the elevated plus-maze). The extinction/reinstatement tests and elevated plus-maze tests were conducted in counterbalanced order on days 6 and 7 of cocaine deprivation.
Extinction and cue-induced reinstatement procedures
Cocaine seeking behavior was evaluated according to a between-within reinstatement procedure (for review, see Shaham et al, 2003). Thus, the extinction and cue-induced reinstatement tests were conducted on the same day. During the extinction period, responses at the previously active lever had no scheduled consequences. Immediately following completion of 2 h of extinction testing, 2 h conditioned reinstatement tests began under contingencies identical to those in effect during the self-administration phase except that lever presses did not result in cocaine delivery.
Elevated plus-maze procedure
Two separate groups of rats, having received the same subchronic treatment with WIN 55,212-2 were tested. One group, the same rats as above, was tested following cocaine self-administration and WIN 55,212-2 treatment during abstinence. Rats in the other group were not subjected to cocaine self-administration and served as cocaine-naïve controls. Rats were placed individually onto the center of the apparatus facing one of the open arms. Plus-maze behavior was recorded by video camera interfaced with a monitor and a VCR in an adjacent room. % Time spent in the open arms/(% time in open arms+% time in the closed arms) and number of entries into the closed arm were analysed. In addition, the number of closed arm entries was evaluated to provide a measure of locomotor activity (Cruz et al, 1994).
Statistical Analysis
The data are presented as mean±SEM. Extinction, reinstatement, and elevated plus maze data were separately analysed by one-way between subjects analysis of variance (ANOVA). After confirmation of significant main effects by ANOVA (p<0.05), Tukey HSD post hoc tests were employed to verify significant differences among individual groups.
RESULTS
Extinction and Cue-Induced Reinstatement Tests
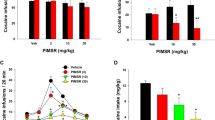
A main effect of treatment was observed in responding at the active lever between the different groups during extinction (F3,28=3.53; p<0.05). The effect of subchronic WIN 55,212-2 was dose-dependent, with the lowest dose producing the highest responding at the previously active lever during extinction. Subsequent Tukey HSD post hoc test revealed significant differences in responding at the active lever (p<0.05) between the lowest (0.3 mg/kg) and the highest dose (3 mg/kg) of subchronic WIN 55,212-2 but there was no significant difference in responding between rats subchronically treated with WIN 55,212-2 and vehicle controls. Responding at the inactive lever was negligible and not altered (F3,28=1.19; p=0.34; NS) by subchronic WIN 55,212-2 (Figure 1).
Lever-press responses during extinction at an active and inactive lever. Rats subchronically pretreated with the lowest dose of WIN 55,212-2 showed the highest responding at the active lever (ie, higher resistance) to extinction. Data represents mean±SEM responses for N=7–8 per group. *p<0.05 vs 3 mg/kg (Tukey HSD post hoc test).
A main effect of treatment was observed in responding at the active lever between the different groups during conditioned reinstatement (F3,28=5.28; p<0.01). Similar to its effects on extinction, subchronic treatment with WIN 55,212-2 modified reinstatement responses in a dose-dependent manner, with the lowest dose producing the highest level of responding at the active lever. The increase in responding with the lowest dose not only was higher than that seen with the highest dose of subchronic WIN 55,212-2 but as well as compared with vehicle (*p<0.05 vs vehicle, **p<0.01 vs 3 mg/kg). Responding at the inactive lever was negligible and not altered (F3,28=3.63; p=0.78; NS) by subchronic WIN 55,212-2 (Figure 2).
Lever-press responses during cue-induced reinstatement at the previously active and inactive lever. The lowest dose of subchronic WIN 55,212-2 produced the highest responding at the active lever during conditioned-reinstatement. Data represent mean±SEM responses for N=7–8 per group. *p<0.05 vs vehicle, **p<0.01 vs 3 mg/kg (Tukey HSD post hoc test).
