ABSTRACT
Arachidonic acid cytochrome P-450 (CYP) hydroxylase 4A isoforms, including 4A1, 4A2, 4A3 and 4A8 in the rat kidney, catalyze arachidonic acid to produce 19/20-Hydroxyeicosatetraenoic acids (20-HETE), a biologically active metabolite, which plays an important role in the regulation of blood pressure. However, controversial results have been reported regarding the exact role of 20-HETE on blood pressure. In the present study, we used recombinant adeno-associated viral vector (rAAV) to deliver CYP 4A1 cDNA and antisense 4A1 cDNA into Sprague-Dawley (SD) rats and spontaneously hypertensive rats (SHR), respectively, to investigate the effects of long-term modifications of blood pressure and the potential for gene therapy of hypertension. The mean systolic pressure increased by 14.2±2.5 mm Hg in rAAV·4A1-treated SD rats and decreased by 13.7±2.2 mm Hg in rAAV·anti4A1-treated SHR rats 5 weeks after the injection compared with controls and these changes in blood pressure were maintained until the experiments ended at 24 weeks. In 4A1 treated animals CYP4A was overexpressed in various tissues, but preferentially in the kidney at both mRNA and protein levels. In anti-4A1-treated SHR, CYP4A mRNA in various tissues was probed, especially in kidneys, but 4A1 protein expression was almost completely inhibited. These results suggest that arachidonic acid CYP hydroxylases contribute not only to the maintenance of normal blood pressure but also to the development of hypertension. rAAV-mediated anti4A administration strategy has the potential to be used as targeted gene therapy in human hypertension by blocking expression of CYP 4A in kidneys.
Similar content being viewed by others
INTRODUCTION
It is well known that arachidonic acid (AA) exists extensively in eukaryotic cells and it is metabolized by cycloxygenases and lipoxygenases to produce prostaglandins (PGs) and hydroperoxyeicosatetraenoic acid (HPETE) 1, 2, 3. In the 1980's, a third pathway of arachidonic acid metabolism was identified 3 as the cytochrome p450-dependent monooxygenase pathway (including epoxygenases and hydroxylases). Epoxygenases catalyze AA to produce epoxyeicosatrienoic acids (EETs) 4. The CYP ω/ω-1 hydroxylases then metabolize AA to produce 19-, 20-hydroxyeicosatetranoic acid (19-, 20-HETE) and 20-HETE is considered critically important in blood pressure regulation as first described by J. C. McGiff and co-workers as well as Makita et al. 2, 5. However, the mechanism of action in vivo remains controversial, as different research groups have reached completely opposite conclusions by employing different animal models and research modes. Ma and colleagues 6 reported that the production of 20-HETE by outer medullary microsomes was lower in prehypertensive Dahl salt-sensitive (SS/Jr) than in Dahl salt-resistant (SR/Jr) rats and considered that the deficiency in the production of 20-HETE in the outer medulla of SS/Jr rats might contribute to the development of hypertension in these animals. The fact that induction of renal p450 fatty acid ω-hydroxylase activity with clofibrate prevented hypertension in SS/Jr rats supports this hypothesis 7, 8. By contrast, Schwartzman reported that i) p450 4A1 and 4A3 can be detected in the kidney of 3-wk-old male SHR, ii) this increases with age and peaks at 5 to 7 weeks, iii) the production of 20-HETE is at its maximum when severe hypertension occurred 2, 9, 10. Inhibition of renal arachidonic acid ω-hydroxylase activity with 1-aminobenzotriazole (ABT) (an inhibitor of CYP activity that can spare ω-hydroxylase) can reduce blood pressure in the SHR 11, 12, which suggested that excessive 19-, 20-HETE production in the kidney can cause hypertension. These paradoxical results regarding the role of ω, ω-1hydroxylases in the development and regulation of hypertension require further investigations to identify the precise role CYP 4a plays in the regulation of blood pressure and the development of hypertension. Previously, we used an expression plasmid pcDNA3.1 to deliver CYP 4A1 cDNA and 4A1 antisense to SD rats, and found that pcDNA·4A1 significantly increased blood pressure whereas pcDNA·anti4A1 significantly reduced blood pressure in SD rats 13. These results suggested that CYP4A is involved in maintenance of normal blood pressure and contributes to development of hypertension 13, 14. This conclusion has been further supported by Wang and colleagues 15. In the present study, we further explored the long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats (SHR) by gene delivery of i) rAAV-mediated cytochrome P450 arachidonic acid hydroxylase to overexpress CYP4A1 protein or ii) antisense to block the expression of CYP4A. We also explored the possibility of hypertension gene therapy using the anti-CYP4A strategy. We found that rAAV-mediated CYP 4A1 overexpression induced a significant increase in arterial blood pressure in SD rats and, by contrast, rAAV-mediated anti-CYP 4A1 suppression resulted in a remarkable reduction in blood pressure in SHR.
