Abstract
Glucose-6-phosphate dehydrogenase-deleted embryonic stem (ES) cells (G6pdΔ) proliferate in vitro without special requirements, but when challenged with oxidants fail to sustain glutathione disulphide reconversion to reduced glutathione (GSH), entering a condition of oxidative stress. Here, we investigate the signalling events downstream of GSH oxidation in G6pdΔ and wild-type (wt) ES cells. We found that G6pdΔ ES cells are very sensitive to oxidants, activating an apoptotic pathway at oxidant concentrations otherwise sublethal for wt ES cells. We show that the apoptotic pathway activated by low oxidant concentrations is accompanied by mitochondria dysfunction, and it is therefore blocked by the overexpression of Bcl-XL. Bcl-XL does not inhibit the decrease in cellular GSH and reactive oxygen species formation following oxidant treatment. We also found that oxidant treatment in ES cells is followed by the activation of the MEK/extracellular signal-regulated kinase (ERK) pathway. Interestingly, ERK activation has opposite outcomes in G6pdΔ ES cells compared to wt, which has a proapoptotic function in the first and a prosurvival function in the latter. We show that this phenomenon can be regulated by the cellular GSH level.
Similar content being viewed by others
Introduction
Apoptosis is a genetically programmed form of cell death that ends in the complete disassembly of the main cellular structures through the activation of a family of cysteine proteases, the caspases. Activation of caspases is generally the result of a complex integration of death-inducing signals and survival signals, where the control of mitochondrial integrity has been shown to play a central role.1, 2, 3 Cell death by apoptosis can be triggered by a variety of different stimuli, ranging from a developmental stimulus to the detection of an unsustainable amount of DNA damage. One of the major threats to the life of cells is represented by atmospheric oxygen. Evolutionary adaptation to life in an oxidant atmosphere represented a big achievement in the efficiency of bioenergy production, but at the same time a serious threat for life itself. Cells were required to learn that the oxygen should be ‘handled with care’ in order to avoid irreversible damage to most of the cellular components. To be able to cope with the highly reactive oxygen species (ROS), the main side effect of life in an oxidant atmosphere, cells therefore had to evolve an efficient antioxidant defence capability. Oxidative stress occurs in the cell when the antioxidant defence cannot keep pace with the rate at which ROS are generated. The response of the cell to oxidative stress can be very different, depending on the intensity of the stress and its duration, and goes from the stimulation of cell proliferation to cell cycle arrest, to cell death by apoptosis or necrosis.4, 5
The source of oxidant generation can be external to the cell or the side product of normal aerobic metabolism. In all cases, cells respond to the redox imbalance by producing more reducing power. The most abundant and readily available reducing power molecules are glutathione (GSH) and the phosphorylated form of the reduced nicotinamide adenine dinucleotide (NADPH). These two molecules are strictly interdependent, given that glutathione reductase, the enzyme responsible for the recycling of the oxidised form of GSH (glutathione disulphide, GSSG), uses NADPH as a cofactor. NADPH is also used as a cofactor by catalase, another antioxidant enzyme that converts hydrogen peroxide (H2O2) into water and oxygen.6
The establishment of a condition of oxidative stress has been implicated in the process of ageing and as the main cause of several human age-related degenerative diseases, including neurodegenerative disorders, like Parkinson and Alzheimer,7 the genesis of type II diabetes,8 heart failure and stroke.9 At the cellular level, production of ROS affects various signal transduction pathways and redox-responsive transcription factors. A large number of mitogen-activated protein kinases (MAPK) signalling pathways are involved in coordinating the response to ROS formation, therefore influencing cell destiny. MAPKs encompass a large number of serine/threonine kinases involved in regulating a wide array of cellular processes including proliferation, differentiation, stress adaptation and apoptosis.10 The extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38 MAPK subfamilies have all been shown to be activated in response to oxidant injury, and therefore could potentially contribute to influencing survival or apoptosis following oxidative insults.11 Although the ERK pathway was initially described to be unresponsive to stress stimuli, it is now clear that oxidative stress can lead to substantial activation of ERK in some cases, and that growth factor receptors play an important role in mediating this effect. A number of studies carried out on a variety of cell types and diverse model agents demonstrated that elevated ERK activity enhanced survival of cells treated with oxidants. However, other studies using different model systems suggest that ERK activation can contribute to apoptosis in response to oxidant injuries.5, 12
Glucose 6-phosphate dehydrogenase (G6PD) is the first and rate-limiting enzyme of the pentose phosphate pathway (PPP), which catalyses the oxidation of glucose-6-phosphate to glucono-δ-lactone-6-phosphate, concomitantly producing NADPH. This pathway represents an alternative shunt for glucose catabolism downstream of glucose phosphorylation. The choice between glucose oxidation through the glycolitic pathway, or its channelling into the alternative shunt of the PPP, depends on the conditions in which the cell grows, as well as on specific metabolic requirements.6 Although mouse embryos deleted of the G6PD enzyme fail to develop normally,13 we and others have shown that G6pd-deleted embryonic stem (ES) cells can grow without particular requirements under normal tissue culture conditions.14, 15 However, if the cells are challenged with oxidants, they fail to sustain GSSG reconversion to GSH, underlying a central role for NADPH production in GSH homeostasis.15 In this paper, we show that, after oxidant treatment, G6pd-deleted (G6pdΔ) ES cells enter into a condition of oxidative stress that strongly increases the level of cellular ROS and activates a cell death pathway with all the features of a mitochondria-dependent apoptotic pathway. Furthermore, we show that activation of the ERK kinase plays a central role in the signalling events downstream of the GSH/GSSG imbalance, establishing the fate of the cell in terms of life and death.
