Abstract
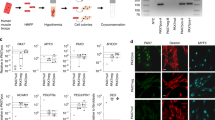
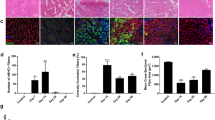
Muscle precursor cell (myoblasts) transplantation is considered as a potential approach to restore dystrophin expression in Duchenne muscular dystrophy (DMD) patients. The study purpose was to verify the implication of hypoxia in the myoblast death observed after their transplantation and also to evaluate the potential beneficial effects of vascular endothelial growth factor (VEGF) overexpression on myoblast engraftment in a murine model. Pimonidazole hydrochloride (hypoxyprobe-1) was used to mark selectively myoblasts to evaluate their hypoxia in vivo. In vitro, hypoxia was induced by culturing human myoblasts in hypoxic environment. In vitro effects of VEGF165 on survival of human cells was assessed by Hoescht-PI labeling. Tibialis anterior (TA) female mouse muscles were electroporated with a plasmid containing the VEGF165 or with an empty vector. Circulating VEGF concentration was assessed by ELISA. After 2 weeks of electroporation, severe combined immunodeficient (SCID) mice were transplanted with 800 000 human male myoblasts labeled with radioactive thymidine. Mouse muscles were harvested 2 and 4 days later and myoblast survival and proliferation were evaluated by scintigraphy and Y chromosome quantitative PCR. The long-term graft success was evaluated using γ-radiograph imaging and by counting the dystrophin positive muscle fibers. Hypoxyprobe labeling has shown that most of the transplanted myoblasts were hypoxic. The transplantation of radioactive male myoblasts in female mice electroporated with the VEGF165 plasmid demonstrated that VEGF reduced their death by 10% but did not improve their proliferation. VEGF165 enhanced human myoblast survival in vitro under hypoxic conditions. Electroporation of TA muscles of SCID mouse with the vector coding for VEGF165 promoted angiogenesis and improved by 1.5-fold the success of myoblast transplantation in comparison with the control mice that were electroporated with the empty vector. These results indicate that hypoxia is partially responsible for the death of the transplanted myoblasts. VEGF can be used to improve myoblast survival and the graft success.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard JP, Roy R et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther 2004; 9: 475–482.
Skuk D, Tremblay JP . Complement deposition and cell death after myoblast transplantation. Cell Transplant 1998; 7: 427–434.
Maurel A, Azarnoush K, Sabbah L, Vignier N, Le Lorc'h M, Mandet C et al. Can cold or heat shock improve skeletal myoblast engraftment in infarcted myocardium? Transplantation 2005; 80: 660–665.
Skuk D, Goulet M, Tremblay JP . Use of repeating dispensers to increase the efficiency of the intramuscular myogenic cell injection procedure. Cell Transplant 2006; 15: 659–663.
Skuk D, Tremblay JP . Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil 2003; 24: 285–300.
Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L et al. VEGF is required for growth and survival in neonatal mice. Development 1999; 126: 1149–1159.
Corley KM, Taylor CJ, Lilly B . Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem 2005; 96: 971–985.
Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res 2004; 21: 15–25.
Huard J, Acsadi G, Jani A, Massie B, Karpati G . Gene transfer into skeletal muscles by isogenic myoblasts. Hum Gene Ther 1994; 5: 949–958.
Fan Y, Maley M, Beilharz M, Grounds M . Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve 1996; 19: 853–860.
Merly F, Huard C, Asselin I, Robbins PD, Tremblay JP . Anti-inflammatory effect of transforming growth factor-beta1 in myoblast transplantation. Transplantation 1998; 65: 793–799.
Guerette B, Skuk D, Celestin F, Huard C, Tardif F, Asselin I et al. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J Immunol 1997; 159: 2522–2531.
Beauchamp JR, Morgan JE, Pagel CN, Partridge TA . Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 1999; 144: 1113–1122.
Skuk D, Caron NJ, Goulet M, Roy B, Tremblay JP . Resetting the problem of cell death following muscle-derived cell transplantation: detection, dynamics and mechanisms. J Neuropathol Exp Neurol 2003; 62: 951–967.
Neumann RA, Knobler RM, Pieczkowski F, Gebhart W . Enzyme histochemical analysis of cell viability after argon laser-induced coagulation necrosis of the skin. J Am Acad Dermatol 1991; 25: 991–998.
Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafe F et al. H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Lett 2003; 536: 85–91.
Tantini B, Fiumana E, Cetrullo S, Pignatti C, Bonavita F, Shantz LM et al. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J Mol Cell Cardiol 2006; 40: 775–782.
Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant 2005; 14: 67–76.
