Abstract
The apoptotic effector Bid regulates cell death at the level of mitochondria. Under its native state, Bid is a soluble cytosolic protein that undergoes proteolysis and yields a 15 kDa-activated form tBid (truncated Bid). tBid translocates to mitochondria and participates in cytochrome c efflux by a still unclear mechanism, some of them at least mediated by Bax. Using mitochondria isolated from wild-type and cardiolipin (CL)-synthase-less yeast strains, we observed that tBid perturbs mitochondrial bioenergetics by inhibiting state-3 respiration and ATP synthesis and that this effect was strictly dependent on the presence of CL. In a second set of experiments, heterologous coexpression of tBid and Bax in wild-type and CL-less yeast strains showed that (i) tBid binding and the subsequent alteration of mitochondrial bioenergetics increased Bax-induced cytochrome c release and (ii) the absence of CL favors Bax effects independently of the presence of t-Bid. These data support recent views suggesting a dual function of CL in mitochondria-dependent apoptosis.
Similar content being viewed by others
Introduction
Cardiolipin (CL) is a unique phospholipid with four acyl chains. It has been known for long that it is associated with mitochondria and, more specifically, with proteins driving oxidative phosphorylation.1, 2 CL has been shown to copurify with different mitochondrial proteins like cytochrome c oxidase,3, 4 the adenine nucleotides carrier,5, 6 the F0F1-ATP synthase,7, 8 the orthophosphate carrier9 and the bc1 complex.4 For most of them, CL has also been shown to be required for their optimal function. For example, strong evidences suggest that CL participates in the interaction of cytochrome c with the outer face of the inner mitochondrial membrane, thereby ensuring the presence of a pool with limited solubility close to respiratory complexes III and IV, increasing the efficiency of electron transport.10
During the past decade, it has become unequivocally clear that most proapoptotic stimuli require a mitochondria-dependent step involving the disruption of mitochondrial bioenergetics11, 12, 13, 14 and outer membrane permeabilization,15, 16 leading to the release of apoptogenic factors, including cytochrome c.
Bid, a widespread proapoptotic member without a proper mitochondrial targeting sequence, has attracted increasing interest since it was identified as a substrate of caspase-8 in apoptotic pathways triggered by death receptors like Fas.17, 18 It has also been identified as a target for other regulatory proteases such as granzyme B,19 cathepsin20 and calpain.21 Caspase-8 cleavage of full-length Bid (flBid) generates a 15 kDa fragment corresponding to the C-terminal side of flBid, termed truncated Bid (tBid), which is considered as the active form of the protein, able to interact with mitochondria.17, 18, 22 However, it remains unclear as to how proteolysis activates the protein.23 The determination of solution structure of Bid showed that it consists of eight α-helices (designated αH1 to αH8), of which αH3 contains the BH3 domain, common to all Bcl-2 family members.24, 25, 26 αH6 and αH7 form an antiparallel hairpin structure, which is surrounded by the other six α-helices. tBid lacks αH1 and αH2, and the hydrophobic hairpin structure formed by αH6 and αH7 becomes exposed, which may explain their binding to the mitochondrial membranes.27 Compared to flBid, tBid also exhibits a 10-fold higher affinity for Bcl-XL, and a 100-fold higher capacity to trigger the release of cytochrome c.22
Mutagenesis studies have shown that the translocation of tBid to mitochondrial membranes is independent of its BH3 domain.17 It has been demonstrated that the successful targeting of tBid depends on the presence of the CL, in what might be a unique structure at the contact sites between the inner and the outer mitochondrial membranes.28 At the contact sites, lipids are likely to form a hexagonal-inverted structure, namely in the presence of calcium ion. It has also been observed that tBid can bind with high affinity to products of CL degradation, namely monolysocardiolipin,29 which are normally present in low amounts in mitochondrial membranes, but increase after the first damage to mitochondria induced during early apoptosis. Uncleaved Bid can also move to mitochondria via a still unclear mechanism, but it remains inactive.30 A new hypothesis31 established that tBid interacts with CL at contact sites and that this disturbs the electron transport chain enough to inhibit state-3 respiration in association with a mild uncoupling of the state-4 respiration. These damages may also be linked to superoxide anion production,32 leading to modification of the mitochondrial membrane composition and favor Bax or Bak oligomerization and cytochrome c release.31
At the mitochondrial surface, tBid participates in the formation and/or activation of channels or pores, in association with other Bcl-2 family proteins such as Bax and Bak: these channels/pores allow the relocalization,23 from the intermembrane space to the cytosol, of apoptogenic factors, such as cytochrome c. The exact nature and mechanism underlying this relocalization is still a matter of debate but might involve, in addition to protein interactions, a reorganization of mitochondrial membranes at the level of contact sites and/or cristae.33 The role of tBid in this general scheme remains unknown: direct action on Bax/Bak activation and have indirect effects on the organization of mitochondrial membranes has been proposed.