Results
Targeted deletion of the G6pd gene sensitises ES cells to the apoptotic and necrotic effect of oxidants
We have recently generated, by homologous recombination, ES cells deleted of the gene coding for G6pd (G6pdΔ) and shown that diamide (Dia), a sulphidryl group oxidising agent, drastically decreases G6pdΔ ES cell viability. 15 To investigate the features of this loss of viability, wild-type (wt) and G6pdΔ ES cells were challenged with a 30?min oxidative burst using increasing concentrations of Dia. After 8?h, cells were collected and analysed for markers of apoptosis. As shown in Figure 1a, both caspase-3 and poly(ADP-ribose) polymerase (PARP) appear to be cleaved at Dia doses as low as 100?μM in G6pd-deleted cells. A similar level of caspase-3 activation was evident in wt ES cells only at Dia concentrations eight-fold higher. Interestingly, the amount of caspase-3 and PARP cleavage seemed to be increased by an increased Dia dose, but only up to 300?μM concentration (Figure 1a). A further increase had the opposite effect, resulting in poor or null caspase-3 activation, although the cells completely detached from the cell culture dish and became permeable to the Trypan blue dye (Figure 1b). This suggests that G6pdΔ ES cells activate an apoptotic pathway if triggered with a relatively low oxidative pulse, but become necrotic if the oxidative power reaches a certain threshold.
ES cells deleted of the G6pd gene are extremely sensitive to Dia treatment. (a) Wt or G6pdΔ were incubated with different concentrations of Dia for 30?min. After 8?h, total proteins were extracted and separated on SDS-PAGE, and their respective content in cleaved PARP and caspase-3 were analysed by Western blotting. (b) ES cells, treated as in (a), were analysed for their ability to incorporate the cell-permeable dye Trypan blue. The percentage of Trypan blue-positive cells was calculated over the total number of cells and plotted against the concentration of Dia
Diamide is a thiol oxidant that rapidly oxidises GSH to GSSG.16 This creates a redox imbalance inside the cell, because GSH represents the most abundant source of readily available reducing power. On the other hand, Dia has been shown to interfere with other cellular processes, like initiation of protein synthesis.16, 17 In order to investigate if the apoptotic effect of low Dia doses on G6pdΔ ES cells was due to redox imbalance or to other redox-unrelated effects, we studied the effect of the GSH precursor N-acetyl-cysteine (NAC) on the apoptosis induced by Dia, arguing that an increase in the GSH pool would titrate down a possible redox-related Dia effect. As shown in Figure 2, G6pdΔ ES cells are completely protected from Dia-induced apoptosis in the presence of 1?mM NAC. Moreover, the oxidant sensitivity of G6pdΔ ES cells was completely reverted to wt levels when they were stably transfected with an expression vector containing the cDNA encoding for mouse G6pd under the control of the ß-actin promoter (G6pdΔpG6pd) (Figure 2, lane 4).
Reintroduction of the G6pd gene or treatment with the antioxidant NAC protects G6pdΔ ES cells from the apoptotic effect of Dia. G6pdΔ and G6pdΔpG6pd ES cells were treated for 30?min with 150?μM Dia in the presence or absence of the antioxidant NAC. After 8?h, cells were harvested and extracts were analysed by Western blotting for PARP and caspase-3 cleavage
The extreme sensitivity to apoptosis of G6pdΔ ES cells is specific for oxidative insults
GSH metabolism is intimately linked to the rate of NADPH production, because NADPH is used by glutathione reductase to regenerate GSH from its oxidised form, GSSG. In order to study the importance of G6PD-mediated NADPH production on the cellular protection from redox imbalance, we studied the effect of oxidants, other than Dia, on the viability of G6pdΔ ES cells. In particular, we focused on H2O2, by itself an ROS acting at the cytoplasmic level, and on menadione (Mq), a quinone compound that induces the production of mitochondrial superoxide radicals. In order to have more reproducible concentrations of H2O2 in the cell culture medium, we used glucose oxidase (Gox), an enzyme that produces H2O2 from glucose and water. As shown in Figure 3, G6pdΔ ES cells are extremely sensitive, compared with wt ES cells, to both Gox and Mq. Again, as observed with Dia, this effect was reverted by the reintroduction of G6pd or by the presence of NAC (data not shown).
