Abstract
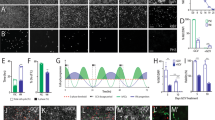
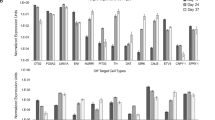
Glial cell line-derived neurotrophic factor (GDNF) has been shown to increase the survival and functioning of dopamine neurons in a variety of animal models and some recent human trials. However, delivery of any protein to the brain remains a challenge due to the blood/brain barrier. Here we show that human neural progenitor cells (hNPC) can be genetically modified to release glycosylated GDNF in vitro under an inducible promoter system. hNPC-GDNF were transplanted into the striatum of rats 10 days following a partial lesion of the dopamine system. At 2 weeks following transplantation, the cells had migrated within the striatum and were releasing physiologically relevant levels of GDNF. This was sufficient to increase host dopamine neuron survival and fiber outgrowth. At 5 weeks following grafting there was a strong trend towards functional improvement in transplanted animals and at 8 weeks the cells had migrated to fill most of the striatum and continued to release GDNF with transport to the substantia nigra. These cells could also survive and release GDNF 3 months following transplantation into the aged monkey brain. No tumors were found in any animal. hNPC can be genetically modified, and thereby represent a safe and powerful option for delivering growth factors to specific targets within the central nervous system for diseases such as Parkinson's.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F . GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993; 260: 1130–1132.
Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996; 380: 252–255.
Bjorklund A, Rosenblad C, Winkler C, Kirik D . Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease. Neurobiol Dis 1997; 4: 186–200.
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws Jr ER et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003; 60: 69–73.
Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann Neurol 1999; 46: 419–424.
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 2003; 9: 589–595.
Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS . Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 2005; 57: 298–302.
Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS . Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 2005; 11: 703–704.
Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B . Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 2005; 102: 216–222.
Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 1997; 275: 838–841.
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 2000; 290: 767–773.
Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ . Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res 2000; 886: 82–98.
Hsich G, Sena-Esteves M, Breakefield XO . Critical issues in gene therapy for neurologic disease. Hum Gene Ther 2002; 13: 579–604.
Gage FH . Cell Therapy. Nature 1998; 392 (Suppl): 18–24.
Blesch A, Tuszynski MH . Gene therapy and cell transplantation for Alzheimer's disease and spinal cord injury. Yonsei Med J 2004; 45 (Suppl): 28–31.
Tuszynski MH, Thal L, Pay M, Salmon DP, HS U, Bakay R et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 2005; 11: 551–555.
Lindvall O . Neural transplants in Parkinson's disease. Dunnett SB, Björklund A (eds). Functional Neural Transplantation. Raven Press: New York, 1994, pp 103–137.
Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods 1998; 85: 141–152.
Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN . Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J Neurochem 2003; 86: 179–195.
Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol 1997; 148: 135–146.
McBride JL, Behrstock SP, Chen EY, Jakel RJ, Siegel I, Svendsen CN et al. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J Comp Neurol 2004; 475: 211–219.
Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 1999; 156: 71–83.
Fricker RA, Carpenter MK, Winkler C, Greco C, Gates MA, Bjorklund A . Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci 1999; 19: 5990–6005.
Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol 1998; 16: 1033–1039.
Englund U, Fricker-Gates RA, Lundberg C, Bjorklund A, Wictorin K . Transplantation of human neural progenitor cells into the neonatal rat brain: extensive migration and differentiation with long-distance axonal projections. Exp Neurol 2002; 173: 1–21.
Burnstein RM, Foltynie T, He X, Menon DK, Svendsen CN, Caldwell MA . Differentiation and migration of long term expanded human neural progenitors in a partial lesion model of Parkinson's disease. Int J Biochem Cell Biol 2004; 36: 702–713.
Ostenfeld T, Tai YT, Martin P, Deglon N, Aebischer P, Svendsen CN . Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons. J Neurosci Res 2002; 69: 955–965.
Akerud P, Canals JM, Snyder EY, Arenas E . Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci 2001; 21: 8108–8118.
Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY . Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol 2002; 20: 1103–1110.
Mohajeri MH, Figlewicz DA, Bohn MC . Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum Gene Ther 1999; 10: 1853–1866.
Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD . Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci 2001; 21: 6136–6146.
Behrstock S, Svendsen CN . Combining growth factors, stem cells, and gene therapy for the aging brain. Ann NY Acad Sci 2004; 1019: 5–14.
Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther 2005; 16: 509–521.
Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira dA et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Hum Gene Ther 2000; 11: 179–190.
Ostenfeld T, Horn P, Aardal C, Orpen I, Caldwell MA, Svendsen CN . Mouse epidermal growth factor-responsive neural precursor cells increase the survival and functional capacity of embryonic rat dopamine neurons in vitro. NeuroReport 1999; 10: 1985–1992.
Kirik D, Rosenblad C, Bjorklund A . Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 1998; 152: 259–277.
Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol 2003; 461: 250–261.
Matsuura K, Makino H, Ogawa N . Cyclosporin a attenuates the decrease in tyrosine hydroxylase immunoreactivity in nigrostriatal dopaminergic neurons and in striatal dopamine content in rats with intrastriatal injection of 6-hydroxydopamine. Exp Neurol 1997; 146: 526–535.
Georgievska B, Kirik D, Bjorklund A . Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol 2002; 177: 461–474.
Levi-Montalcini R, Angeletti PU . Essentiality of nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol 1963; 7: 653–659.
Airaksinen MS, Saarma M . The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002; 3: 383–394.
Corti O, Sabate O, Horellou P, Colin P, Dumas S, Buchet D et al. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat Biotechnol 1999; 17: 349–354.
Georgievska B, Jakobsson J, Persson E, Ericson C, Kirik D, Lundberg C . Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum Gene Ther 2004; 15: 934–944.
Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 2002; 99: 2344–2349.
Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL et al. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol 2005; 23: 19–20.
Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN et al. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol 2000; 164: 215–226.
Martin MJ, Muotri A, Gage F, Varki A . Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 2005; 11: 228–232.
Acknowledgements
We gratefully acknowledge Kristin Hoffman for technical assistance and Nicole Deglon for development of the lentiviral constructs. This work was supported by the Department of Defense (DAMD17-03-1-0122, CNS), The Michael J Fox Foundation (CNS) and the Kinetics Foundation (CNS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behrstock, S., Ebert, A., McHugh, J. et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther 13, 379–388 (2006). https://doi.org/10.1038/sj.gt.3302679
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302679
Keywords
This article is cited by
-
Sleeping Beauty transposon system for GDNF overexpression of entrapped stem cells in fibrin hydrogel in a rat model of Parkinson’s disease
Drug Delivery and Translational Research (2023)
-
Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial
Nature Medicine (2022)
-
The role of nonhuman primate models in the development of cell-based therapies for Parkinson’s disease
Journal of Neural Transmission (2018)
-
Systemic injection of AAV9-GDNF provides modest functional improvements in the SOD1G93A ALS rat but has adverse side effects
Gene Therapy (2017)
-
Effects of striatal transplantation of cells transfected with GDNF gene without pre- and pro-regions in mouse model of Parkinson’s disease
BMC Neuroscience (2016)