In the present study, yeast was used as a simplified cellular model system to investigate the role of CL in the initial steps of the interaction of tBid with mitochondria and the subsequent bioenergetic changes, by comparing Bid-effects on mitochondria isolated from wild-type and, from CL-less yeast strains. Furthermore, the effect of tBid on Bax-induced release of cytochrome c was studied in these two strains, and a model of a self-amplifying loop involving CL was proposed.
Results
Lipid composition and bioenergetic characterization of Δcls1 mitochondria
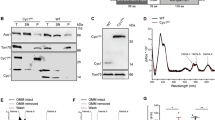
The investigation of the role of CL on the effects of tBid on mitochondria first required a careful characterization of the properties of Δcls1 mitochondria in comparison to wild type. Wild-type strain W303-1B and the Δcls1 mutant were grown on a strictly nonfermentable carbon source (glycerol+ethanol) in order to obtain an optimal differentiation status of mitochondria. Mitochondria were isolated and their phospholipid content was analysed (Figure 1a). Under these conditions, CL represented 12±3% of total phospholipids in wild-type mitochondria, while no CL was detected in Δcls1 mitochondria. As previously described, CL was replaced by its precursor monophophatidylglycerol (PG) since the amount of (phosphatidylethanolamine(PE)+PG) increased from 31% in wild-type to 49% in Δcls1 mitochondria. Interestingly, the proportions of the other phospholipids (phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS)) were not significantly different in wild type and mutant mitochondria, showing that the mutation exclusively modified the repartition between PG and CL. As noted in the introduction, CL is thought to play a crucial role in the existence of contact sites between the inner and outer mitochondrial membranes. In accordance to this hypothesis, the CL proportion in contact sites from wild-type mitochondria was higher than in whole mitochondria (18±2% as compared to 12±3%; Figure 1b) and, as expected, the proportion of PG in contact sites of Δcls1 strain was also higher than in whole mitochondria (58% PE+PG as compared to 49%; Figure 1b). Concerning the fatty acid composition of phospholipids, no significant differences on the saturated/unsaturated ratio and the C18/C16 ratio was observed (Figure 1c), showing that differences of the behavior of wild-type and Δcls1 mitochondria are exclusively caused by the modification of the polar heads. The absence of CL in Δcls1 mitochondria would also be evidenced on whole cells by a cytofluorometric assay on spheroplasts stained with the CL-selective fluorescent probe nonyl acridine orange (NAO; Figure 1d, lower panel). A parallel staining of the cells with the mitochondrial membrane potential (ΔΨm)-sensitive probe DiOC(6)3 showed that the ΔΨm was reduced, but not completely collapsed, in the Δcls1 strain (Figure 1d, upper panel), predicting that the bioenergetic capacity of Δcls1 mitochondria is moderately altered.
Lipid analyses of wild-type and Δcls1 mitochondria and their mitochondrial network. (a) Proportion of each class of phospholipids in whole mitochondria from wild-type and Δcls1 strains separated by TLC. Data are means from three independent experiments±S.D. (b) Proportion of each class of phospholipids in mitochondrial contact sites. Data are means of two independent experiments±S.E.M. (c) Fatty acid analysis from phospholipids from whole mitochondria. C16 : 0, C16 : 1, C18 : 0 and C18 : 1 were separated by gas chromatography-mass spectrometry. Data are means from two independent experiments±S.E.M. (d) Flow cytometry analysis of spheroplasts for ΔΨm (DiOC6(3) (3,3′-dihexyloxacarbocyanine iodide), left panel) and CL content (NAO, right panel). The upper panel describes CL+ and CL− mitochondrial membrane potential, whereas the middle panel and the lower panel show the drop in ΔΨm induced by the uncoupling agent FCCP (at 10 μM). The upper right panel shows the CL content of the CL+ strain via NAO staining, whereas the lower right panel shows the absence of CL in the CL-synthase-less strain (CL−). Data are representative from four independent experiments. (e) Fluorescence microscopy of the mitochndrial network related to early exponential growing phase or late exponential growing phase. The mitochondrial network appeared as threads during early exponential growth phase, both in wild-type and Δcls1 strains. When cells reached late exponential growth phase, the network appeared as grains in the wild-type strain but remained as threads in the mutant strain
Bioenergetic characteristics of isolated mitochondria were determined by measuring simultaneously respiration rate, ΔΨm and ATP synthesis (Figure 2). The basal respiration rate (state 4) of Δcls1 mitochondria oxidizing NADH was slightly increased as compared with wild type (150 versus 129 nmol O2/min/mg protein) in accordance to a slight decrease of ΔΨm (−171 versus −187 mV). Upon addition of ADP, phosphorylation-coupled respiration (state 3) was significantly lower in Δcls1 than in wild type (510 versus 650 nmol. O2/min/mg protein), in accordance with a decreased ATP synthesis (786 compared with 866 nmol H+/min/mg protein) and a slight decrease in ΔΨm (−137 versus −141 mV). The efficiency of coupling between respiration and ATP synthesis was clearly decreased in Δcls1 mitochondria, as indicated by the lower respiratory control ratio (3.4 versus 5.0 in wild-type mitochondria) and the lower ADP/O ratio (1.3 versus 1.5 in wild-type mitochondria). These data indicate that Δcls1 mitochondria exhibit a slight alteration of their bioenergetic function. It is not caused by the isolation of mitochondria, since the lower ΔΨm could also be visualized in whole cells (Figure 1d, upper panel). In addition to the effects on mitochondrial transmembrane potential, the absence of CL also induced some alterations in mitochondrial dynamics. Expression of a mitochondria-addressed GFP allows to visualize the mitochondrial network by fluorescence microscopy. In the wild-type strain, the morphology of the network is changing when the cells approach the stationary phase of growth (thread-grain transition). Such a change does not occur in the Δcls1 strain (Figure 1e), suggesting that the absence of CL may hamper the reorganization of the inner mitochondrial membrane.