MATERIALS AND METHODS
Materials
All standard cell culture reagents were obtained from Gibco BRL (Life Technologies, Inc., Grand Island, NY) including Dulbecco's modified Eagle's medium (DMEM), trypsin and fetal bovine serum (FBS). Phenylmethylsulfonyl fluoride (PMSF), leupeptin, aprotinin, dithiothreitol (DTT), HEPES, Tween-20, and goat anti-rabbit horseradish peroxidase antibody were supplied by Jackson Immuno Research Lab (West Grove, PA). Enhanced chemiluminescent substrate (SuperSignal Substrate) used in Western blotting was purchased from Pierce (Rockford, IL). Protein molecular weight pre-stained SDS-PAGE standards were obtained from Bio-Rad (Hercules, CA). Hybrisol solution used in northern blotting was purchased from Intergen (Purchase, NY). PVDF and nylon membranes (positive electron charged) were purchased from Schleicher and Schuell (Dassel, Germany). [α-32P] dCTP (3000 μCi/mmol) was purchased from Yahui Nuclear (Beijing, China). Superfect cell transfection reagent was supplied by Qiagen (Hilden, Germany). TRIzol reagent for protein and total RNA isolation was purchased from Life Technologies (USA). Primer-It II Random Primer Labeling Kit was obtained from Stratagene Company (USA) and QIAquickTM Nucleotide Removal Kit for purification of probe was from QIAGEN (Germany). Anti-CYP4A1 antisera and rat kidney microsome, which contains abundant CYP 4A, were purchased from GENTEST Corporation (USA).
Experimental animals
Sprague-Dawley (SD) rats were provided by the Experimental Animal Center of Shanghai, China (approved by the Academy of Sciences of China) and Spontaneously Hypertensive rats (SHR) were purchased from the Shanghai Institute of Hypertension. Throughout the study period, all animals were housed at 25°C with a 12-h light/dark cycle and allowed free access to normal rat chow and water ad libitum. All animal experimental protocols complied with standards stated in the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by The Academy of Sciences of China.
Cloning of CYP4A1 cDNA
Reverse transcription was performed in 20 μl reaction mixture containing 2 μl 10×PCR buffer, 2 μl of 1 mM dNTPs, 2.5 U/μl of reverse transcriptase (Gibco BRL), 5 μg of total RNA isolated from male SD rat kidney using Trizol reagent according to the manufa-cturer's instructions, 2.5 μmol/L of 16-mer oligo-dT (Perkin-Elmer). After 1 h at 40°C, the reactions were terminated by heating (100°C, 2 min) and the product utilized as PCR template. PCR was carried out in a 50 μl reaction mixture containing 5 μl of 10×PCR buffer, 5 μl of 2 mmol/L dNTPs, the following primers each at 50 pmol/L: sense primer, 5′-GTA TAG AAT TCC GAG GAG TGG CTG CAC C-3′ and antisense primer (5′-GTT ACG TCG ACA CCA CCA ACT CAG CTT-3′) designed from the published cDNA sequences 16, and 2.5 U of Taq DNA polymerase. Thirty cycles of PCR were performed in a thermal cycler with a denaturing phase of 1 min at 94°C, annealing phase of 40 s at 65°C, and an extension phase of 1 min at 72°C. The resulting PCR products were purified by gel electrophoresis, and the fragment of ∼1600 bp base pairs was subcloned into pBluescript vector by Eco RI/Sal I, and sequenced by the dideoxy chain termination method. Sequence analysis demonstrated that the sequence of the gel-purified PCR fragment is identical to CYP4A1 cDNA as published in GenBank 16.