G6pdΔ ES cells show an increased sensitivity to the apoptotic effect of oxidants, more than to apoptosis in general. Wt and G6pdΔ ES cells were treated with Dia, Gox, Mq, Chx, CisPt and UV light as described in Materials and methods. Apoptosis was measured by determining the amount of cytoplasmic DNA–histone complexes using the commercial Cell Death Elisa kit (Roche)
We have shown that G6pdΔ ES cells undergo programmed cell death when triggered with doses of oxidants otherwise sublethal for wt ES cells. This observation could possibly be explained by invoking a general increased sensitivity to apoptosis given by the absence of G6PD activity. To investigate if G6pdΔ ES cells show an increased sensitivity to apoptosis, we treated wt and G6pd-deleted ES cells with different apoptotic insults. As shown in Figure 3, the DNA-damaging agents UV and cisplatin (CisPt) induced a comparable level of cell death in both wt and G6pd-deleted ES cells. A similar result was observed with cycloheximide (Chx). This was not due to a saturating amount of the agents used, because the same result was obtained over a large range of doses of the tested stimuli (data not shown). These data clearly support a specific role for G6PD in the defence against oxidative stress-causing agents, although a role in the protection from apoptotic insults not tested cannot be excluded.
Diamide-induced apoptosis proceeds through a mitochondria-dependent apoptotic pathway
In most of the signal transduction pathways that lead to apoptotic cell death, an early marker of the engagement of the apoptotic machinery is represented by the release of cytochrome c from the mitochondrial intermembrane space into the cytosol. Therefore, we analysed if the apoptotic pathway triggered by Dia in G6pdΔ ES cells involved mitochondria dysfunction, by studying cytochrome c redistribution following the apoptotic stimulus. Figure 4a shows that after Dia treatment, G6pdΔ ES cells release cytochrome c into the cytosol in a time-dependent manner, probably as a consequence of lost mitochondrial permeability. Cytochrome c release was followed by the activation of caspase-9, presumably through the induction of the apoptosome formation.
Dia-induced apoptosis in G6pdΔ ES cells proceeds through the mitochondrial pathway. (a) G6pdΔ ES cells were treated with 150?μM Dia for 30?min and then collected at the indicated time points. Cytosolic fractions were analysed by Western blot for the presence of cytochrome c or for the cleavage of caspase-9. (b) G6pdΔ and G6pdΔpBclXL ES cells were treated for 30?min with 150?μM Dia. After 8?h, cells were harvested and extracts were analysed by Western blotting for PARP and caspase-3 cleavage
is considered to be a general regulator of mitochondrial homeostasis, and protects from essentially most of the apoptotic triggers. As Dia induced an apparent mitochondria dysfunction, assayed by detecting of cytochrome c release, we addressed the possibility that G6pdΔ ES stably transfected with an expression vector containing the cDNA encoding for Bcl-XL under the control of the ß-actin promoter (G6pdΔBcl−XL) could be protected from oxidant-induced apoptosis. When the cells were triggered with 150?μM Dia, Bcl-XL-expressing G6pdΔ ES cells showed a complete protection from the apoptotic effect of the Dia, as suggested by the absence of caspase-3 and PARP cleavage (Figure 4b).
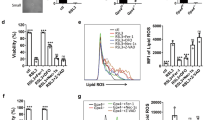
Diamide treatment increases cellular ROS in G6pdΔ ES cells, independent of Bcl-XL expression
We have previously shown that G6pdΔ ES cells triggered with low doses of Dia fail to re-establish a correct ratio of cellular GSH/GSSG.15. GSH metabolism is tightly linked to ROS scavenging. In fact, glutathione peroxidase catalyses the conversion of H2O2 into water using the reducing power of GSH. Furthermore, other forms of ROS, namely, mitochondrial or cytosolic superoxide anions, can be ultimately neutralised by cellular GSH through their conversion to H2O2, mediated by superoxide dismutase (SOD) 1 and 2. Low levels of cellular GSH could therefore be accompanied by an increase in the intracellular concentration of ROS. We made use of ROS-sensitive fluorescent probes to detect the amount of ROS generated by the treatment with 150?μM Dia of both wt ES cells and G6pdΔ ES cells, transfected with an expression vector encoding for Bcl-XL. As expected, Dia treatment produced an appreciable increase in cellular ROS only in G6pdΔ ES cells (Figure 5). Interestingly, G6pdΔ ES cells showed an increased basal ROS content compared to wt ES cells. Forced expression of Bcl-XL completely abolished Dia-induced death, but did not prevent the production of cellular ROS. Bcl-XL, therefore, would act downstream of ROS production in the apoptotic pathway triggered by low doses of Dia in G6pdΔ ES cells. In agreement with this, also basal levels of ROS in G6pdΔ ES cells were not influenced by Bcl-XL expression.