Bioenergetic characteristics of wild-type and Δcls1 mitochondria. (a, b) On-line recordings of oxygen concentration (black line, respiration values in nmol O2/min/mg protein), concentration of TPP+ (green line, – ΔΨm values in mV) and proton production linked to ATP synthesis (blue line, values in nmol H+/min/mg protein) for wild-type (a) and Δcls1 (b) mitochondria
CL-dependent effects of tBid on bioenergetic function of isolated mitochondria
The effect of 20 nM tBid on the bioenergetic function of wild-type mitochondria was measured. Both state-3 respiration and ATP synthesis were decreased (from 650–470 nmol O2/min/mg−1 protein, and from 866 to 715 nmol H+/min mg protein, respectively) (Figure 3a). Interestingly, ΔΨm values (both under state 4 and state 3) were not affected showing that the effect of tBid is not a simple detergent-like effect on the membranes. This effect of tBid was completely absent when the protein was assayed on Δcls1 mitochondria (Figure 3b). A titration of tBid has been carried out, which demonstrates that the tBid-binding sites, that is, CLs, were hardly saturable (Figure 4a). Effectively, almost all the tBid added to the mitochondrial suspension (0.5–20 nM with reference to 25 nM tBid alone as a 100% for the Western blot reference) is recovered at the mitochondrial level. Concerning the changes in mitochondrial respiration, the state-3 inhibition by tBid is detected at 5 nM and above, whereas the mild uncoupling leading to state-4 enhancement is only apparent at 10 nM (Figure 4a). flBid at 200 nM is without effect on such parameters.
Effect of tBid addition on the mitochondrial respiration and ATP synthesis. (a) Wild-type mitochondria were preincubated for 20 min with different concentration of tBid (0.5–20 nM). The Western blots with a 25 nM tBid as a reference were scanned and the amount of tBid bound to mitochondria was estimated by comparison to the reference. The plots presented are the mean±S.D. of five blots. The data concerning the state-3 inhibition and the state-4 increase induced by tBid were selected from seven different experiments conducted with the clark electrode (cf. Materials and methods). (b) For the ATP synthesis, the experimental conditions were identical to those in Figures 2 and 3 except that ADP concentration was 1 mM. Aliquots were taken out at 0, 15, 30 and 45 s after ADP addition for the measurement of ATP by bioluminescence
The rate of ATP synthesis was directly measured with a bioluminometric method (Figure 4b): tBid dramatically inhibited the rate of ATP synthesis in wild-type mitochondria, whereas it had no effect on Δcls1 mitochondria.
These demonstrate that, in the absence of any other Bcl-2 family protein, tBid alters mitochondrial function in a CL-dependent way.
CL-dependent synergetic action of tBid and Bax on cytochrome c release
The main proapoptotic function of tBid is thought to occur via its stimulating effect on the conformational change of Bax leading to the acquisition of a structure able to trigger the release of cytochrome c.23 This early step of apoptosis can be reproduced in yeast, following the expression of a Bax variant that has already acquired the active conformation: this was simply obtained by adding a c-myc tag at the C-terminal end of the protein.34 This Bax-variant targets yeast mitochondria, is inserted in the outer membrane and is able to form a large cytochrome c-permeable channel, identical to that observed in apoptotic mammalian cells.35
This simplified cellular system allows testing the effect of tBid on the function of Bax, independently of the apoptotic network. Following the expression of the proteins alone or in combination, mitochondria were isolated and the spectrophotometric measurement of the amount of mitochondrial cytochromes allows visualization of the release of cytochrome c, while the amounts of membrane-attached cytochromes b and aa3 serve as internal controls (Figure 5a). Quantitative results of these series of experiments are given in Figure 5b.