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 2003; 93: 1074–1081.
Jiang J, Jiangl N, Gao W, Zhu J, Guo Y, Shen D et al. Augmentation of revascularization and prevention of plasma leakage by angiopoietin-1 and vascular endothelial growth factor co-transfection in rats with experimental limb ischaemia. Acta Cardiol 2006; 61: 145–153.
Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P et al. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol 2003; 163: 1417–1428.
Graells J, Vinyals A, Figueras A, Llorens A, Moreno A, Marcoval J et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol 2004; 123: 1151–1161.
Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG et al. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun 1999; 264: 781–788.
Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 2000; 6: 851–860.
Matter ML, Ruoslahti E . A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem 2001; 276: 27757–27763.
Retuerto MA, Beckmann JT, Carbray J, Patejunas G, Sarateanu S, Kane BJ et al. Angiogenic pretreatment to enhance myocardial function after cellular cardiomyoplasty with skeletal myoblasts. J Thorac Cardiovasc Surg 2007; 133: 478–484, e472.
Avivi A, Resnick MB, Nevo E, Joel A, Levy AP . Adaptive hypoxic tolerance in the subterranean mole rat Spalax ehrenbergi: the role of vascular endothelial growth factor. FEBS Lett 1999; 452: 133–140.
Becker C, Lacchini S, Muotri AR, da Silva GJ, Castelli JB, Vassallo PF et al. Skeletal muscle cells expressing VEGF induce capillary formation and reduce cardiac injury in rats. Int J Cardiol 2006; 113: 348–354.
Haider H, Ye L, Jiang S, Ge R, Law PK, Chua T et al. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med 2004; 82: 539–549.
Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation 2001; 104: I207–212.
Guerette B, Asselin I, Skuk D, Entman M, Tremblay JP . Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant 1997; 6: 101–107.
Bouchentouf M, Benabdallah BF, Rousseau J, Schwartz LM, Tremblay JP . Induction of Anoikis following myoblast transplantation into SCID mouse muscles requires the Bit1 and FADD pathways. Am J Transplant 2007; 7: 1491–1505.
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998; 273: 30336–30343.
Kalka C, Asahara T, Krone W, Isner JM . Angiogenesis and vasculogenesis. Therapeutic strategies for stimulation of postnatal neovascularization. Herz 2000; 25: 611–622.
Winkel R . NEMaSU. Endothelial cells of implanted veins to neovascularize free muscle grafts in a rat model. Eur j plast surg 1995; 18: 201–208.
Gowdak LH, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A et al. Adenovirus-mediated VEGF(121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation 2000; 102: 565–571.
Zachary I, Gliki G . Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 2001; 49: 568–581.
Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther 2004; 10: 844–854.
Skuk D, Paradis M, Goulet M, Tremblay JP . Ischemic central necrosis in pockets of transplanted myoblasts in nonhuman primates: implications for cell transplantation startegies in the skeletal and cardiac muscles. Transplantation 2007; in press.
Shah GM, Shah RG, Poirier GG . Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem Biophys Res Commun 1996; 229: 838–844.
Ukropec JA, Hollinger MK, Woolkalis MJ . Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress. Exp Cell Res 2002; 273: 240–247.
Bouchentouf M, Benabdallah BF, Dumont M, Rousseau J, Jobin L, Tremblay JP . Real-time imaging of myoblast transplantation using the human sodium iodide symporter. Biotechniques 2005; 38: 937–942.
Acknowledgements
We thank Dr Roy PH (CHUL-Quebec city) for giving us the access to Corbett Rotorgen Q-PCR machine and Dr Piedboeuf B (CHUL-Quebec city) for providing us the hypoxic chamber. This work was supported by AFM (Association Française contre les Myopathies).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouchentouf, M., Benabdallah, B., Bigey, P. et al. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther 15, 404–414 (2008). https://doi.org/10.1038/sj.gt.3303059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303059
Keywords
This article is cited by
-
Systemic cell therapy for muscular dystrophies
Stem Cell Reviews and Reports (2021)
-
Protein disulfide isomerase as a prosurvival factor in cell therapy for muscular and vascular diseases
Stem Cell Research & Therapy (2018)
-
An experimental study of muscular injury repair in a mouse model of notexin-induced lesion with EPI® technique
BMC Sports Science, Medicine and Rehabilitation (2015)
-
VEGF induces stress fiber formation in fibroblasts isolated from dystrophic muscle
Journal of Cell Communication and Signaling (2015)
-
In vivo Fluorescence Imaging of Muscle Cell Regeneration by Transplanted EGFP-labeled Myoblasts
Molecular Therapy (2010)