Construction and preparation of rAAV
The rAAV vector plasmid, pXXUF1, and a rAAV plasmid containing report gene, pdxII-lacZ, were provided by Dr. Xiao XIAO 17. The pXXUF1 contains 2 inverse terminal repeats, a cytomegalovirus promotor (CMV) and a poly A tail. The AAV packaging plasmids were constructed based on the plasmid pAAV/Ad as reported previously 17, 18. Packaging plasmid pXX2 was constructed from pACG2 by inserting a promotor p5 element downstream of the capsid gene. The adenovirus helper plasmid pXX6 was constructed by inserting the large ClaI/SalI fragment of pXX5 into plasmid pBluescript KS (+). A 1600 bp CYP4A1 fragment (Not I/Not I) containing the open frame was subcloned into pXXUF1 downstream of CMV to produce the resultant plasmids pUF1·4A1 and pUF1·anti4A1.
Human 293 cells (adenovirus-5 sheared DNA transferred embryonic kidney epithelial cells) were used for packaging. Cells were grown in Dulbecco's modified Eagle medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) and antibiotics in a 5% CO2 atmosphere at 37°C. One to two hours before transfection, each 15 cm diameter plate of cells (70 to 80% confluent) was supplemented with 15 ml of fresh DMEM containing 10% fetal bovine serum. Initial optimization experiments showed that the best rAAV yields were obtained at a 1:1:2.3 ratio (μg: μg: μg DNA) of the three constructs (vector/packaging/helper) which was roughly in a 1:1:1 molar ratio, given the sizes of the vector and helper plasmids. 140 ug plasmid DNA (4A1/UF3·plasmid 32.6 ug or pdxII-lacZ, pXX2 packaging plasmid 32.6 μg and pXX6 helper plasmid 74.8 μg) were dissolved in 2.64 ml of 0.25 M CaCl2, and mixed well with 2.64 ml of 2×BBS buffer (50 mM BES, 280 mM NaCl, 1.5 mM Na2HPO4, pH 6.95). After incubation for 20 min, the Calcium phosphate-DNA solution was added dropwise into the medium-containing 293 cells at 80% confluence in 15 cm plates, with swirling, and the cells were then incubated in a 35°C, 3% CO2 incubator. After 18 h, the medium was replaced with fresh DMEM containing 10% fetal bovine serum and antibiotics. The cells were harvested at 48-72 h postinfection. After low-speed centrifugation on a tabletop centrifuge, the cell pellets were resuspended in 1-2 ml of 100 mM NaCl-10 mM Tris-HCl (pH 8.5) and subjected to four cycles of freeze-thaw. Cell debris was removed by centrifugation. In large-scale rAAV preparation, 40×15 cm plates, each containing ∼5×106 293 cells were used and a single-step gravity-flow column purification method was carried out according to a previously published method 19, 20. The titers of rAAV·4A1, rAAV·anti4A1 and rAAV·LacZ were determined by dot blot hybridization. The eluted rAAV was aliquoted and stored at -80°C for experiments. The resultant rAAVs were assigned as rAAV·4A1, rAAV·anti4A1 and rAAV·LacZ.
Effect of delivery of rAAV·4A1 and rAAV·anti4A1 on systolic blood pressure of SD rats and SHR
Twenty-four male Sprague- Dawley (SD) rats (weighing 210-225 g) and 18 SHRs were used in this study. All the rats were housed in an air-conditioned room with 12-h light/dark cycle, received a standard rat chow (0.4% sodium chloride) and drank tap water. All the procedures complied with standards for care and use of animal subjects as stated in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). One week before gene delivery, the SD rats were randomly divided into 3 groups, which were administered rAAV·4A1, rAAV·LacZ (control), and saline, respectively, via sublingual vein (5×1010/rat). SHRs were randomly divided into 3 groups (6 for each group), which received intravenous injection of rAAV·anti4A1, rAAV·LacZ (control) and saline, respectively (5×1010 p.f.u. for each animal). The systolic blood pressure of rats was measured weekly with a manometer-tachometer (Rat Tail NIBP System, ADI Instruments, Australia) by the tail- cuff method 21 immediately after injection until the experiments ended. At 25 weeks after gene delivery, all the animals were sacrificed (euthanasia), and the major organs including brain, heart, lungs, liver, and kidneys were collected, frozen in liquid nitrogen followed by storage in -80°C for later morphology and gene expression analysis.