Effect of tBid on Bax-induced cytochrome c release. (a) Redox spectrum of mitochondria from the wild-type strain (thin trace) or mitochondria from the wild strain coexpressing tBid and Bax (bold trace). The peaks of absorbance difference corresponding to cytochromes c, b and aa3 are indicated. (b) Quantification of cytochrome c in mitochondria isolated from wild-type or Δcls1 strains expressing Bax, Bid and tBid. Data are expressed as the ratio cytochrome c/cytochrome b, which is an unreleased membrane protein and is thus taken as an internal standard. Data are means from three to seven independent experiments±S.D. Bid and tBid were expressed under the control of the strong promoter GAL1/10, while Bax was expressed under the control of the weak promoter tet-off.39, 40 (c) Quantification of cytochrome c in mitochondria isolated from wild-type or Δcls1 strains expressing Bax under the control of the strong promoter GAL1/10. Data are expressed as in (b) and are means of three independent experiments±S.D.
Full-length Bid (flBid) or tBid were expressed under the control of the strong promoter GAL1/10: none of them were able to induce any release of cytochrome c. When expressed in yeast, flBid does not spontaneously localize to mitochondria. On the contrary, tBid is localized in mitochondria and this localization is higher when tBid is coexpressed with Bax-c-myc,34 suggesting that both proteins are able to interact in the mitochondrial membrane. Bax was first expressed in the wild-type strain under the control of the low-strength promoter tet-off, under conditions where it does not induce a significant release of cytochrome c. The combined expression of flBid with weakly expressed Bax did not induce any release either. On the contrary, the combined expression of tBid with weakly expressed Bax induced the release of cytochrome c (which is also visualized in Figure 5a). This result is a clear support to the hypothesis that the presence of tBid actually helps in the acquisition of the ultimate active conformation of Bax.
The same series of experiments was carried out in the Δcls1 strain (Figure 5b, gray bars). Like in wild-type strain, neither weakly expressed Bax nor strongly expressed Bid or tBid induced any release of cytochrome c. The combination of weakly expressed Bax and strongly expressed tBid induced a release of cytochrome c, with a higher extent than in wild-type mitochondria. Accordingly, the coexpression of weakly expressed Bax and strongly expressed Bid induced a slight but significant release of cytochrome c in Δcls1 strain, while this combination was inefficient in wild type.
A recent paper described that the killing effect of Bax expression in yeast does not require CL.36 To support this observation, we expressed Bax under the control of the high-strength promoter GAL1/10 in wild-type and Δcls1 strains (Figure 5c): under these conditions, Bax alone was able to induce the release of cytochrome c, independently of the presence of CL.
Discussion
Data reported in this paper are a strong support to the hypothesis that CL plays a crucial role in the effects of tBid on mitochondria. Yeast provides a simplified cellular system that allows testing of the interaction of tBid and Bax with mitochondria and a possible synergy between them, independently from the apoptotic network, and evaluates the role of CL in this interaction.
In vitro experiments demonstrated that, in the absence of any other Bcl-2 family members, tBid is able to target the mitochondrial membrane and to induce moderate but significant alterations of oxidative phosphorylation. An important observation is the lack of effect on ΔΨm, which shows that tBid does not act via a simple detergent-like effect. The second important characteristic of this in vitro effect of tBid is the requirement for CL.
The extensive bioenergetic characterization of CL-less mitochondria showed that, although slightly altered, these mitochondria are still able to support vigorous oxidative phosphorylation. This is an important point since it demonstrates that the lack of effect of tBid is not caused by a dramatic collapse of the bioenergetic function. This was particularly apparent from direct measurements of ATP synthesis (Figure 4): tBid induced a dramatic collapse of ATP synthesis in wild-type mitochondria and was almost inefficient on Δcls1 mitochondria.
Since the proapoptotic action of tBid is thought to involve the activation of Bax towards a conformation changeable to trigger the release of cytochrome c,30 the role of CL on this activation was investigated. It clearly appeared that tBid was actually able to activate the cytochrome c-release activity of Bax, and that the absence of CL further amplified this stimulating effect of tBid. This last point is confirmed by the fact that the less-efficient flBid, which had no stimulating effect in wild type, gained a weak but significant stimulating effect in Δcls1 mutant. This observation is in apparent contradiction with the effect of tBid in vitro but is consistent with the hypothesis that a first damage to mitochondria (visualized by the effect on oxidative phosphorylation) further helps the action of Bax. It has been shown that tBid exhibits a strong affinity for CL28 and even more for by-products of CL degradation, namely monolysocardiolipin.29 It has also been shown that Bax expression, even at low-expression levels, induced the oxidation of mitochondrial lipid, including CL, an effect which is prevented by hydrophobic antioxidants, such as α-tocopherol and resveratrol.37 It is important to notice that yeast expression of tBid alone has no effects on the whole yeast cell respiration (data not shown) nor on cell growth.34 However, in the presence of Bax-c-myc, the expression of tBid increased the kinetics of Bax-c-myc-induced cell death,34 showing that tBid is able to amplify the killing function of an active form of Bax.
