Abstract
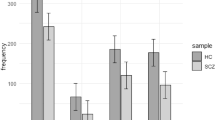
Disturbances in GABAergic system have been observed in schizophrenics.1,2,3 In the present study, population association analysis was performed on 19 SNPs in the α1, β2, γ2, ɛ and π subunit genes of GABAA receptor. Five SNPs in GABRB2, namely B2I7G1584T, rs1816071, rs194072, rs252944 and rs187269, were found to be significantly associated, and their haplotypes in linkage disequilibrium, with schizophrenia. This represents the first report on any disease association of SNPs in the human GABAA receptor genes, and focuses attention on the GABAergic hypothesis of schizophrenia etiology.3,4
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C . et al. Changes in serotonin2A and GABAA receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem 1999; 72: 1593–1599.
Guidotti A, Auta J, Davis JM, Giorgi-Gerevini V, Dwivedi Y, Grayson DR . et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 2000; 57: 1061–1069.
Benes FM, Berretta S . GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001; 25: 1–27.
Roberts E . Prospects for research on schizophrenia. A hypothesis suggesting that there is a defect in the GABA system in schizophrenia. Neurosci Res Program Bull 1972; 10: 468–482.
Tsuang MT, Stone WS, Faraone SV . Genes, environment and schizophrenia. Br J Psychiatry Suppl 2001; 40: s18–s24.
Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S . et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002; 71: 877–892.
Makino C, Fujii Y, Kikuta R, Hirata N, Tani A, Shibata A . et al. Positive association of the AMPA receptor subunit GluR4 gene (GRIA4) haplotype with schizophrenia: linkage disequilibrium mapping using SNPs evenly distributed across the gene region. Am J Med Genet 2003; 116B: 17–22.
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G . et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 1998; 50: 291–313.
Mehta AK, Ticku MK . An update on GABAA receptors. Brain Res Rev 1999; 29: 196–217.
Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H . Distribution, prevalence, and drug binding profile of gamma-aminobutyric acid type A receptor subtypes differing in the beta-subunit variant. J Biol Chem 1994; 269: 27100–27107.
Nusser Z, Sieghart W, Somogyi P . Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 1998; 18: 1693–1703.
Wisden W, Seeburg PH . GABAA receptor channels: from subunits to functional entities. Curr Opin Neurobiol 1992; 2: 263–269.
Gurling HM, Kalsi G, Brynjolfson J, Sigmundsson T, Sherrington R, Mankoo BS . et al. Genome-wide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21–22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3–24 and 20q12.1–11.23. Am J Hum Genet 2001; 68: 661–673.
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I . et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, Part II: Schizophrenia. Am J Hum Genet 2003; 73: 34–48.
Raymond M, Rousset F . GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Heredity 1995; 86: 248–249.
Zhao JH, Curtis D, Sham PC . Model-free analysis and permutation tests for allelic associations. Hum Hered 2000; 50: 133–139.
Xie X, Ott J . Testing linkage disequilibrium between a disease gene and marker loci. Am J Hum Genet 1993; 53: 1107.
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H . et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 2002; 99: 13675–13680.
Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A . et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 2002; 71: 1296–1302.
McKinley DD, Lennon DJ, Carter DB . Cloning, sequence analysis and expression of two forms of mRNA coding for the human beta 2 subunit of the GABAA receptor. Brain Res Mol Brain Res 1995; 28: 175–179.
Abe S, Suzuki T, Ito T, Baba A, Hori T, Kurita H . et al. Differential expression of GABAA receptor subunit mRNAs and ligand binding sites in rat brain following phencyclidine administration. Synapse 2000; 38: 51–60.
Huntsman MM, Tran BV, Potkin SG, Bunney Jr WE, Jones EG . Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci USA 1998; 95: 15066–15071.
American Psychiatric Association. Schizophrenia and other psychiatric disorders. In: Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Press: Washington, DC, 2000 pp 155–165.
Nickerson DA, Tobe VO, Taylor SL . PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res 1997; 25: 2745–2751.
Sham PC, Curtis D . Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann Hum Genet 1995; 59: 97–105.
Murray R . The essentials of postgraduate psychiatry. In: Murray R, Hill P, McGuffin P (eds) Schizophrenia. Cambridge University Press: London, 1997 pp 281–309.
Excoffier L, Slatkin M . Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 1995; 12: 921–927.
Lewontin RC . The interaction of selection and linkage. I. General considerations: heterotic models. Genetics 1964; 49: 49–67.
Slatkin M, Excoffier L . Testing for linkage disequilibrium in genotypic data using the expectation-maximization algorithm. Heredity 1996; 76: 377–383.
Acknowledgements
We are grateful to Professor Laszlo Endrenyi and Professor J Tze-Fei Wong for helpful discussion, Professor Huimin Zhu for assistance in sample collections, and Miss Peggy Lee for technical support. We wish to thank the Innovation and Technology Fund of the Government of Hong Kong, and PharmacoGenetics Limited, for financial support.
Competing interests statement: We declare that we have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic-database information
URLs for databases and program used herein are as follows:
ARLEQUIN, http://lgb.unige.ch/arleguin/
Blastn, http://www.ncbi.nlm.nih.gov/BLAST/
dbSNP, http://www.ncbi.nlm.nih.gov/SNP/
GENEPOP, http://wbiomed.curtin.edu.au/genepop/
Primer3, http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi
Rights and permissions
About this article
Cite this article
Lo, WS., Lau, CF., Xuan, Z. et al. Association of SNPs and haplotypes in GABAA receptor β2 gene with schizophrenia. Mol Psychiatry 9, 603–608 (2004). https://doi.org/10.1038/sj.mp.4001461
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001461
Keywords
This article is cited by
-
A gene–brain–behavior basis for familiarity bias in source preference
Theory and Decision (2022)
-
The association of GABRB2 SNPs with cognitive function in schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2020)
-
Gabrb2-knockout mice displayed schizophrenia-like and comorbid phenotypes with interneuron–astrocyte–microglia dysregulation
Translational Psychiatry (2018)
-
Akting up in the GABA hypothesis of schizophrenia: Akt1 deficiency modulates GABAergic functions and hippocampus-dependent functions
Scientific Reports (2016)
-
AluScan: a method for genome-wide scanning of sequence and structure variations in the human genome
BMC Genomics (2011)