Elevated Plus-Maze Test
We examined whether the ability of a subchronic treatment with WIN 55,212-2 to alter drug seeking behavior in the extinction and cue-induced reinstatement tests is associated with modulation of anxiety-like behavior.
In rats with a history of cocaine self-administration (n=7–8 per group) statistical analysis revealed a main effect of subchronic WIN 55,212-2 treatment (F3,28=5.20; p<0.01) on plus maze performance expressed as % time in open arms/(% time in open arms+% time in close arms). WIN 55,212-2 modulated this behavior in a dose-dependent manner with the lowest subchronic dose resulting in the lowest % time spent in open arms. The increase in % time spent in open arms associated with the highest subchronic dose not only was higher than that seen after subchronic treatment with the lowest dose of WIN 55,212-2 but also compared to vehicle (*p<0.05 vs vehicle, **p<0.01 vs 0.3 mg/kg). The number of crossing into the closed arms, a realiable measure of locomotor activity (Cruz et al, 1994), was not altered (F3,28=2.26; p=0.11; NS) by subchronic WIN 55,212-2 (Figure 3).
Effects of subchronic treatment with WIN 55,212-2 on anxiety-like behavior and locomotor activity in the elevated plus-maze test in rats with a history of cocaine self-administration. (a) Rats subchronically treated with the highest dose of WIN 55,212-2 showed the highest anxiolytic-like effect. (b) Locomotor activity was not altered by subchronic WIN 55,212-2. Data represent mean±SEM (N=7–8 per group). *p<0.05 vs vehicle, **p<0.01 vs 0.3 mg/kg (Tukey HSD post hoc test).
Furthermore, a separate group of rats that underwent the identical subchronic treatment with WIN 55,212-2 (n=9 per group) but without a history of cocaine self-administration (or catheter implantation) was used. Statistical analysis did not reveal significant effects of subchronic treatment with WIN 55,212-2 on either % time spent in the open arms (F3,35=0.45; p=0.72; NS) or on number of crossing into closed arms (F3,35=0.79; p=0.51; NS) (Figure 4).
Effects of subchronic treatment with WIN 55,212-2 on anxiety-like behavior and locomotor activity in the elevated plus-maze test in rats without a history of cocaine self-administration. (a) Neither anxiety-like behavior (b) nor locomotor activity was altered by subchronic WIN 55,212-2. Data represent mean±SEM (N=9 per group).
DISCUSSION
The major finding was that subchronic treatment with the cannabinoid agonist WIN 55,212-2 during a period of abstinence following intravenous cocaine self-administration results in a dose-dependent modification of cocaine-seeking behavior in extinction and conditioned reinstatement tests and produces anxiolytic-like effects as measured by the elevated plus-maze test. The direction of these dose-dependent modulations was inverted. Although subchronic pretreatment with the lowest dose of WIN 55,212-2 produced the greatest resistance to extinction and reinstatement, the highest dose of WIN 55,212-2 was associated with the greatest anxiolytic-like effect without significantly affecting motor activity, as inferred by the absence of differences between WIN 55,212-2-treated rats and vehicle controls in either the number of entries into the closed arm of the elevated-plus maze or in the number of responses at the inactive lever in the extinction and reinstatement tests.
To our knowledge, this is the first study demonstrating effects of subchronic cannabinoid agonist treatment implemented during forced abstinence from daily cocaine self-administration on extinction of cocaine-reinforced behavior. The modification of resistance to extinction and cue-induced reinstatement following subchronic cannabinoid agonist treatment observed here confirms a role of the cannabinoid system in cocaine-seeking and vulnerability to relapse. In this context, it should be pointed out the subchronic WIN 55,212-2 pretreatment across doses altered resistance to extinction and reinstatement in the same direction, consistent with existing evidence that resistance to extinction predicts cue-induced reinstatement (Shaham et al, 2003).