Considering these observations and data reported herein, the following self-amplification loop model can be proposed.
-
1)
tBid binds to mitochondria in a CL-dependent fashion. This initial injury to mitochondria has been suggested to induce the oxidation of lipid,29 leading to the formation of lysophospholipids, including monolysocardiolipin. This might help the further binding of tBid.29
-
2)
The presence of tBid has destabilizing effect on mitochondrial membranes, as evidenced by the effects on oxidative phosphorylation reported in the present paper. The nature of this effect remains unclear. It is not an acute detergent-like effect, but might be a more subtle reorganization of the membranes induced by CL reorganization (like change in membrane curvature38) and subsequent destabilization of the CL/enzymatic complexes in the electron transport chain, such as has been observed in apoptotic cells.31
-
3)
The destabilizing effect of tBid on mitochondrial membranes leads to the activation of Bax towards its final active conformation, resulting in its oligomerization, which is able to trigger the release of cytochrome c. It should be noted that the binding of tBid to yeast mitochondria did not increase the binding of Bax (while the binding of Bax did increase that of tBid).34 This further supports the view that tBid acts by favoring the activating conformational change of Bax, which allows the acquisition of the capacity to release cytochrome c, but that it does not act by increasing the amount of Bax bound to mitochondria.
It should be noted that, both in vitro and in yeast, tBid is not absolutely required for Bax function since Bax alone is able to target mitochondria, form a channel in the outer mitochondrial membrane and trigger the release of cytochrome c, but this does not preclude a crucial role in vivo, in a genuine apoptotic network. Data presented in this paper are now a basis for further investigation into the molecular mechanisms involved in the interaction between tBid, Bax and CL, since yeast represents a simplified living model for evaluating the action of Bid and Bax mutants in the absence or in the presence of CL.
Materials and Methods
Strains and plasmids
The strain WKEN011B (matα, ade2, his3, leu2, trp1, ura3, cls1::kanMX4, termed Δcls1 in the following) was derived from the wild-type strain W303-1B, and was obtained from Euroscarf (Frankfurt, Germany). The wild-type strain HT444 (mat a, his3, leu2, lys2, ura3) expressing Bax-c-myc under the control of GAL1/10 promoter was described previously.39 The strain SM400 was derived from HT444 by introducing the cls1::kanMX4 construction, amplified by PCR from the WKEN011B genome, at the original CLS1 locus. The construction of the plasmid pCM189/bax, expressing Bax-c-myc under the control of the tet-off promoter was described in Priault et al.40 The construction of the plasmids pYES3/Bid and pYES3/tBid, expressing Bid and tBid under the control of the GAL1/10 promoter, was described in Priault et al.34 PCR, cloning, and transformations were performed by standard procedures.
Mitochondria isolation and bioenergetic measurements
Cells were grown aerobically in a complete medium YPGE (1% yeast extract, 1% Bacto-peptone, 0.1% potassium phosphate, 0.12% ammonium sulfate, 3% ethanol, 3% glycerol, pH 5.0) until mid-exponential growth phase. The use of glycerol/ethanol as a carbon source ensured a full differentiation of mitochondria. Mitochondria were isolated from zymoliase-treated cells, as described in Law et al.41
For bioenergetic measurements, mitochondria (0.33 mg/ml) were suspended in respiration buffer (0.6 M mannitol, 5 mM tris/maleate, 2 mM EGTA, 3 mM potassium phosphate, 5 μM tetraphenylphosphonium bromide, pH 6.7) in a thermostatically controlled chamber (28°C) connected to three electrodes, allowing the simultaneous measurement of oxygen concentration, tetraphenylphosphonium (TPP+) concentration42 and pH, respectively.43 Respiration noncoupled to ADP-phosphorylation (state 4) was initiated by the addition of 1 mM NADH (yeast mitochondria are able to oxidize directly cytosolic NADH via two intermembrane space-facing NADH : ubiquinone oxidoreductases). Respiration coupled to ADP phosphorylation (state 3) was initiated by the addition of 110 μM ADP. Respiration rates were calculated from the slopes of oxygen concentration versus time curves. ΔΨm was calculated from TPP+ concentration according to the Nernst equation corrected for TPP+ binding.44 The rate of ATP synthesis was extrapolated from the rate of acidification caused by the extrusion of the scalar proton in the reaction of ADP phosphorylation.43 Alternatively, ATP synthesis was measured directly by a bioluminometric method. Aliquots (100 μl) were sampled at different times after ADP addition and precipitated in 100 μl of 50 mM EDTA in 7% perchloric acid. After immediate centifugation (3 min, 12 000 g), the pH of the supernatant was adjusted at 7.0 with KOMO (2 M MOPS, 0.2 N potassium hydroxide). ATP was monitored with a luciferin/luciferase kit (BioOrbit) in an LKB bioluminometer against ATP standard solutions.