Preparation of RNA and northern blot analysis
Total RNA and protein from brain, heart, lungs, liver, and kidney samples were extracted by a single step method using Trizol reagent according to manufacturer's instructions.
1.6 kb CYP4A1 cDNA and 500 bp of GAPDH cDNA were labeled with [α-32p]dCTP, using prime-a-gene labeling system (Promega, USA). Total RNA (20 μg for each sample) was separated by 1.2% formaldehyde-agarose gel electrophoresis followed by transfer overnight onto hybond nylon membranes The blot was baked for 2 h at 80°C, followed by prehybridization for 2 h with Hybrisol I (Intergene Comp. USA). Labeled probe was then added to hybridize using the same conditions. All blots were subjected to stringent washing conditions (2×SSC/0.1%SDS and 0.5×SSC/ 0.1%SDS 20 min, two times for each washing solution) prior to autoradiography with x-ray film using an intensifying screen at -80°C overnight.
Western blot analysis
Animals were sacrificed by lethal intraperitoneal injection of pentobarbital (100 mg/kg). Following whole body perfusion with 0.9% NaCl, hearts, kidneys, lungs, livers and spleens were excised, frozen and stored at -80°C for analysis. Proteins were extracted using TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA) and protein concentrations were estimated by the Bradford method 22. The tissue protein samples (50 μg per lane) were then separated by 10% SDS/PAGE and transferred onto nylon membrane (PVDF, Schleicher and Schull) in a transfer buffer consisting of 25 mmol/L Tris-HCl, 192 mmol/L glycine, and 20% methanol at 4°C overnight. After blocking for 2 hours at room temperature with 5% non-fat dried milk in 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.1% Tween-20 (TBS-T), the membrane was incubated overnight at 4°C with primary antibodies of goat anti-CYP4A1 antisera (Daiichi pure chemicals, USA) in a 1:800 dilution in TBS-T, followed by washing of the blots and then incubation with donkey anti-goat antibodies conjugated with horseradish peroxidase at room temperature. Labeled bands were visualized using an enhanced chemiluminescence kit.
Statistical Analysis
Data were analyzed by standard statistical methods. Repeated blood pressure measurements at each time point (at least 5 times) were taken after rAAV injection and data were analyzed with the use of either unpaired Student's t test or ANOVA and Fisher's protected least significant differences. Group data are expressed as mean ± SEM. Differences were considered significant at a value of P<0 .05.
RESULTS
Effect of rAAV·4A1 and rAAV·anti4A1 injection on blood pressure of SD rats and SHR, respectively
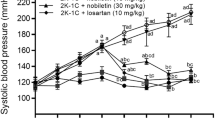
The effects of rAAV·4A1 and rAAV·anti4A1 on the blood pressure of SD rats and SHR, respectively, were monitored weekly for 25 w after injection. The rAAV·LacZ and saline solution (1 ml), respectively, were injected intravenously as a control. Fig. 1 summarizes the effects of delivery of rAAV·4A1 on blood pressure in SD rats throughout the experiment. The results show that there was no significant difference in basal blood pressure among all groups, and rAAV·LacZ injection did not lead to a significant change in blood pressure. Single rAAV·4A1 delivery, however, significantly increased blood pressure by 4 w in SD rats after injection up to a maximum increase of 14.3±2.3 mm Hg at 5 w, which was maintained stable until the end of the experiment. By contrast, single injection of rAAV·anti4A1 significantly decreased blood pressure in SHR by 4 w (13.7±2.2 mmHg) and the hypotensive effects were maintained stable until the end of the experiment at 25 w, while blood pressure in rAAV-LacZ- and saline-treated animals remained unchanged at high levels (Fig. 2). These results suggest that Arachidonic acid-P450 ω/ω- hydroxylase may involve in development of hypertension.
Line graph showing stable increase of systolic pressure in long-term after injection of rAAV·CYP4A1 into SD rats via sublingual vein. rAAV·CYP4A1 (•), rAAV·lacZ (○) and saline (▾). From the third weeks, blood pressure of rAAV·CYP4A1-treated rats raised significantly compared with rAAV-LacZ- and the saline-treated rats (n=8 for each group).