Different from JNK and p38 SAPKs, ERK activation reflects the oxidative stress sensitivity of G6pdΔ with respect to wt ES cells
The data presented here suggest that ES cells respond specifically to a proapoptotic signal transduction pathway that senses a drop in the GSH/GSSG ratio. The amount of GSH oxidant needed to reach a GSH/GSSG ratio capable of triggering this pathway might depend on the NADPH-producing capability of the cell. Cellular redox imbalance caused by altered GSH metabolism was shown to be sensed, in other cell systems, by specific MAPK pathways.4, 5 In order to study the activation status of MAPK pathways in response to Dia in ES cells, we used antibodies raised against phosphorylated ERK, JNK and p38. As expected, treatment of wt and G6pdΔ ES cells with different concentrations of Dia markedly resulted in the activation of different MAPK pathways (Figure 6). JNK 1/2 were found to be phosphorylated using 50?μM Dia in G6pdΔ and 100?μM Dia in wt ES cells. Similarly, p38 showed exactly the same activation profile as JNK1/2 in wt and G6pdΔ ES cells. As shown in Figure 1a, these two oxidant concentrations do not correlate with the induction of apoptosis either in G6pdΔ or in wt ES cells. Interestingly, ERK 1/2 activation profile was strikingly different between wt and G6pdΔ ES cells. In wt cells, phosphorylated ERK 1/2 could be detected at 500?μM Dia, while in G6pdΔ ES cells, a 10-fold lower concentration of Dia was sufficient (Figure 6). Therefore, ERK activation reflected the difference in oxidative stress sensitivity between wt and G6pdΔ ES cells.
Analysis of JNK1/2, p38 and ERK1/2 following Dia treatment in Wt and G6pdΔ ES cells. Wt and G6pdΔ ES cells were incubated for 30?min with the indicated amount of Dia. Total cell lysates were prepared and analysed by Western blotting using antibodies against the phosphorylated forms of JNK1/2, p38 and ERK1/2. The analysis of actin content was used as a loading control
Inhibition of MEK/ERK activation decreases Dia-induced apoptosis in G6pdΔ, but not wt in ES cells
Activation of MAPK pathways following oxidative stress has been shown to produce different outputs. According to the intensity and persistence of the stimulus, they can either increase the survival potential of cells by inducing the transcription of several antioxidant enzymes or determine cell death through mechanisms in part still to be elucidated.4, 5 The pattern of ERK activation suggested a possible role for this kinase in the induction of apoptosis. To address this hypothesis, we made use of U0126, an inhibitor of the activation of the upstream kinase MEK, thus abolishing ERK phosphorylation. Wt and G6pdΔ ES cells were treated with a 30?min Dia pulse at the doses known to induce apoptosis (see above) in the presence or absence of the MEK inhibitor U0126. Cells were then analysed either after 30?min, to detect ERK phosphorylation, or after 8?h, to investigate PARP and the caspase-3 cleavage status, as a marker for apoptotic cell death. As shown in Figure 7a, treatment with U0126 while completely inhibiting ERK phosphorylation in both cell lines, had remarkably the opposite effect on apoptosis markers in wt and G6pdΔ ES cells. When we treated wt ES cells with a lethal dose of Dia, in the presence of the MEK inhibitor, we could reproducibly observe an increased amount of the faster migrating PARP cleavage product, as well as an increase in the amount of activated caspase 3, suggesting a role of MEK/ERK in an antiapoptotic pathway. On the contrary, in G6pdΔ ES cells, the presence of U0126 strongly decreased the level of activated caspase-3 and cleavage of PARP, as well as the release of cytochrome c from mitochondria and the activation of caspase-9 (Figure 7b), suggesting an active role for the MEK/ERK pathway in mediating the apoptotic effect of Dia.
Inhibition of Dia-induced ERK activation has different outputs in wt and G6pdΔ ES cells. (a) The effect of the MEK inhibitor U0126 was analysed on the apoptosis induced by 800 and 150?μM Dia on wt and G6pdΔ ES cells, respectively. Cells were treated for 30?min with the indicated Dia concentrations in the presence or absence of the MEK inhibitor U0126 and, either harvested immediately, to measure the phosphorylation status of ERK, or collected after 8?h to measure the cleavage status of PARP and caspase-3. The figure is a Western blot analysis using antibodies specific for the phosphorylated form of ERK, for PARP or for the cleaved form of caspase-3. (b) G6pdΔ ES cells were harvested 4?h after Dia treatment in the presence or absence of U0126, and cytosolic extracts were analysed by Western blot for the presence of cytochrome c or for the cleavage of caspase-9
These results, therefore, show that MEK/ERK activation plays a proapoptotic role in the G6pdΔ ES cells, while in the wt cell lines, ERK activation is either insufficient or possibly implicated in an antiapoptotic response.