Cocaine has been reported to produce anxiety in humans and anxiogenic-like behavioral effects in animals (Blanchard and Blanchard, 1999; Ettenberg, 2004). These effects are associated with both acute and chronic administration of the drug (Yang et al, 1992) and cocaine withdrawal (Wood and Lal, 1987). Importantly, cues associated with repeated cocaine treatment can elicit anxiety-like behavior in the absence of the drug (DeVries and Pert, 1998). Drug cues also can induce both cocaine craving and anxiety, paired with activation of the hypothalamic-pituitary-adrenal axis (Sinha et al, 2003), suggesting that overlap exists between neural substrates controlling craving evoked by drug cues and stress (Sinha et al, 2000; Weiss, 2005). Finally, the anxiogenic effects of cocaine can be attenuated by CB1 agonists (Hayase et al, 2005). Considering this evidence, one may speculate that the anxiolytic consequences of subchronic treatment with the highest dose of WIN 55,212-2 in the elevated plus maze test may have contributed to the attenuation of anxiogenic effects exerted by environmental cues during the extinction and cue reinstatement tests. Biphasic dose–effect curves for the behavioral effects of cannabinoids have been reported previously (Chaperon and Thiebot, 1999). Consistent with this dose-response profile, the lowest dose of subchronic WIN 55,212-2 provoked the greatest resistance to extinction and reinstatement while at the same time producing the highest anxiety-like response in the elevated plus maze. Hypersensitization to drug and drug-associated stimuli due to neuroadaptations is a crucial factor in relapse (Robinson and Berridge, 2001). To date, behavioral cross-sensitization between cocaine and cannabinoids and CB1 participation in psychomotor sensitization to cocaine have not yet been demonstrated (Arnold, 2005). However, it cannot be fully ruled out that an enhanced response (sensitization) to the incentive actions of the cocaine cues contributed to the increased cocaine-seeking response in rats treated with the lowest dose of subchronic WIN 55,212-2.
Controversial data exist concerning the ability of cannabinoids to modulate emotional states such as anxiety. Both anxiolytic- and anxiogenic-like effects have been reported with both enhancers and blockers of endocannabinoid neurotransmission (Witkin et al, 2005). Although it is well documented that acute administration of CB1 agonists induce anxiolytic effects at low doses and anxiety-like behavior at high doses (Navarro et al, 1997; Valverde, 2005; Viveros et al, 2005), the modulation of emotional responses by repeated administration of cannabinoid agonists, as in the present study, still remains to be systematically elucidated. It is interesting to note that the subchronic treatment with WIN 55,212-2 did not affect anxiety levels in the elevated plus-maze test in rats without a history of cocaine self-administration, but had dose-dependent anxiolytic-like consequences in rats with a history of self-administration. A very recent report indicates that, in mice, acute administration of WIN 55,212-2 produces anxiolytic effects on the elevated plus-maze only at low doses (Patel and Hillard, 2006). Although slight spontaneous withdrawal signs (wet-dog shakes and facial rubs) have been reported 24 h after a 4-day treatment period with an intermediate dose of WIN 55,212-2 (Aceto et al, 2001), chronic treatment with the agonist HU-210 did not alter behavior on the elevated plus-maze (Hill and Gorzalka, 2006). The finding that subchronic WIN 55,212-2 pretreatment produced anxiolytic effects only in rats with a history of cocaine self-administration suggests a specific link between the consequences of chronic cocaine exposure and endocannabinoid function in the modulation of susceptibility to anxiety.