Flow cytometry measurements and fluorescence microscopy
All measurements were carriedout with a FACScalibur (Becton-Dickinson) equipped with a 488 nm laser. For ΔΨm measurements, spheroplasts obtained from zymoliase treatment of the cells were incubated for 30 min in the presence of 5 nM DiOC(6)3 in the absence or in the presence of 10 μM fluorocarbonyl cyanide m-chlorophenylhydrazone (FCCP), and fluorescence was detected at 530±30 nm in the FL1 channel. For CL-content measurements, spheroplasts were incubated for 30 min in the presence of 1 μM NAO and fluorescence was detected at 585±40 nm in the FL2 channel.
For fluorescence microscopy experiments, cells were transformed with plasmid pGAL-CLbGFP, which allows the galactose-driven expression of GFP fused downstream the mitochondria-addressing sequence of mitochondrial citrate synthase.44 Cells were grown aerobically on a medium supplemented with 2% galactose and observed during the early (O.D.550 nm=1) or late (O.D.550 nm=10) exponential phases of growth, with a Leica fluorescence microscope connected to an SIS camera.
Heterologous expression of proapoptotic proteins and measurement of cytochrome c release
The expression of c-myc-tagged Bax was achieved with two systems giving different levels of expression.34, 35 High-level expression was carried out with the GAL1/10 promoter as follows: HT444/Bax and SM400/Bax cells were grown aerobically in a complete medium YPL (1% yeast extract, 0.1% potassium phosphate, 0.12% ammonium sulfate, 2% lactate, pH 5.0) until the early-exponential growth phase. Galactose (1%) was added to induce Bax expression, cells were harvested after 90 min and mitochondria were isolated for cytochrome c measurement. Low-level expression of Bax, in the presence of Bid or tBid, was carried out as follows: W303-1B and WKEN011B cells, carrying or not pCM189/Bax, pYES3/Bid and pYES3/tBid plasmids, were grown aerobically on a minimal medium YNBGal (0.17% yeast nitrogen base, 0.1% potassium phosphate, 0.5% ammonium sulfate, 0.2% Drop-Mix, 0.01% auxotrophic requirements, 2% galactose, pH 5.0) with 10 μg/ml doxycyclin: these conditions allowed the expression of flBid or tBid and the repression of Bax. When cultures reached mid-exponential growth phase, cells were harvested, washed three times and resuspended in the same medium with 0.1 μg/ml doxycyclin to induce a low level of Bax expression.34 Cells were harvested after 12 h and mitochondria were isolated for cytochrome c measurement.45
Cytochrome c content of mitochondria was measured spectrophotometrically. Mitochondria suspension (5 mg/ml) was poured into the two cuvettes of a double-beam spectrophotometer (Aminco DW2000). The reference cuvette was oxidized with potassium ferricyanide and the sample cuvette was reduced with sodium dithionite. Difference spectra were acquired between 500 and 650 nm. The amount of each cytochrome was measured from the difference in the difference spectra between maxima and isobestic points: 550–540 nm for cytochrome c (Δɛ=18 000/M), 561–575 nm for cytochrome b (Δɛ=18 000/M) and 603–630 nm for cytochromes a+a3 (Δɛ=24 000/M). Previous reports have shown that the amount of cytochrome c which disappeared from mitochondria following Bax expression was found in the postmitochondrial supernatant.39
Contact sites, outer and inner mitochondrial membranes were isolated according to Simbeni et al46 and Pon et al47, respectively, from isolated mitochondria.
Synthesis of tBid
Full-length His-tagged flBid and tBid were purified as described by Desagher et al.30 The protein was stored in 25 mM tris-HCl, 100 mM NaCl, 1% octyl D-glucopyranoside, 0.2 mM dithiothreitol, 30% glycerol, pH 7.5, at −80°C. Stock solution of tBid (1.9 mg/ml) was diluted in the nM range in the respiration buffer. An identical dilution of the storage buffer has no effect on mitochondrial functions.
Lipid extraction and fatty acid analysis
Mitochondria were suspended in methanol (10 mg/ml) and lipids were extracted twice by a chloroform/methanol/water (2/1/1) (v/v/v) mixture. The organic phases were pooled, evaporated to dryness and resolubilized in 50 μl chloroform/methanol (2/1) (v/v). Polar lipids were resolved by one-dimensional thin layer chromatography on 10 × 10 cm silica gel plates (Merck) using the solvent system as described.48 Lipids were located by spraying the plates with a solution of 0.001% primuline in 80% acetone, visualized under UV light, scrapped and quantified by UV absorbance versus known standards.