ERK activation has multiple roles in cell fate decisions after Dia treatment
The observation that G6pdΔ ES cells activated different MAPK pathways in response to oxidative stress with respect to the parental wt ES cells was, in part, expected, given the strong difference in apoptosis sensitivity of the two cell lines. The results shown in Figure 7, though, suggest that the absence of G6PD does not simply lower the threshold for oxidative stress sensitivity. Diamide concentrations that induce apoptotic cell death in wt and G6pdΔ ES cells with similar kinetics (data not shown) activate the same pathway with opposite outputs. One possibility, to explain this apparent discrepancy, is that a change in GSH/GSSG ratio is sensed by the MEK/ERK pathway in ES cells and that the effect of this activation, in terms of life or death for the cell, could then depend on the persistence of the stress as well as from the intensity. We have already shown15 that after 30?min of Dia treatment, most of the cellular GSH is converted to GSSG. To investigate whether stress persistence might play a role in the output of MEK/ERK activation, we treated G6pdΔ ES cells with shorter pulses of 150?μM Dia. As shown in Figure 8 (lanes 1 and 2), a 10?min Dia pulse was already able to induce caspase-3 activation and PARP cleavage in G6pdΔ ES cells. Strikingly, when we analysed the role of the MEK/ERK pathway by treating the cells with U0126 during the two different Dia pulses (Figure 8, compare lanes 2/3 and 4/5), we observed that U0126 treatment increased the level of caspase-3 activation at the 10?min incubation time and inverted its effect to an inhibitor of caspase-3 activation, as already shown in Figure 7, at the 30?min incubation time point, suggesting that ES cells try to rescue the death induced by the change in GSH/GSSG ratio by activating an MEK/ERK-dependent survival pathway, but if the GSH oxidation persist, they convert the MEK/ERK-dependent survival pathway into a proapoptotic one.
Inhibition of Dia-induced ERK activation in G6pdΔ ES cells has different outputs, depending on the duration of the stress. The effect of the MEK inhibitor U0126 was analysed on the apoptosis induced by 150?μM Dia on G6pdΔ ES cells. Cells were treated for 10 and 30?min with 150?μM Dia in the presence or absence of the MEK inhibitor U0126 and collected after 8?h to measure the cleavage status of PARP and caspase-3. The figure is a Western blot analysis using antibodies specific for PARP or for the cleaved form of caspase-3
Diamide treatment induces the formation of GSSG in G6pdΔ but not in wt ES cells
The results shown in Figures 6 and 7 suggest that, although 800 and 150?μM Dia kill wt and G6pdΔ ES cells with similar kinetics, respectively, they probably induce cell death through different signal transduction pathways. This difference could reflect a difference in the amount of GSH oxidised or in its oxidation rate in wt and G6pdΔ ES cells treated with the respective lethal doses of Dia. We therefore measured the kinetics of GSH decrease and GSSG appearance in wt and G6pdΔ ES cells upon incubation with 800 and 150?μM Dia, respectively. As shown in Figure 9, after 10?min of Dia incubation there was already a dramatic drop in the GSH cell content both in wt ES cells treated with 800?μM Dia and in G6pdΔ ES cells treated with 150?μM Dia, although the level of decrease was much more pronounced in the cells devoid of G6PD activity. Analysis of GSSG cell content during the time after Dia treatment also revealed a very different picture between wt and G6pdΔ ES cells: we were unable to detect any traces of GSSG in wt ES cells even after 30?min of incubation with 800?μM Dia. GSSG, instead, reached its maximum level in G6pdΔ ES cells already after 10?min of 150?μM Dia treatment, suggesting that the conditions of oxidative stress induced in wt and G6pdΔ ES cells upon incubation with 800 and 150?μM Dia, respectively, have strongly different features.
Contrary to what was observed in G6pdΔ ES cells, GSH decrease in Dia-treated wt ES cells is not accompanied by a GSSG increase. Wt and G6pdΔ ES cells were treated for the indicated amount of time with 800 and 150?μM Dia, respectively. After acidic extraction, changes in cellular GSH and GSSG content were analysed by HPLC
Discussion
By targeting the gene coding for G6PD in mouse ES cells for deletion, we have previously shown that the reducing potential produced by G6PD in the form of NADPH is essential for an efficient response to oxidant treatment. G6pd-deleted ES cells fail to sustain high GSH levels when treated with low levels of oxidating agents. In this paper, we analyse the signalling events downstream of GSH oxidation in terms of apoptosis and/or survival in G6pdΔ ES cells in comparison to wt ES cells. Furthermore, we investigate if the condition of oxidative stress that we observe at low oxidant concentrations in G6pdΔ ES cells are equivalent to the oxidative stress induced by high oxidant doses in ES cells wt for G6PD.