The present data provide no direct insight into the neural mechanisms by which subchronic WIN 55,212-2 produced the behavioral changes observed. The alterations in cocaine-seeking and anxiety-like behavior may have been the result of CB1 cannabinoid receptor activation. However, modulation of neural systems other than cannabinoid receptors by subchronic WIN 55,212-2 may also be responsible to a certain extent for these results. For example, WIN 55,212-2 and other agents acting at the CB1 receptor interact with GABAA receptors, the corticotrophin-releasing factor, cholecystokinin, endogenous opioid, and serotonergic systems (Viveros et al, 2005). Interestingly, the hypothalamic-pituitary-adrenal axis may have a common role in anxiety-related responses (Sinha et al, 2003), cue-induced reinstatement of extinguished cocaine seeking (Goeders and Clampitt, 2002), and behavioral effects of cannabinoids (Rodriguez de Fonseca et al, 1997). In this line, recent studies have shown that chronic cocaine exposure decreased the levels of CB1 receptor mRNA in the ventromedial hypothalamic nucleus (González et al, 2002b), which forms part of the hypothalamic–pituitary–adrenal axis, and that orexin neurons in the lateral hypothalamus, probably by a regulatory role of the endocannabinoid system (Hilairet et al, 2003), became activated by cues associated with cocaine and altered relapse (Harris et al, 2005). Future research will be needed to confirm this suspicion and to elucidate the role of these neural substrates in the neurobiological effects produced by pharmacological manipulation of the cannabinoid system.
In conclusion, the present findings indicate that subchronic treatment with the cannabinoid agonist WIN 55212-2 alters, in a biphasic and dose-sensitive fashion, cocaine seeking behavior in extinction and cue-induced reinstatement tests in association with modulation of anxiety-like behavior. The direction of changes in cocaine-seeking, inversely linked to the direction of changes in anxiety-like behavior as measured on the elevated plus maze, suggests an important role of the endocannabinoid system in the vulnerability to relapse. The anxiolytic effects of subchronic treatment with the highest dose of WIN 55,212-2, only shown in the group with a history of cocaine self-administration, is suggestive of a specific link between the consequences of chronic cocaine exposure and endocannabinoid function in the modulation of susceptibility to anxiety. By contrast, the high resistance to extinction and enhanced reinstatement produced by subchronic treatment with the lowest dose of WIN 55,212-2 suggests that cannabinoid antagonists may prove to offer treatment promise for craving and relapse prevention. However, further research will be required to understand the precise implications of these findings with respect to the treatment target potential of the cannabinoid system.
References
Aceto MD, Scates SM, Martin BB (2001). Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55,212-2. Eur J Pharmacol 416: 75–81.
Arnold JC (2005). The role of the endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav 81: 396–406.
Arnold JC, Topple AN, Hunt GE, McGregor IS (1998). Effects of pre-exposure and co-administration of the cannabinoid receptor agonist CP 55,940 on behavioral sensitization to cocaine. Eur J Pharmacol 354: 9–16.
Blanchard DC, Blanchard RJ (1999). Cocaine potentiates defensive behaviours related to fear and anxiety. Neurosci Biobehav Rev 23: 981–991.
Caine SB, Lintz Z, Koob GF (1993). Intravenous drug self-administration techniques in animals. In: Sahgal A (ed). Behavioral Neuroscience. IRL at Oxford UP: New York. pp 117–143.
Chaperon F, Thiebot MH (1999). Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol 13: 243–281.
Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M et al (2001). Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res 118: 61–65.
Cruz AP, Frei F, Graeff FG (1994). Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49: 171–176.
De Vries TJ, Schoffelmeer AN (2005). Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26: 420–426.
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J et al (2001). A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7: 1151–1154.
DeVries AC, Pert A (1998). Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology 137: 333–340.
Ettenberg A (2004). Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 27: 721–728.
Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W (1999). CB1 cannabinoid receptor agonist WIN 55,212-2 decrease intravenous cocaine self-administration in rats. Behav Brain Res 104: 141–146.
Ferrari F, Ottani A, Giuliani D (1999). Influence of the cannabinoid agonist HU-210 on cocaine- and CQP 201–403-induced behavioral effects in rat. Life Sci 65: 823–831.
Foltin RW, Fischman MW, Pippen PA, Kelly TH (1993). Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend 32: 93–106.
Freund TF, Katona I, Piomelli D (2003). Role of endogenous cannabinoids in synaptic signalling. Physiol Rev 83: 1017–1066.
Goeders NE, Clampitt DM (2002). Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology 161: 222–232.
González S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA (2002a). Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res 954: 73–81.
González S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA (2002b). Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend 66: 77–84.