For fatty acid analysis, aliquots were mixed with 900 μl methanol containing 2.5% H2SO4 and 10 μg C17 : 0 as an external standard. Transesterification was carried out at 80°C for 1 h and, after cooling on ice, methyl ester fatty acids were extracted with 1 ml hexane. Separation of methyl ester fatty acids was carried out by gas chromatography (Hewlett-Packard 5890 series II) on a 15 m × 0.53 mm Carbowax column (Alltech). The oven temperature is programmed for 1 min at 160°C, followed by a 20°C/min ramp up to 190°C, a 5°C/min ramp up to 210°C and a final 5 min at 210°C. Methyl ester fatty acids were identified and quantified by comparison of their retention time with those of standards.
Abbreviations
- CL:
-
cardiolipin
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PG:
-
phosphatidylglycerol
- PI:
-
phosphatidylinositol
- PS:
-
phosphatidylserine
- DiOC6(3):
-
3,3′-dihexyloxacarbocyanine iodide
- ΔΨm:
-
mitochondrial membrane potential
- FCCP:
-
fluorocarbonyl cyanide m-chlorophenylhydrazone
- NAO:
-
nonylacridine orange
- TPP+:
-
tetraphenylphosphonium
References
Hoch FL (1998) Cardiolipins and mitochondrial proton-selective leakage. J. Bioenerg. Biomembr. 30: 511–532
Schlame M, Rua D and Greenberg ML (2000) The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39: 257–288
Dale MP and Robinson NC (1988) Synthesis of cardiolipin derivatives with protection of the free hydroxyl: its application to the study of cardiolipin stimulation of cytochrome c oxidase. Biochemistry 27: 8270–8275
Robinson NC (1993) Functional binding of cardiolipin to cytochrome c oxidase. J. Bioenerg. Biomembr. 25: 153–163
Horvath LI, Drees M, Beyer K, Klingenberg M and Marsh D (1990) Lipid–protein interactions in ADP–ATP carrier/egg phosphatidylcholine recombinants studied by spin-label ESR spectroscopy. Biochemistry 29: 10664–10669
Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N and Greenberg ML (2000) Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 275: 22387–22394
Kagawa Y, Kandrach A and Racker E (1973) Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J. Biol. Chem. 248: 676–684
Serrano R, Kanner BI and Racker E (1976) Purification and properties of the proton-translocating adenosine triphosphatase complex of bovine heart mitochondria. J. Biol. Chem. 251: 2453–2461
Eble KS, Coleman WB, Hantgan RR and Cunningham CC (1990) Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J. Biol. Chem. 265: 19434–19440
Tuominen EK, Wallace CJ, Kinnunen PK, Zhu K, Clark-Lewis I, Craig DB and Rytomaa M (2002) Phospholipid–cytochrome c interaction: evidence for the extended lipid anchorage: ATP induces a conformational change in lipid-bound cytochrome c. J. Biol. Chem. 277: 8822–8882
Vayssière J-L, Petit PX, Risler Y and Mignotte B (1994) Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl. Acad. Sci. USA 91: 11752–11756
Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B and Gougeon ML (1995) Alterations of mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 130: 157–167
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev. 15: 2922–2933
Newmeyer DD, Ferguson-Miller S, Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R and Green DR (2003) Mitochondria: releasing power for life and unleashing the machineries of death Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 112: 481–490
Gross A, McDonnell JM and Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911
Martinou JC and Green DR (2001) Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2: 63–67
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X (1998) Bid, a bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface receptors. Cell 94: 481–490
Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P and Korsmeyer SJ (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274: 1156–1163
Barry M, Heibein JA, Pinkoski MJ, Lee SF, Moyer RW, Green DR and Bleackley RC (2000) Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid (in process citation). Mol. Cell. Biol. 20: 3781–3794
Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V and Salvesen GS (2001) Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276: 3149–3157
Chen M, He H, Zhan S, Krajewski S, Reed JC and Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J. Biol. Chem. 276: 30724–30728
Li H, Zhu H, Xu CJ and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Esposti MD (2002) The roles of Bid. Apoptosis 7: 433–440
Chou JJ, Li H, Salvesen GS, Yuan J and Wagner G (1999) Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96: 615–624
McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ and Cowburn D (1999) Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96: 625–634
Esposti MD, Erler JT, Hickman JA and Dive C (2001) Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol. Cell. Biol. 21: 7268–7276
Hu X, Han Z, Wyche JH and Hendrickson EA (2003) Helix 6 of tBid is necessary but not sufficient for mitochondrial binding activity. Apoptosis 8: 277–289
Lutter M, Perkins GA and Wang X (2001) The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2: 22