Line graph showing stable decrease in systolic blood pressure in long-term after injection of rAAV·anti4A1 into SHR via sublingual vein, rAAV·anti4A1 (▾ ), rAAV·lacZ (○) and saline (•). From the third weeks, blood pressure of rAAV·anti4A1-treated rats decreased significantly compared with rAAV-LacZ- and the saline-treated rats (n=6 for each group).
Northern blot analysis after gene delivery
To determine the effects of rAAV·4A1 and rAAV·anti4A1 on the expression level of 4A1 in SD rats and SHR as well as on the dynamics of CYP expression in various organs, Northern blot analysis was performed. Results showed that twenty-five weeks after injection of rAAV, CYP4A1 or anti4A1 mRNA expression was significantly increased in kidney and liver, especially in the former (Fig. 3 and 4). Interestingly, CYP4A1 and anti4A1 were preferentially transcribed in the liver and especially in the kidneys, compared with the control rats. CYP4A1 mRNA levels in brain, heart and lungs were relatively very low in both basal and treated groups, although the transcription of CYP4A1 increased to some extent after delivery of rAAV compared to the level in the brain, heart and lungs of control rats (data not shown). The antisense 4A1 transcription in kidney provides a possibility to silence native 4A1 expression in kidney and observe effect of specific inhibition of P450 4A gene expression in kidney where P450 is most abundant.
Northern blot analysis showing changes of anti4A1 mRNA levels in liver and kidney of SHR rats 25 weeks after rAAV·anti4A1 delivery. (A) Representative Northern blot images of CYP4A1 and anti4A1 mRNA levels in response to anti4A1 treatment using double 4A1 DNA probe. RNA loading was determined by hybridization to GAPDH; (B) Autoradiographs were scanned and relative 4A1 mRNA levels normalized to GAPDH were determined. Data shown are mean±SE of three separate experiments. **P<0.05 vs. transfection with rAAV·LacZ (n=3).
Northern blot analysis showing changes of CYP4A1 mRNA levels in liver and kidney of SD rats 25 weeks after delivery. A representative Northern blot images of CYP4A1 mRNA levels in response to rAAV CYP4A1 treatment. RNA loading was determined by hybridization to GAPDH; (B) Autoradiographs were scanned and relative 4A1 mRNA levels normalized to GAPDH were determined. Data shown are mean ± SE of three separate experiments. **P<0.05 vs. transfection with rAAV·LacZ (n=3).
Western blot analysis
Western blots were performed to investigate the effects of rAAV·4A1 or rAAV·anti4A1 on expression levels of CYP4A1 in various organs in SD rats and SHR, respectively, at 25 w after gene delivery. The results showed that CYP4A1 expression increased significantly in kidneys of rAAV·4A1-treated SD rats as compared with rAAV·LacZ-treated SD rats (Fig. 5). By contrast, in rAAV·anti4A1-treated animals, CYP4A expression was almost completely blocked in liver, and especially in the kidneys (Fig. 6). The pattern of protein expression for CYP4A1 was similar to that of the mRNA expression pattern for brain, heart and lungs, both before and after treatment with rAAVs (data not shown). These results demonstrate that CYP4A1 overexpression, especially in kidneys, was likely responsible for induction of hypertension in SD rats. On the other hand, the reduction in CYP4A expression in kidneys by anti4a1 delivery resulted in hypotension in SHR, and thus further suggests that CYP4A expression in kidneys contributes to the development of hypertension.
Western Blot analysis of CYP4A1 in kidney of SD rats 25 weeks after rAAV·4A1 delivery. (A) Representative Western blot images of CYP4A1 protein levels in response to rAAV 4A1 treatment. Standard indicates rat kidney microsome containing abundant CYP 4A from GENTEST Corporation as positive control. (B) Autoradiographs were scanned and relative 4A1 expression levels normalized to GAPDH were determined. Data shown are mean ± SE of three separate experiments. **P<0.05 vs. infection with rAAV·LacZ (n=3).
Western Blot analysis of CYP4A1 expression in SHR kidney 25 weeks after rAAV·anti4A1 delivery. (A) Representative Western blot images of CYP4A1 protein levels in response to rAAV anti4A1 treatment. Standard indicates rat kidney microsome from GENTEST Corporation as positive control. (B) Autoradiographs were scanned and relative 4A1 expression levels normalized to GAPDH were determined, showing rAAV·anti4A1 blocked CYP4A1 expression in kidney of SHR. Data shown are mean±SE of three separate transfections with rAAV·LacZ. **P<0.05 vs. transfection with rAAV·LacZ (n=3).