Harris GC, Wimmer M, Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559.
Hayase T, Yamamoto Y, Yamamoto K (2005). Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behav Pharmacol 16: 395–404.
Hilairet S, Bouaboula M, Cerriere D, Le Fur G, Casellas P (2003). Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem 278: 23731–23737.
Hill MN, Gorzalka BB (2006). Increased sensitivity to restraint stress and novelty-induced emotionality following long-term, high dose cannabinoid exposure. Psychoneuroendocrinology 31: 526–536.
Koob GF, Le Moal M (2006). Psychostimulants. In: Koob GF, Le Moal M (eds). Neurobiology of Addiction. Elsevier Inc.: San Diego. pp 69–120.
Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerritis MA (2004). Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioral sensitization. Eur Neuropsychopharmacol 15: 31–37.
Li CS, Milivojevic V, Constable RT, Sinha R (2005). Recent cannabis abuse decreased stress-induced BOLD signals in the frontal and cingulate cortices of cocaine dependent individuals. Psychiatry Res 140: 271–280.
Lopez-Moreno JA, Gonzalez-Cuevas G, Rodriguez de Fonseca F, Navarro M (2004). Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation. J Neurosci 24: 8245–8252.
Lukas SE, Sholar M, Kouri E, Fukuzako H, Mendelson JH (1994). Marihuana smoking increases plasma cocaine levels and subjective reports of euphoria in male volunteers. Pharmacol Biochem Behav 48: 715–721.
Maldonado R, Valverde O, Berrendero F (2006). Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29: 225–232.
Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W (1998). Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naïve mice. Neuroscience 85: 327–330.
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2000). Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci 12: 4038–4046.
Marx J (2006). Drugs inspired by a drug. Science 311: 322–325.
Navarro M, Hernandez E, Muñoz RM, Del Arco I, Villanua MA, Carrera MR et al (1997). Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport 8: 491–496.
Patel S, Hillard CJ (2006). Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318: 304–311.
Robinson TE, Berridge KC (2001). Mechanisms of action of addictive stimuli. Incentive-sensitization and addiction. Addiction 96: 103–114.
Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F (1997). Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276: 2050–2054.
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168: 3–20.
Sinha R, Fuse T, Aubin LR, O'Malley SS (2000). Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152: 140–148.
Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ (2003). Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology 170: 62–72.
Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M et al (2005). Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30: 1670–1680.
Valverde O (2005). Participation of the cannabinoid system in the regulation of emotional-like behavior. Curr Pharm Des 11: 3421–3429.
Viveros MP, Marco EM, File SE (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81: 331–342.
Weiss F (2005). Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 5: 9–19.
Witkin JM, Tzavara ET, Nomikos GG (2005). A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol 16: 315–331.
Wood DM, Lal H (1987). Anxiogenic properties of cocaine withdrawal. Life Sci 41: 143–146.
Yang XM, Gorman AL, Dunn AJ, Goeders NE (1992). Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav 41: 643–650.
Acknowledgements
This work was supported by NIH/NIDA grant DA 07348 (FW) and by the Spanish Fondo de Investigación Sanitaria (Red de Trastornos Adictivos G03/05), the Comunidad Autónoma de Madrid GR/SAL/0541/2004, the Plan Nacional Sobre Drogas (Ministerio de Sanidad), The European Fifth Framework Programme QLRT-2000–01691 and the MEC SAF2005–04926, (MN). Gustavo González-Cuevas is a predoctoral fellow of the Ministerio de Educación y Cultura in Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Cuevas, G., Aujla, H., Martin-Fardon, R. et al. Subchronic Cannabinoid Agonist (WIN 55,212-2) Treatment during Cocaine Abstinence Alters Subsequent Cocaine Seeking Behavior. Neuropsychopharmacol 32, 2260–2266 (2007). https://doi.org/10.1038/sj.npp.1301365
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301365
Keywords
This article is cited by
-
Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats
Psychopharmacology (2016)
-
A neuropeptide‐centric view of psychostimulant addiction
British Journal of Pharmacology (2008)