We have previously reported that G6pdΔ ES cells become permeable to Trypan blue staining after treatment with Dia doses otherwise sublethal for wt ES cells.15 Trypan blue permeability could be the consequence either of apoptotic cell death or necrotic cell death. It has been shown, in different cell systems, that oxidant treatment, if not counterbalanced by an appropriate antioxidant response, can induce either an apoptotic pathway or necrosis. The decision between apoptosis and necrosis is usually dictated by the strength of the oxidative insult.18 Accordingly, we found that low doses of Dia engage an apoptotic pathway in G6pdΔ ES cells, as measured by the appearance of the cleaved forms of caspase-3 and PARP. By increasing the Dia concentration over 300?μM, all cells became Trypan blue positive without caspase-3 activation, a condition that is characteristic of necrotic cell death. Interestingly, at these Dia concentrations, the control wt ES cells did not show any signs of caspase-3 activation or permeability to Trypan blue. To induce a similar level of caspase-3 activation in wt ES cells, it was necessary to increase Dia concentration up to eight times more. This increased sensitivity of G6pd-deleted ES cells against the effect of Dia is likely due to the redox imbalance that we previously observed in these cells, characterised by low GSH and low NADPH content, because the effect can be reversed by treating the cells with the GSH precursor, NAC.
The establishment of a condition of redox imbalance was followed by destabilisation of the outer mitochondrial permeability that presumably rapidly triggered the apoptotic pathway. We could indeed completely block caspases activation by overexpressing the antiapoptotic protein Bcl-XL in G6pdΔ ES cells. On the other hand, Bcl-XL expression prevented ROS formation following Dia treatment only to a very small extent (Figure 5), suggesting a role in blocking the apoptotic signal transduction pathway rather than in preventing oxidative stress. In line with this, also the decrease in GSH levels and ERK activation (see below) was not influenced by Bcl-XL expression (data not shown). If Bcl-XL expression does not prevent oxidative stress, but only the activation of the apoptotic pathway, this would imply that life is compatible with a certain amount of oxidation of cellular structures. Otherwise Bcl-XL expression, similar to what has been observed with Bcl-2,19 might play a role also as an antioxidant, by blocking the damaging effects of peroxides, rather than preventing their formation.
Although a possible involvement of G6PD in the protection from apoptosis was previously indirectly suggested by the use of the G6PD inhibitors dehydroepiandrosterone and 6-aminonicotinamide,20 to our knowledge for the first time a role for G6PD in the protection from redox imbalance-induced apoptosis and necrosis has been clearly assigned, also in view of the complex and multiple physiological effects triggered by these inhibitors.21 That G6PD could be a mediator of a survival pathway was also previously suggested by the fact that G6PD overexpression in HeLa cells increased their resistance to H2O2 and TNFα treatment.22
G6PD is not the only NADPH-producing enzyme in nucleated cells, although we have previously shown that it is the only one strongly activated by oxidant treatment. Probably, the peculiarity of G6PD lies in the fact that it uses a highly abundant and readily available source of reducing power, glucose, and this makes it the rate-limiting factor in antioxidant defence. This should be taken into account when considering the importance of glucose in cell survival. In the absence of glucose, in fact, cells are not only unable to proceed through the glycolytic pathway but they are also affected in the production of the main reducing equivalent, NADPH. For instance, the oncogene Akt has a broad antiapoptotic activity but, different from Bcl-XL, strictly requires glucose availability to afford protection against apoptosis.23, 24 It has been suggested that glucose phosphorylation is sufficient to promote cell survival by Akt, based on the fact that 2-deoxyglucose, a glucose analogue that can be phosphorylated by hexokinase but not further metabolised through the glycolytic pathway, restores Akt ability to prevent apoptosis.23. Given the importance for G6PD activity in cell survival and given that 2-deoxyglucose-6-phosphate, although not further metabolised during glycolysis, is accepted as a substrate by G6PD,25 we propose that the strict glucose requirement of Akt-induced survival could depend on the activity of the PPP shunt.
Although G6PD seems to have a central role in protection from apoptosis, this effect seems to be specific for apoptosis induced by oxidating agents. G6pd-deleted ES cells were equally sensitive to Chx-induced cell death (Figure 3) and serum starvation (data not shown), and even slightly more resistant to the death induced by UV irradiation or CisPt. On the other hand, G6PD-deleted cells showed increased sensitivity to agents inducing, directly or indirectly, ROS, and ROS production has been suggested to be and intermediated in several apoptotic pathways.4 It will be interesting to investigate if G6pd-deleted cells could also be sensitive to conditions accompanied by physiological levels of intracellular ROS production, as it has been suggested to occur during TNFα treatment or prolonged p53 activation.26, 27, 28 We are currently deriving an epithelial-like cell line from in vitro differentiated ES cells to address this possibility.