DISCUSSION
Four isoforms of cytochrome p-450 in the 4A family (4A1, 4A2, 4A3 and 4A8) have been identified in rats to date 23, 24. All of the isoforms catalyze the ω- and ω-1 hydroxylation of arachidonic acid to produce 20-HETE 2, 5, 25, 26, 27. Ito et al 28 used isoform specific primers to amplify 4A isoforms individually and found that the expression of CYP4A1 mRNA was very low but that CYP 4A2 and 4A3 mRNA are constitutively expressed throughout various nephron segments and the renal vessels in male SD rats. Schwartzman and his colleagues 2 also demonstrated, by Northern blot analysis, that CYP 4A2 and 4A3 mRNA are constitutively expressed in rat kidney, whereas the expression of 4A1 mRNA is nearly undetectable in the kidney of 3 to 7-wk-old male SD rats. However renal cortical CYP4A1 protein levels were higher in SHR compared to SD and Wistar-Kyoto rats. These data demonstrate that the normal expression of 4A1 mRNA is not high in control SD rats. Additionally, CYP4A1 is expressed in the arterial system at low levels but is almost undetectable in other organs. Taken together, this data implicate important roles for CYP 4A and 20-HETE in the development of hypertension. By contrast, however, in Dahl salt-sensitive (Dahl S) rats, 20-HETE production is reduced and induction of renal cytochrome P4504A (P4504A) activity and 20-HETE production with clofibrate prevents the development of hypertension 6, 8, 9. Further studies have indicated that induction of renal P4504A activity with clofibrate improves the pressure natriuresis relationship in Dahl S rats by primarily increasing GFR 29, 30. These contradictory results remain to be explained, and may, in fact, reflect the differences among various animal models 31.
In previous study, we found plasmid-mediated delivery of CYP4A1 and anti4A1 cDNA significantly raised and reduced blood pressure, respectively, in normatensive SD rats 13. In order to further investigate the relationship of CYP4A expression level and blood pressure in rats, the present study used recombinant adeno-associated virus (rAAV) as vector to overexpress 4A1 or to block the expression of CYP4A by injection of either rAAV·CYP4A1 or anti4A1, respectively, via the sublingual vein. The rAAV vector system has several unique advantages for gene transfer over other viral vectors (such as plasmid and adenoviral vectors), including a high efficiency of infection, minimal induction of host immune and inflammatory response and driving genes to express in vivo and in vitro in long-term and stably 19, 32. To our surprise, both Northern blots and Western blots demonstrated that after rAAV injection, CYP4A1 was preferentially expressed in the kidney in long-term, with only low level of expression in the liver and poorly in all the other organs. In addition, the expression of CYP4A on the protein level was almost completely blocked by anti4A1 delivery through expression of antisense 4A1 mRNA in the kidney compared with control rats. The CMV-driven gene expression dynamics in this study is quite different from what we observed in other studies. Regardless of the plasmid- and adenovirus-vector-mediated gene transfection, liver generally expresses more than kidneys and other organs, as long as the promoter is not organ-specific and the delivery route is not vessel-selective. The exact mechanisms through which CYP4A1 and anti4A1 are highly kidney-specific require further study. One possible explanation is that the binding site for kidney-specific expression might be within CYP4A. The kidney-selective CYP4A expression, however, does allow a convenient way to investigate the relationship between expression of kidney CYP4A1 and blood pressure.
Schwartzman et al 3 showed that the expression pattern of CYP4A1 as well as 4A2 and 3 in spontaneously hypertensive rats (SHR) is very high, which increases the production of 20-HETE in kidneys and results in the increase in blood pressure. In our study, rAAV·4A1 delivery led to kidney-specific overexpression of CYP4A1. This new transgenic SD rat model with CYP4A high kidney expression of CYP4A1 shows significant increase in systolic blood pressure that was observed over the long term. Therefore, we consider that overexpression of 4A isoforms in kidneys substantially contributes to the elevation of arterial blood pressure in SHR and CYP4A1-treated SD. Altogether, this suggests that overexpression of CYP4A1 may play an important role in the development of hypertension.
