Abstract
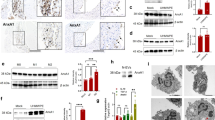
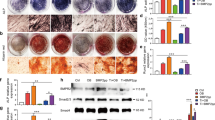
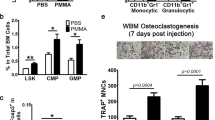
The current study evaluated the protective effects of anti-inflammatory cytokine gene transfer on osteolysis provoked by orthopedic biomaterial particles using a murine model of inflammatory bone loss. A section of bone was surgically implanted into an air pouch established on a syngeneic recipient mouse. Inflammation was provoked by introduction of ultra-high-molecular-weight polyethylene (UHMWPE) particles into the pouch, and retroviruses encoding for interleukin-1 receptor antagonist (hIL-1Ra), viral interleukin-10 (vIL-10), or LacZ genes were injected. Pouch fluid and tissue were harvested 7 days later for histological and molecular analyses. The results indicated that IL-1Ra or vIL-10 gene transfer significantly inhibited IL-1β and tumor necrosis factor (TNF) expression at both mRNA and protein levels. There were significantly lower mRNA expressions of calcitonin receptor and cathepsin K in RNA isolated from hIL-1Ra- or vIL-10-transduced pouches than LacZ-transduced and virus-free controls. Both anti-inflammatory cytokine gene transfers significantly reduced the mRNA expression of M-CSF (70–90%) and RANK (>65%) in comparison with LacZ- and virus-free controls. Histological examination showed that hIL-1Ra or vIL-10 gene transfer dramatically abolished UHMWPE-induced inflammatory cellular infiltration and bone pit erosion compared to LacZ-transduced and virus-free controls. Histochemical staining revealed significantly fewer osteoclast-like cells in samples treated with IL-1Ra or vIL-10 gene transfer. In addition, bone collagen content was markedly preserved in the groups with anti-inflammatory cytokine gene transfers compared with the other two groups. Overall, retrovirus-mediated hIL-1Ra or vIL-10 gene transfer effectively protected against UHMWPE-particle-induced bone resorption, probably due to the inhibition of IL-1/TNF-induced M-CSF production and the consequent osteoclast recruitment and maturation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harris WH . Wear and periprosthetic osteolysis: the problem. Clin Orthop 2001: 66–70.
Sabokbar A et al. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis 1997; 56: 414–420.
Neale SD et al. Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J Orthop Res 1999; 17: 686–694.
Wooley PH et al. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res 1997; 15: 874–880.
Chiba J et al. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop 1994: 304–312.
Jones LC, Frondoza C, Hungerford DS . Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. J Biomed Mater Res 1999; 48: 889–898.
Glant TT et al. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res 1993; 8: 1071–1079.
Jiranek WA et al. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization [see comments]. J Bone Joint Surg Am 1993; 75: 863–879.
Stea S et al. Cytokines and osteolysis around total hip prostheses. Cytokine 2000; 12: 1575–1579.
Sud S et al. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation 2001; 25: 361–372.
Yang S et al. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res 2002; 51: 342–350.
Yang SY et al. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum 2002; 46: 2514–2523.
Yang SY et al. A novel model for osteolysis in aseptic loosening; femoral implants within the inflammatory murine air pouch. Arthritis Rheum 2000; 43: S884.
Wooley PH et al. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials 2002; 23: 517–526.
Gelb H et al. In vivo inflammatory response to polymethylmethacrylate particulate debris: effect of size, morphology, and surface area [published erratum appears in J Orthop Res 1994 Jul;12(4):598]. J Orthop Res 1994; 12: 83–92.
Bottomley KM et al. A modified mouse air pouch model for evaluating the effects of compounds on granuloma induced cartilage degradation. Br J Pharmacol 1988; 93: 627–635.
Nita I et al. Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum 1996; 39: 820–828.
Qin L et al. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J Immunol 1996; 156: 2316–2323.
Ren W, Yang SY, Wooley PH . A novel model of orthopaedic wear debris-associated osteolysis. J Orthop Res 2003, (in press).
Bauer TW . Particles and periimplant bone resorption. Clin Orthop 2002; 405: 138–143.
Schwarz EM et al. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res 2000; 18: 472–480.
