ABSTRACT
To explore the molecular mechanism of chromatin remodeling involved in the regulation of transcriptional activation of specific genes by a myogenic regulatory factor Myogenin, we used NIH3T3 fibroblasts with a stably integrated H1.1-GFP fusion protein to monitor histone H1 movement directly by fluorescence recovery after photobleaching (FRAP) in living cells. The observation from FRAP experiments with myogenin transfected fibroblasts showed that the exchange rate of histone H1 in chromatin was obviously increased, indicating that forced expression of exogenous Myogenin can induce chromatin remodeling. The hyperacetylation of histones H3 and H4 from myogenin transfected fibroblasts was detected by triton-acid-urea (TAU)/SDS (2-D) electrophoresis and Western blot with specific antibodies against acetylated N-termini of histones H3 and H4. RT-PCR analysis indicated that the nAChR a-subunit gene was expressed in the transfected fibroblasts. These results suggest that the expression of exogenous Myogenin can induce chromatin remodeling and activate the transcription of Myogenin-targeted gene in non-muscle cells.
Similar content being viewed by others
INTRODUCTION
Most eukaryotic DNA, including transcriptionally active and inactive genes, is assembled into beadlike structures termed nucleosomes, which are folded into higher-order chromatin fibres1, 2. The linker histone H1 is generally viewed as a repressor of transcription as it prevents the access of transcription factors and chromatin remodeling complexes to DNA3, 4. Zhao and his colleagues visualized the structure of the nucleosome core particle of chromatin in chicken erythrocytes by using atomic force microscopy5. The physical properties of nucleosomes depend on solution condition such as ionic strength and divalent-ion concentration as well as on histone-modification state. The nucleosomes lacking histone H1 and H5 are mobile in physiological condition6. Increased acetylation of core histones (H2A H2B, H3 and H4 octamer) is a hallmark of transcriptionally active chromatin. Alternatively, the 'active' chromatin is relatively depleted in histone H1 and enriched in acetylated core histones7, 8. A point of view considers that the acetylation of the amino termini of histones can decrease the positive electric charge on the surface of nucleosomes and weaken the interaction of histones and DNA. Thus, the nucleosomes can hardly form the higher order structure. On the contrary, the systematic and compact chromatin structure can affect the recognition of specific DNA sequences by transcriptional regulatory factors, thus stop or weaken their regulatory effects. Most recently, Misteli and his colleagues established the fluorescence recovery after photobleaching (FRAP) technique and observed that the residence time of the histone H1 on chromatin of living cells treated by TSA (Trichostatin A, histone deacetylase inhibitor) was shortened from 220-250s to 100-150s, suggesting a higher rate of chromatin remodeling9.
Myogenin is a member of myogenic regulatory factors (MRFs), which possess the ability to activate the muscle differentiation program in non-myogenic cells, and functions as a trans-activator of muscle-specific genes, such as nAChR10, 11, 12 and muscle creatine kinase13, 14. Whether the transcription activation of Myogenin-targeted genes is directly associated with the acetylation modification of histones and the mechanism for remodeling chromatin is still unknown. In this study, Myogenin cDNA was transfected into NIH3T3 fibroblasts and we observed that the acetylated modification degree of histones H3 and H4 was enhanced dramatically and the exchange frequency of histone H1 on chromatin increased significantly. These changes were associated with the transcriptional activation of nAChRa-subunit gene, one of Myogenin target gene.
MATERIALS AND METHODS
Vectors and chemicals
The H l.l-GFP fusion protein expression vector was the generous gift by Dr. Misteli, Hochest33342 was bought from Beijing Jing Ke Company, the pEMSV-Myogenin fusion protein expression vector and NIH3T3 cell lines were kept in our laboratory.
Cell culture and transfection
NIH3T3 cell culture and transfection were performed as described previously11, pEMSV vector lacking Myogenin cDNA was used as the control.