On the other hand rAAV·anti4A1 delivery induced a persistent decrease in systolic blood pressure in SHR due to the blocked expression of CYP4A1 mediated by antisense interference. The antisense to CYP4A1 produced can hybridize mRNA of CYP4A1, 2 and 3 to form double-strand RNA, which is easily digested by RNase H in the cells 33. This result further suggests that the CYP4A1 also plays an important role in the development of hypertension and moreover, hints that rAAV-mediated anti4A (such as 4A9 or 4A11) could be targeted for the gene therapy in the treatment of human hypertension.
20-HETE production in the kidney may increase blood pressure in rats through at least three mechanisms: i) 20-HETE might constrict the renal artery through inhibition Ca2+-activated K+ channels in smooth muscle directly, ii) 20-HETE also acts indirectly as a second messenger for ET-1 and iii) it serves as mediator of selective renal effects of angiotensin II 1, 33, 34, 35, 36. This is supported by the discovery in the unx/salt/DOCA model, which rats that were uni-nephrectonized, given excess salt and treated with DOCA, had increased blood pressure, which increased progressively over the 3-wk study. Between the second and third week, urinary excretion of ET-1 and HETE had increased by three to four fold. Blockade of the ETA receptor could lower blood pressure and attenuate organ hypertrophy and proteinuria while decreasing the excretion of 20-HETE 37.
In summary, in normotensive SD rats, the delivery of rAAV·4A1 via the sublingual vein could lead to CYP4A1 overexpression in the kidneys and elevate the blood pressure of the rats five weeks after injection. The delivery of antisense CYP4A1 specifically blocked CYP4A1 expression in the kidney, and significantly decreased the blood pressure. Altogether, we interpret this data with our previous study 13 to conclude cytochrome p450-arachidonic acid hydroxylases of the rat substantially contribute to the development of high blood pressure as well as the maintenance of normal blood pressure.
References
McGiff JC, Quilley J . 20-HETE and the kidney: resolution of old problems and new beginnings. Am J Physiol Regul Integr Comp Physiol 1999; 277:R607–23.
Schwartzman ML, da Silva JL, Lin F, Nishimura M, Abraham NG . Cytochrome P450 4A expression and arachidonic acid omega-hydroxylation in the kidney of the spontaneously hypertensive rat. Nephron 1996; 73:652–63.
Schwartzman M, Carroll MA, Ibraham NG, et al. Renal arachidonic acid metabolism. The third pathway. Hypertension 1985; 7(3 Pt 2):I136–44.
Zeldin DC . Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 2001; 276:36059–62.
Makita K, Falck JR, Capdevila JH . Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. Faseb J 1996; 10:1456–63.
Ma YH, Schwartzman ML, Roman RJ . Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 1994; 267(2 Pt 2):R579–89.
Wilson TW, Alonso-Galicia M, Roman RJ . Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension 1998; 31(1 Pt 2):225–31.
Roman RJ, Alonso-Galicia M, Wilson TW . Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 1997; 10(5 Pt 2): S63–7.
Omata K, Abraham NG, Escalante B, Schwartzman ML . Age-related changes in renal cytochrome P-450 arachidonic acid metabolism in spontaneously hypertensive rats. Am J Physiol Renal Physiol 1992; 262:F8–16.
Sacerdoti D, Abraham NG, McGiff JC, Schwartzman ML . Renal cytochrome P-450-dependent metabolism of arachidonic acid in spontaneously hypertensive rats. Biochem Pharmacol. 1988; 37:521–7.
Su P, Kaushal KM, Kroetz DL . Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regul Integr Comp Physiol 1998; 275(2 Pt 2):R426–38.
Wang MH, Zand BA, Nasjletti A, Laniado-Schwartzman M . Renal 20-hydroxyeicosatetraenoic acid synthesis during pregnancy. Am J Physiol Regul Integr Comp Physiol 2002; 282:R383–9.
Zhang F, Qian JQ, Wang DW . Arachidonate CYP hydroxylases of kidney contribute to formation of hypertension and maintenance of blood pressure. Acta Pharmacol Sin 2002; 23:497–502.
Wang DW, Zhang F, Qian JQ . Contribution of cytochrome P450 4a1 to the long term control of arterial blood pressure: evidence from somatic CYP 4a1 gene modidications. FASEB J 2002; 16:A486 (abstract).
Wang MH, Zhang F, Marji J, Zand BA, Nasjletti A, Laniado-Schwartzman M . CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am J Physiol Regul Integr Comp Physiol 2001; 280:R255–261.
