Abstract
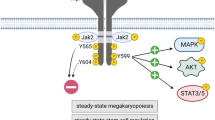
Thrombopoietin (TPO) is a recently cloned growth and differentiation factor implicated in megakaryocytopoiesis. Here, we show that TPO, interleukin-3 (IL-3) and, at least in short-term assays, also interferon γ (IFN γ) induced proliferation in acute myeloid leukemia (AML-M7)-derived M-07e cells. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway was activated after stimulation with any of the three cytokines. Thus, the TPO-receptor (TPO-R) MPL was tyrosine phosphorylated after a short-term stimulation with TPO, followed by tyrosine phosphorylation of STAT 3 and STAT 5, but not of STAT 1. IL-3 and IFN γ induced phosphorylation of STAT 5 or STAT 1, respectively, without affecting the other STATs. As STATs are thought to regulate proliferation by modulating expression of inhibitors of cyclin-dependent kinases (Cdk), we analyzed p21 and p27 expression after stimulation with TPO or IL-3. In contrast to the constitutively low p21 expression, p27 mRNA levels were high in synchronized, cytokine-deprived cells in G0/1 phase. Stimulation with TPO or IL-3 induced a rapid decrease of p27 mRNA. The phosphorylation cycle of the retinoblastoma protein (Rb) was inversely correlated with the level of p27 mRNA. Hyperphosphorylation of Rb was detectable 9 h after onset of stimulation, concomitantly with the decrease of p27 mRNA and shortly before transition of the cells into S phase. As phosphorylation of Rb is a key event for transition of cells into S phase, our observations support the notion of p27 being an important regulator during cytokine-induced proliferation. Whether the JAK/STAT pathway is directly involved in p27 expression or not, remains to be elucidated. The JAK inhibitor AG-490 blocked cytokine-induced STAT 5 phosphorylation and proliferation of M-07e cells in a dose-dependent manner. Although these data indicate a role for the JAK/STAT pathway in cytokine-induced proliferation, a direct influence on the p27 mRNA downregulation has to be confirmed. The second major effect of TPO, polypoidization, could not be observed in M-07e cells. Even long-term culture with TPO did not induce endomitosis in these cells. However, polyploidization could be brought about by the kinase inhibitor K-252a. After 3 days of exposure to this reagent, 17% of the originally mononucleated cells contained two to five nuclei. K-252a-induced polykaryon formation was not preceded by STAT 5 phosphorylation. Thus, K-252a did not mimic TPO stimulation at the early steps of the signaling chain. Taken together, our experiments confirm a role for the JAK/STAT pathway in cytokine-induced proliferation; TPO and IL-3 induce downregulation of the Cdk inhibitor p27, hyperphosphorylation of Rb and subsequently transition of the cells into S phase; the kinase inhibitor K-252a induces polyploidization of M-07e cells, but this effect is independent of STAT 5 phosphorylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Quentmeier, H., Zaborski, M. & Drexler, H. Effects of thrombopoietin, interleukin-3 and the kinase inhibitor K-252a on growth and polyploidization of the megakaryocytic cell line M-07e. Leukemia 12, 1603–1611 (1998). https://doi.org/10.1038/sj.leu.2401170
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401170
Keywords
This article is cited by
-
Inhibition of Tropomyosin Receptor Kinase A Signaling Negatively Regulates Megakaryopoiesis and induces Thrombopoiesis
Scientific Reports (2019)
-
Staurosporine analogs promote distinct patterns of process outgrowth and polyploidy in small cell lung carcinoma cells
Tumor Biology (2015)
-
MEF2C is activated by multiple mechanisms in a subset of T-acute lymphoblastic leukemia cell lines
Leukemia (2008)
-
JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders
Leukemia (2006)