Screening of the stable cell lines
NIH3T3 cells were cultured to 70-90% confluence and H l.l-GFP plasmids were transfected with the calcium phosphate co-precipitation technique, then shocked by glycerol for 2.5 min, switched to DMEM medium with 10% calf serum for 48h. After that, stable cell lines expressing H1.1-GFP fusion proteins were generated from NIH3T3 fibroblasts by using 600 μg/ml G-418.
Hoechst 33342 loading of NIH3T3 cells
The NIH3T3-Hl.l-GFP stable cell line was cultured with DMEM growth medium without phenol red (Hyclone) and then transfected with pEMSV-Myogenin fusion protein expression vector. Thirty minutes before loading, the growth culture medium was renewed. Then Hoechst 33342 was added at the final concentration of 500 ng/ml, incubated for l hr at 37°C and washed twice with PBS solution. After that, the growth medium without phenol red was added again, incubated for 30 min at 37°C and observed with a laser confocal microscope.
Fluorescence recovery after photobleaching (FRAP)
The type of confocal microscope is Leica TCS-CP and the test was carried out using 488-nm laser line of an argon laser (20 mW nominal output, beam width at specimen 0.2 μm, detection 500-575 nm)9, 15, 16. The selected heterochromatin regions of nuclei were photobleached by a single bleach pulse of 8s and then images were acquired, SAAM software was used to calculate the intensity and duration of fluorescence recovery. Single section images were then collected at 5s intervals (5 images), followed by 1s intervals (15 images) and then by 10s intervals (10 images).
The standard Student's t-test was used to determine the statistical significance of results. All quantitative values represent averages from at least 8 nuclei from 3 independent experiments. All experiments were done at 37°C
Protein extraction and Western blot analysis
Immunoblotting for nucleic proteins (including histones) were carried out as described17. In brief, 60 μg of total protein extracted from NIH3T3 cells or transfected cells were separated by SDS-PAGE, transferred onto nitrocellulose membrane and probed with primary rabbit polyclonal antibodies of anti-acetylated histone H3 and anti-acetylated histone H4 (Upstate Biotechnology, NY). The secondary antibody was goat anti rabbit Ig-HRP (Zhongshan Co. China).
For 2-D gel electrophoresis, the nucleus isolation and histone extraction were performed as slightly modified18. All manipulations were performed without delay, with samples kept at 4°C
2-D gel electrophoresis and Western blot analysis
Histones (60 μg) were electrophoresed on 2-D gels as described by Davie21 with a slight modification. Triton urea-acetic acid slab gels (8.0×10.0×1.0 cm) were as the first direction and 15% SDS-PAGE slab gels (8.0×10.0×1.5 cm) as the second direction. Antiacetylated H3 and antiacetylated H4 antibodies were used to detect acetylated species of H3 and H4.
RT-PCR
The total RNA was extracted from cultured cells by Trizol reagent. RT-PCR was performed according to the reagent kit instruction (Gibco). The forward primer for amplifying 541bp fragment of nAChRa-subunit transcript was 5 -AACCAGATCATGGAAACCAAC-3' and the downstream primer was 5'-GGTGTAGAACAGCGGCAGGCG-3'. The reaction conditions of PCR were as follows: 94°C 30s; 52°C 30s; 72°C 45s; 30 cycles. The internal control was β-actin cDNA (275bp), the upstream primer was 5'-CAGGAGATGCGCACCACTGCCGCA-3', and the downstream primer was 5'-TCCTTCTGCA TCCTGTCAGCA-3'. Five ml of PCR products were identified with 1% agarose gel electrophoresis.
RESULTS
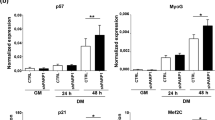
Expression and distribution of H1.1-GFP
The NIH3T3-H1.1-GFP cell lines expressed H1.1-GFP fusion proteins stably and continuously, and the proteins were distributed within nuclei (Fig 1). The chromatin UV localization images after Hoechst 33342 loading were consistent with the expression distribution of H1.1-GFP fusion protein analyzed by laser confocal microscope, indicating that the movement of H1.1-GFP can represent the changes of chromatin.