Kimura S Hardwick JP, Kozak CA, Gonzalez FJ . The rat clofibrate-inducible CYP4A gene subfamily. I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA 1989; 8:503–16.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72:2224–32.
Monahan PE, Samulski RJ . Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today 2000; 6:433–40.
Xiao X, Li J, Samulski R . Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol 1996; 70:8098–108.
Auricchio A, Hildinger M, O'Connor E, Gao GP, Wilson JM . Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12:71–6.
Wang J, Xiong W, Yang Z, et al. Human tissue kallikrein induces hypotension in transgenic mice. Hypertension 1994; 23:236–43.
Bradford M . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–54.
Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML . Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol Regul Integr Comp Physiol 1999; 276:R1691–700.
Wang MH, Wang J, Chang HH, et al. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol Renal Physiol 2003; 285:F295–302.
Zou AP, Fleming JT, Falck JR, et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol Regul Integr Comp Physiol 1996; 270:R228–237.
Lange A, Gebremedhin D, Narayanan J, Harder D . 20-Hydroxyeicosatetraenoic Acid-induced Vasoconstriction and Inhibition of Potassium Current in Cerebral Vascular Smooth Muscle Is Dependent on Activation of Protein Kinase C. J Biol Chem 1997; 272:27345–52.
Imig JD, Zou AP, Stec DE, et al. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol Regul Integr Comp Physiol 1996; 270:R217–27.
Ito O, Alonso-Galicia M, Hopp KA, Roman RJ . Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol Renal Physiol 1998; 274:F395–404.
Escalante B, Erlij D, Falck JR, McGiff JC . Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991; 251:799–802.
Alonso-Galicia M, Frohlich B, Roman RJ . Induction of P4504A activity improves pressure-natriuresis in Dahl S rats. Hypertension 1998; 31(1 Pt 2):232–6.
Shatara RK, Quest DW, Wilson TW . Fenofibrate lowers blood pressure in two genetic models of hypertension. Can J Physiol Pharmacol 2000; 78:367–71.
Wang T, Li H, Zhao C, et al. Recombinant adeno-associated virus-mediated kallikrein gene therapy reduces hypertension and attenuates its cardiovascular injuries. Gene Ther 2004; 11:1342–50.
Gunzl A, Palfi Z, Bindereif A . Analysis of RNA–protein complexes by oligonucleotide-targeted RNase H digestion. Methods 2002; 26:162–9.
Zhao X, Imig JD . Kidney CYP450 enzymes: biological actions beyond drug metabolism. Curr Drug Metab 2003; 4:73–84.
Escalante BA, McGiff JC, Oyekan AO . Role of cytochrome P-450 arachidonate metabolites in endothelin signaling in rat proximal tubule. Am J Physiol Renal Physiol 2002; 282:F144–150.
Carroll MA, Kemp R, Cheng MK, McGiff JC . Regulation of preglomerular microvascular 20-hydroxyeicosatetraenoic acid levels by salt depletion. Med Sci Monit 2001; 7:567–72.
Oyekan AO, McAward K, Conetta J, Rosenfeld L, McGiff JC . Endothelin-1 and CYP450 arachidonate metabolites interact to promote tissue injury in DOCA-salt hypertension. Am J Physiol Regul Integr Comp Physiol 1999; 276(3 Pt 2):R766–75.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (NSFC, No.39870307) and National Basic Research Program of China (973 Program, No. G2000056901). KC was the recipient of an Fonds de la recherche en santé du Québec (FRSQ, Quebec-Canada) - NSFC (China exchange grant).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ZHANG, F., CHEN, C., QIAN, J. et al. Long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats by gene delivery of rAAV-mediated cytochrome P450 arachidonic acid hydroxylase. Cell Res 15, 717–724 (2005). https://doi.org/10.1038/sj.cr.7290341
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290341
Keywords
This article is cited by
-
Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis
Nature Communications (2019)
-
Temporal changes of cytochrome P450 (Cyp) and eicosanoid-related gene expression in the rat brain after traumatic brain injury
BMC Genomics (2013)
-
The anti-tumor effect and increased tregs infiltration mediated by rAAV-SLC vector
Molecular Biology Reports (2013)
-
Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure
Kidney International (2009)