In order to study the signal transduction pathway activated by Dia-induced oxidative stress in ES cells deleted or not of the G6pd gene, we investigated the phosphorylation status of the major cellular MAPK pathways. The pathway that mirrored most closely the difference in oxidative stress sensitivity found in wt and G6pdΔ ES cells was the MEK/ERK pathway. Interestingly, MEK/ERK activation seemed to have a multiple role in ES cells' fate decisions: in G6pdΔ ES cells, it seemed to have a protective role following a very short pulse of oxidant treatment, and clearly a proapoptotic function following a longer oxidant exposure. In wt ES cells, on the contrary, MEK/ERK activation seemed to have a protective role in all the conditions tested. Independent of its role in apoptosis induction, our data clearly indicate that in ES cells the establishment of a condition of redox imbalance is sensed by the MEK/ERK pathway. Although JNK and p38 were also activated at different oxidant concentrations in wt and G6pdΔ ES cells, their activation was transient and did not correlate with redox imbalance and apoptosis induction. We observed, in fact, JNK and p38 activation in wt ES cells treated with 100?μM Dia, a condition that does not induce apoptosis (Figure 1). How does oxidative stress activate MEK/ERK? According to a previously reported study, a possible explanation involves the inactivation, through disulphide bonds formation, of receptor tyrosine phosphatases. These keep normally the activation level of receptor tyrosine kinases low, following prolonged stimulation by receptor ligands. Transient inactivation of the phosphatases results therefore in transient activation of the kinases receptor.29 Receptor tyrosine kinases are among the strongest activators of the MEK/ERK pathway. Indeed, we have preliminary results showing a general increase in phosphotyrosines following Dia treatment (data not shown).
As mentioned above, MEK/ERK activation produces opposite outputs, depending on the persistence of the stress and on the G6PD status of the cells. The fact that a 10?min pulse of 150?μM Dia in G6pdΔ ES cells results in the activation of the pathway with an apparent antiapoptotic function (Figure 8) resembled the condition that we observed in wt ES cells when treated for 30?min with 800?μM Dia (Figure 7). This was initially suggesting that, if there was simply a threshold difference between wt and G6pdΔ ES cells, this was higher than initially thought, and also dependent on the oxidant exposure time. It would be possible to extrapolate from this argument that in wt ES cells treated for a longer time or with a higher Dia dose, the activation of the MEK/ERK pathway would become proapoptotic as well. We were unable to confirm this prediction because of the necrotic effect that Dia has at higher concentrations and exposure times (data not shown). This observation highlights the difference in oxidant sensitivity between wt and G6pdΔ ES cells. This difference became even more evident when we measured the kinetics of GSH decrease after treating wt and G6pdΔ ES cells with their respective lethal dose of oxidant. We found that not only was there a difference in the kinetics of GSH decrease between the two cell lines, but, while the GSH decrease was accompanied by an increase in GSSG level in G6pdΔ ES cells, we could not observe any traces of GSSG in wt cells following oxidant treatment. It is possible to speculate that Dia-treated wt ES cells might use protein thiols instead of a second molecule of GSH as an electron donor and might result in an increased cellular content of glutathionylated proteins,30, 31 more than GSSG. This observation suggests that we cannot explain the difference in oxidant sensitivity of wt and G6pdΔ ES cells with a simple threshold model, but we have to consider the conditions of oxidative stress induced by Dia in ES cells, in the presence or absence of a G6PD activity, qualitatively very different.
The results presented in this paper indicate G6PD as one of the major enzymes involved in cell survival following redox imbalance, and a key player in cellular antioxidant defence. In addition, these findings could be particularly relevant in relation to the fact that G6PD deficiency is one of the most common enzymopathies in the human population.32, 33 As the establishment of a condition of oxidative stress has been implicated as the main cause of several degenerative diseases, including neurodegenerative disorders, like Parkinson and Alzheimer,7 the genesis of type II diabetes,8 heart failure and stroke,9 it will be of great interest to study possible connections between the risk of progression of such diseases and inherited G6PD deficiency.
Materials and Methods
Reagents and cell culture
NAC, GSH, Mq, Dia, Chx, CisPt and Gox were purchased from Sigma-Aldrich. U0126 was from Promega. Antibodies specific for PARP, cleaved caspase-3 (Asp175), caspase-9, phospho-SAPK/JNK (Thr183/Tyr185), phospho-p38 MAP kinase (Thr180/Tyr182) and phospho-p44/42 MAP kinase (Thr202/Tyr204) (phospho-ERK1/2) were purchased from New England Biolabs. Antibodies specific for cytochrome c were from BD Biosciences (clone 7H8.2C12). ES cells were cultured on mouse fibroblasts as described by Robertson.34
Cell death assay
At 48?h before treatment, cells were seeded at equal density on gelatin-coated plates. Cells were treated either with Dia, Mq, Gox or Chx for 30?min and than harvested after 8?h, or with CisPt for 16?h, or they were UV irradiated at 10?mJ/cm2 using a Stratalinker 2400 (Stratagene) and collected after 8?h. Where NAC or U0126 was used, cells were first preincubated for 30?min and then treated as indicated in the presence of the same concentration of the NAC or U0126. For apoptosis assay, cell extracts were either analysed by Western blotting for PARP and caspase-3 cleavage or using the commercial kit Cell Death Elisa (Roche).