Colocalization of histone H1.1-GFP with Hoechst 33342 The NIH3T3-Hl.l-GFP stable cell line was generated by transfection with Hl.l-GFP fusion protein expression vector and G418 selection (see Methods). a, Localization of chromatin stained with Hoechst 33342. Hoechst 33342 was added (500 ng / ml), then chromatin UV localization image was analyzed by laser confocal microscope. b, Distribution of histone H1.1-GFP. After Hoechst 33342 loading and UV analysis, the cells were washed twice with PBS solution, and cultured in the growth medium without phenol red for 30 min at 37°C The distribution of H1.1-GFP fusion protein was observed with confocal microscope. The red arrows indicate colocalization of stable expressed H1.1-GFP with Hoechst 33342 in nuclear foci.
Eexchange rate of histone H1.1-GFP in chromatin
In this study we applied the FRAP technique to probe the dynamic properties of histone H1.1-GFP in chromatin of living cells. After photobleaching an area in the nucleus of a cell expressing H1.1-GFP, the recovery of fluorescence signal in the bleaching area was recorded by time-lapse imaging (Fig 2). The recovery level in normal cells (without transfection) was more than 90% of the basal level.
FRAP analysis of H1.1-GFP A spot photobleaching experiment was performed under untreated condition. A. A cell expressing H1.1-GFP was imaged before and during recovery after bleaching of a nucleoplasmic area (green circle). Images were taken at the indicated time after the bleach pulse. Scale bar: 10 μm. B, Relative intensity plot of the phototobleaching experiment in a. Time scale: 40 seconds. The vertical lines on the curve indicate the fluorescence at the time (pre, 0, 4.1s, 15s, 24s and 65s) shown in Fig 2a.
During independent experiments, eight nuclei were selected randomly. Fluorescence intensity and recovery time were analyzed and recorded as mentioned in methods. As shown in Fig 3 and Tab l, the recovery time after photobleaching of nuclei of myogenin transfected cells was shortened from 167s to 46.8s. The highest recovery intensity for transfected nuclei was 23.4%, while the control was 18%. The mean time for 50% recovery of fluorescence in control cells was 1.5-fold longer than that in transfected cells. Conversely, within half time, the average recovery intensity of fluorescence was 16.0% in control cells, whereas, up to 20% in transfected cells (Tab l).
Recovery profiles of spot bleaching experiments FRAP experiments after bleaching of a nucleoplasmic area transfected with (top curve) or without (lower curve) myogenin transfection were performed (see Methods). Fluorescence recovery was monitored quantitatively at indicated time. A cell expressing H1.1-GFP was imaged before and during recovery after bleaching of a nucleoplasmic area (as shown as the “green circle” in Fig 2a). The initial intensity (before bleaching) represents 1.0 and the intensity of first image collected after bleaching represents 0. Values represent means ±s.d. from 24 nuclei in 3 experiments. Arrows indicate the recovery time.
Acetylation of histones H3 and H4
In order to prove that the change of exchange rate of H1 was associated with the acetylation state of core histones, Western blot was conducted with antibodies specific to acetylated histones H3 and H4 (Fig 4). The results showed that the degree of acetylation of core histones H3 and H4 was gradually increased when exogenous Myogenin was expressed.
Identification the degree of acetylation of core histones by 2-D/Western blot
To furthur identifying the change in acetylation of core hostones H3 and H4, histones were resolved on TAU-SDS (2-D) gels and immunoblotted by the antiacetylated H3 and h4 antibodies. Fig 5 showed that histones were enriched in hyperacetylated H3 and H4 isoforms at 72h posttransfection.
Analysis of acetylated H3 and H4 isoforms of histones Histones were extracted from transfected cells at 72 h posttransfection. After 2-D gel electrophoresis and transfer of histones onto a membrane, the acetylated histones were immunoblotted by anti-acetylated antibodies against N-terminal sequences of histone H3 and H4. S: standard histones, C: control (transfected with empty vector), T: transfected with myogenin. Arrows (right) indicate the isoformes of acetylated histones.