Carmody EE et al. Viral interleukin-10 gene inhibition of inflammation, osteoclastogenesis, and bone resorption in response to titanium particles. Arthritis Rheum 2002; 46: 1298–1308.
Evans CH et al. Gene therapy for autoimmune disorders. J Clin Immunol 2000; 20: 334–346.
Goater JJ et al. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res 2002; 20: 169–173.
Evans CH et al. Gene therapy for rheumatic diseases. Arthritis Rheum 1999; 42: 1–16.
Lubberts E et al. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest 2000; 105: 1697–1710.
Gowen M et al. An interleukin 1 like factor stimulates bone resorption in vitro. Nature 1983; 306: 378–380.
Merkel KD et al. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol 1999; 154: 203–210.
Toumbis CA et al. Total joint arthroplasty and the immune response. Semin Arthritis Rheum 1997; 27: 44–47.
Algan SM, Purdon M, Horowitz SM . Role of tumor necrosis factor alpha in particulate-induced bone resorption. J Orthop Res 1996; 14: 30–35.
Wooley PH et al. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum 1993; 36: 1305–1314.
Joosten LA et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res 1999; 1: 81–91.
van de Loo FA, Den Berg WB . Gene therapy for rheumatoid arthritis. Lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators. Rheum Dis Clin N Am 2002; 28: 127–149.
Henderson B . Therapeutic modulation of cytokines. Ann Rheum Dis 1995; 54: 519–523.
Trindade MC et al. Interleukin-10 inhibits polymethylmethacrylate particle induced interleukin-6 and tumor necrosis factor-alpha release by human monocyte/macrophages in vitro. Biomaterials 2001; 22: 2067–2073.
Roodman GD . Advances in bone biology: the osteoclast. Endocr Rev 1996; 17: 308–332.
Pandey R et al. Arthroplasty implant biomaterial particle associated macrophages differentiate into lacunar bone resorbing cells. Ann Rheum Dis 1996; 55: 388–395.
Wang W et al. Biomaterial particle phagocytosis by bone-resorbing osteoclasts. J Bone Joint Surg Br 1997; 79: 849–856.
Greenfield EM et al. The role of osteoclast differentiation in aseptic loosening. J Orthop Res 2002; 20: 1–8.
Yoshida H et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990; 345: 442–444.
Takahashi N, Udagawa N, Suda T . A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun 1999; 256: 449–455.
Yasuda H et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998; 95: 3597–3602.
Azuma Y et al. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 2000; 275: 4858–4864.
Xu JW et al. Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the periprosthetic tissues in the aseptic loosening of total hip replacement (THR). Clin Rheumatol 1997; 16: 243–248.
Campbell IK, Ianches G, Hamilton JA . Production of macrophage colony-stimulating factor (M-CSF) by human articular cartilage and chondrocytes. Modulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta 1993; 1182: 57–63.
Hamilton JA, Filonzi EL, Ianches G . Regulation of macrophage colony-stimulating factor (M-CSF) production in cultured human synovial fibroblasts. Growth Factors 1993; 9: 157–165.
Kong YY et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999; 402: 304–309.
PE Applied Biosystems. TagMan Cytokine Gene Expression Plate I. Protocol 1998; P/N 4304671: 37–53.
Grynkiewicz G, Poenie M, Tsien RY . A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440–3450.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, SY., Wu, B., Mayton, L. et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther 11, 483–491 (2004). https://doi.org/10.1038/sj.gt.3302192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302192
Keywords
This article is cited by
-
Lactobacilli can attenuate inflammation in mouse macrophages exposed to polyethylene particles in vitro
BMC Research Notes (2018)
-
Leptin Increases Particle-Induced Osteolysis in Female ob/ob Mice
Scientific Reports (2018)
-
Genetic susceptibility of early aseptic loosening after total hip arthroplasty: the influence of TIMP-1 gene polymorphism on Chinese Han population
Journal of Orthopaedic Surgery and Research (2014)
-
Calcineurin/NFAT pathway mediates wear particle-induced TNF-α release and osteoclastogenesis from mice bone marrow macrophages in vitro
Acta Pharmacologica Sinica (2013)
-
Combination gene therapy targeting on interleukin-1β and RANKL for wear debris-induced aseptic loosening
Gene Therapy (2013)