Esposti MD, Cristea IM, Gaskell SJ, Nakao Y, Dive C, Erler JT and Hickman JA (2003) Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 1: 1–10
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou JC (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144: 891–901
Gonzalvez F, Pariselli F, Rameau C, Dupaigne P, Budihardjo I, Leducq N, Lutter M, Antonsson BSM, Martinou JC, Wang X, Diolez P, Bernard S and Petit PX (2005) t-Bid interaction with cardiolipin primarily orchestrate mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 40 (in press)
Ding WX, Ni HM, DiFrancesca D, Stolz DB and Yin XM (2004) Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology 40: 403–413
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA and Korsmeyer SJ (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2: 55–67
Priault M, Cartron PF, Camougrand N, Antonsson B, Vallette FM and Manon S (2003) Investigation of the role of the C-terminus of Bax and of tc-Bid on Bax interaction with yeast mitochondria. Cell Death Differ. 10: 1068–1077
Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA and Kinnally KW (2001) A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell Biol. 155: 725–731
Iverson SL, Enoksson M, Gogvadze V, Ott M and Orrenius S (2004) Cardiolipin is not required for Bax-mediated cytochrome c release from yeast mitochondria. J. Biol. Chem. 279: 1100–1107
Priault M, Bessoule JJ, Grelaud-Coq A, Camougrand N and Manon S (2002) Bax-induced cell death in yeast depends on mitochondrial lipid oxidation. Eur. J. Biochem. 269: 5440–5450
Epand RF, Martinou JC, Fornallaz-Mulhauser M, Hughes DW and Epand RM (2002) The apoptotic protein tBid promotes leakage by altering membrane curvature. J. Biol. Chem. 277: 32632–32639
Priault M, Chaudhuri B, Clow A, Camougrand N and Manon S (1999) Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur. J. Biochem. 260: 684–691
Priault M, Camougrand N, Chaudhuri B and Manon S (1999) Role of the C-terminal domain of Bax and Bcl-XL in their localization and function in yeast cells. FEBS Lett. 443: 225–228
Law RH, Manon S, Devenish RJ and Nagley P (1995) ATP synthase from Saccharomyces cerevisiae. Methods Enzymol. 260: 133–163
Kamo N, Muratsugu M, Hongoh M and Kobatake Y (1979) Membrane potential of mitochondria measured with an electrode to tetraphenylphosphonium and relationship between proton electrochemical gradient and phosphorylation potential in steady state. J. Membr. Biol. 49: 105–121
Leducq N, Delmas-Beauvieux MC, Bourdel-Marchasson I, Dufour S, Gallis JL, Canioni P and Diolez P (2000) Mitochondrial and energetic dysfunctions of the liver during normothermic reperfusion: protective effect of cyclosporine and role of the mitochondrial permeability transition pore. Transplant Proc. 32: 479–480
Rottenberg H (1984) Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J. Membrane Biol. 81: 127–138
Okamoto K, Perlman PS and Butow RA (1998) The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J. Cell Biol. 142: 613–623
Simbeni R, Pon L, Zinser E, Paltauf F and Daum G (1991) Mitochondrial membrane contact sites of yeast. Characterization of lipid components and possible involvement in intramitochondrial translocation of phospholipids. J. Biol. Chem. 266: 10047–10049
Pon L, Moll T, Vestweber D, Marshallsay B and Schatz G (1989) Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J. Cell Biol. 109: 2603–2616
Vitiello F and Zanetta JP (1978) Thin-layer chromatography of phospholipids. J. Chromatogr. 166: 637–640
Acknowledgements
This work was supported by grants from the Centre National de la Recherche Scientifique (CNRS), the ‘Association pour la Recherche contre le Cancer’ (ARC no. 4493 to PXP), the Conseil Régional d'Aquitaine and the Université de Bordeaux 2 (to SM). We also thank Professor JC Martinou for providing us with recombinant tBid (University of Geneva, Switzerland) and E Gottlieb (Beatson Institute for Cancer Research, Glasgow, UK) for a critical point of view.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Edited by JC Martinou
Rights and permissions
About this article
Cite this article
Gonzalvez, F., Bessoule, JJ., Rocchiccioli, F. et al. Role of cardiolipin on tBid and tBid/Bax synergistic effects on yeast mitochondria. Cell Death Differ 12, 659–667 (2005). https://doi.org/10.1038/sj.cdd.4401585
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401585
Keywords
This article is cited by
-
Lost in promiscuity? An evolutionary and biochemical evaluation of HSD10 function in cardiolipin metabolism
Cellular and Molecular Life Sciences (2022)
-
The molecular mechanism of a novel derivative of BTO-956 induced apoptosis in human myelomonocytic lymphoma cells
Apoptosis (2021)
-
Expressing and functional analysis of mammalian apoptotic regulators in yeast
Cell Death & Differentiation (2010)
-
New insights into apoptosis signaling by Apo2L/TRAIL
Oncogene (2010)
-
Giant unilamellar vesicles (GUVs) as a new tool for analysis of caspase-8/Bid-FL complex binding to cardiolipin and its functional activity
Cell Death & Disease (2010)