Expression of nAChR subunit gene
In order to investigate whether the aceylation levels of histones and the changes of chromatin resulted from myogenin transfection affect the expression of the Myogenin-targeted genes, RT-PCR technique was employed to examine the expression level of nAChR a-subunit mRNA. The results shown in Fig 6 revealed that Myogenin did activate the transcription of nAChRa-subunit gene.
DISCUSSION
In this study, we provide direct evidence indicating that the expression of exogenous Myogenin can stimulate chromatin remodeling near to the Myogenin-targeted gene. Firstly, the recovery time of H1-GFP after photobleaching of nuclei of myogenin transfected cells was shortened from 167s to 46.8s, indicating an increase in the exchange rate of linker protein H1 on chromatin. The increased exchange rate was a consequence of myogenin transfection, as empty vector transfection did not have such effect on the recovery rate. Secondly, enhanced exchange rate of H1 on chromatin was coupled to core histone hyperacetylation induced by exogenous Myogenin expression. This is in agreement with the notion that histone H1 mobility and core histone acetylation are implicated in changes in chromatin structure. Thirdly, the nAChR a-subunit gene, a muscle-specific gene expressed in muscle cells, but not in fibroblasts, was activated in fibroblasts transfected by myogenin. Our results strongly demonstrate that exogenous Myogenin expression can stimulate chromatin remodeling and muscle-specific gene expression in fibroblasts.
Misteli and his colleagues used the photobleaching technique to characterize the binding dynamics of the linker histone H1-GFP to unperturbed chromatin in vivo and found that at any given time, most H1-GFP molecules are bound to chromatin and continuously exchanged between chromatin regions9. Having characterized the dynamics of H1-GFP, they proposed the presence of two distinct kinetic pools in the nucleus: a large mobile pool, which represents the continuously exchanging molecules and is responsible for the fluorescence signal recovery; and a smaller, less mobile pool, which does not contribute to the recovery. This less mobile fraction is significantly larger by 2.5 folds in heterochromatin than in euchromatin. In this study, we determined the recovery kinectics of histone H1.1 in heterochromatic region, and found that the fluorescence recovery in myogenin transfected cells was obviously faster than the recovery time in control cells. Therefore, we conclude that the size of less mobile fraction of H1.1-GFP in heterochromatin was reduced when exogenous Myogenin expressed. It has been proposed that core histone acetylation9 and H1 phosphorylation15 may promote the displacement of histone H1.1 from chromatin. By Western and 2-D/Western assays, we found hyperacetylated histones H3 and H4 in myogenin transfected fibroblasts. These results suggest that the increase of H1 exchange rate is relative to core histone acetylation. This time, of cause, we can not exclude the possibility that H1 phosphorylation is induced by myogenin transfection and contributes to fluorescence recovery after photobleaching.
Berger and his colleagues have demonstrated that MyoD can remodel chromatin at binding sites in muscle gene enhancers and activate transcription at previously silent loci. MyoD and Myf-5 are 10-fold more efficient than Myogenin in activating genes in regions of transcriptionally silent chromatin19. These results established a mechanism for chromatin remodeling in the skeletal muscle lineage. However, they did not determine histone hyperacetylation and H1 exchange as such we did. In these experiments, we performed both issues and showed that exogenous Myogenin could stimulate the hyperacetylation of core histones and speed up the exchange rate of histone H1, manifesting that the chromatin is remodeling. Considering the fact that the endogenous myoD gene is not autoactivated in NIH3T3 cells transfected with MyoD expression vectors and the endogenous MyoD is not detected in NIH3T3 cells after transfection with myogenin10,20, we suppose that exogenous Myogenin may activate downstream target genes and remodel chromatin.
It has been demonstrated that specific transcriptional activation factors can recruit active molecules with acetylase activity2, which may explain why the acetylated degree of histones H3 and H4 increased (Fig 4) and different acetylated isoforms were observed (Fig 5) when exogenous Myogenin expressed. Inferred from stated above, the acetylation of core histones decondenses chromatin, then weakens the binding between H1 and core histones, thus the fluorescence recovery time after photobleaching was shortened. These lead us to conclude that Myogenin first enhances the acetylation degree of histones, and then changes the compact state of chromatin near the region of target gene promoters, and activates transcription of down-stream genes.
References
Luger K, Richmond TJ . The histone tails of the nucleosome. Curr Opin Genet Dev 1998; 8:140–6.
Kornberg RD, Lorch Y . Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 1999; 98:285–94.
Strahl BD, Allis CD . The language of covalent histone modifications. Nature 2000; 403:41–5.
Brown DT, Alexander BT, Sittman DB . Differential effect of H1 variant overexpression on cell cycle progression and gene expression. Nucl Acids Res 1996; 24:486–93.
ZHAO H, ZHANG Y, ZHANG SB, JIANG C, HE QY, LI QM, QIAN RL . The structure of the nucleosome core particle of chromatin in chicken erythrocytes visualized by using atomic force microscopy. Cell Res 1999; 9:255–60.
Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ . Crystal structure of the nucleosome core particle at 2.8 ∼ resolution. Nature 1997; 389:251–60.
Grunstein M . Histone acetylation in chromatin structure and transcription. Nature 1997; 389:349–52.
Berger SL . Gene activation by histone and factor acetyltransferases. Curr Opin Cell Biol 1999; 11:336–41.
Misteli T, Gunjan A, Hock R, Bustin M . Dynamic binding of histone H1 to chromatin in living cells. Nature 2000; 408:877–81.
Prody CA, Merlie JP . A development and tissue-specific enhancer in the mouse skeletal muscle AChR a subunit gene regulated by myogenic factors. J Biol Chem 1991; 266:22588–96.
Jia HT, Tsay HJ, Schmidt J . Analysis of binding and activating functions of the chick muscle AChR g subunit upstream sequence. Cell Mol Neurobiol 1992; 12:241–58.
Witzemann V, Sakmann B . Differential regulation of MyoD and myogenin mRNA levels by nerve induced muscle activity. FEBS Lett 1991; 282:259–64.
Edmonson DG, Olson EN . A gene with homology to the myc similarity region of myoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev 1989; 3:628–40.
Brennan TJ, Olson EN . Myogenin resides in the nucleus and acquires high affinity for a conserved enhancer element on heterodimerization. Genes Dev 1990; 4:582–95.
Lever MA, Th'ng JPH, Sun XJ, Hendzel M . Rapid exchange of histone H1.1 on chromatin in living human cells. Nature 2000; 408:873–6.
Phair RD, Misteli T . High mobility of proteins in the mammalian nucleus. Nature 2000; 404:604–9.
Wang J, Jia HT . Expression of mogenin during skeletal muscle development in chick. Chin J Biochem 1996; 12:507–10.
Perry M, Chalkley R . The effect of histone hyperacetylation on the nucleasse sensitivity and the solubility of chromatin. J Biol Chem 1981; 7:3313–8.
Gerber AN, Klesert TR, Bergstrom DA, Tapscott SJ . Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev 1997; 11:436–50
Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H . Positive autoregulation of the myogenic determination gene MyoD1. Cell 1989; 58:241–8.
Davie JR . Two-Dimensional gel systems for rapid histone analysis for use in minislab polyacrylamide gel electrophoresis. Anal Biochem 1982; 120:276–81.
Juan LJ, Utley RT, Adames CC, Vettese-Dadey M, Workman JL . Differential repression of transcription factor binding by histone H1 is regulated by the core histone amino termini. J EMBO 1994; 13:6031–40.
Acknowledgements
This work was supported in part by grant (39870394, 30271448) from National Natural Science Foundation of China, grant (9728) from the Ministry of Education of China, and Peking University 985 Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GONG, M., NI, J. & JIA, H. Increased exchange rate of histone H1 on chromatin by exogenous myogenin expression. Cell Res 12, 395–400 (2002). https://doi.org/10.1038/sj.cr.7290141
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290141