Western blotting
Total cell lysates were prepared from cells grown on gelatin-coated plates. Cells were lysed in 10?mM Tris-HCl pH7.4, 150?mM NaCl, 1?mM EDTA, 1% Triton X-100, 10% glycerol, supplemented with 0.5?mM DTT, 0.5?mM phenylmethylsulphonyl fluoride, 2?mM benzamidine, 20?mg/ml aprotinin, 4?mg/ml pepstatin, 10?mM leupeptin, 10?mM sodium fluoride, 1?mM sodium orthovanadate and 25?mM β-glycerophosphate (Sigma-Aldrich). After 15?min incubation on ice, the lysates were cleared by centrifugation. Total cell extracts were separated on 10% or 12.5% SDS-PAGE and transferred to Immobilon-P transfer membranes (Millipore). After blocking with 5% nonfat milk, membranes were incubated with primary antibodies as recommended by the supplier. The immune complexes were detected by the ECL detection system according to the manufacturer's protocol (Amersham Bioscience).
Detection of cytochrome c release
To evaluate the amount of cytochrome c released into cytosol, cells were trypsinised, washed in PBS and resuspended in 300?μl of isotonic-sucrose buffer (20?mM HEPES/NaOH pH 7.3, 250?mM sucrose, 1.5?mM MgCl2, 10?mM KCl, 1?mM EDTA, 1?mM DTT and 1 × CompleteTM (Roche)) containing 5?mg of digitonin/4 × 106 cells. After incubation for 3?min at room temperature, cells were centrifuged at 9500 × g for 5?min at 4°C. The resulting supernatant was then separated on 12.5% SDS-PAGE, and analysed by Western blotting using a monoclonal antibody specific for cytochrome c.
Generation of G6pd and Bcl-XL overexpressing ES cells
For the generation of G6pd and Bcl-XL ES cells, the wt and G6pdΔ ES cell lines were electroporated with 20?μg of linearised plasmid vector Pallino β-actin G6pd or Pallino β-actin Bcl-XL, a plasmid vector containing, respectively, G6pd or Bcl-XL cDNA driven by β-actin promoter, and the puromycin-resistance gene driven by the mouse phosphoglycerokinase promoter. Transfected clones were selected by growth in the presence of puromycin 1?μg/ml, and the resistant clones were subjected to immunoblot analysis by using anti-rabbit-G6PD antibody (ABCAM) or anti-mouse-Bcl-XL antibody (BD Biosciences).
Fluorescent measurement of intracellular ROSs
For the fluorimetric measure of ROS, cells were trypsinised and washed three times with PBS; 1 × 106 cells were suspended in 2?ml of PBS and incubated with 10?mM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) (DCHF-DA) (Molecular Probes), for 20?min at 37°C. Then, the cells were washed twice with PBS, suspended in 2?ml of PBS and fluorescence levels were measured using a spectrofluorimeter (FP-777 Jasco) with excitation and emission wavelengths set at 495 and 530?nm, respectively.
GSH and GSSG measurements
To determine intracellular levels of oxidised and reduced glutathione (GSH), acidic extracts from cells untreated or treated as described in Figure 9 were subjected to reversed-phase high-performance liquid chromatography using an electrochemical detection system (HPLC/ECD) as described.15
Change history
12 January 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41418-022-01111-y
Abbreviations
- CisPt:
-
cisplatin
- ERK:
-
extracellular signal-regulated kinases
- G6PD:
-
glucose 6-phosphate dehydrogenase
- Gox:
-
glucose oxidase
- GPX:
-
glutathione peroxidase
- GSH:
-
reduced glutathione
- GSSG:
-
glutathione disulphide
- H2O2:
-
hydrogen peroxide
- JNK:
-
c-Jun N-terminal kinases
- MAPK:
-
mitogen-activated protein kinases
- Mq:
-
menadione
- NAC:
-
N-acetyl cysteine
- NADPH:
-
reduced nicotinamide adenine dinucleotide
- PARP:
-
poly(ADP-ribose) polymerase
- PPP:
-
pentose phosphate pathway
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
References
Cory S and Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2: 647–656
Gross A, McDonnell JM and Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911
Vander Heiden MG and Thompson CB (1999) Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1: E209–216
Finkel T (2000) Redox-dependent signal transduction. FEBS Lett. 476: 52–54
Martindale JL and Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192: 1–15
Stryer L (1995) Biochemistry. New York: W.H. Freeman and Company
Lotharius J and Brundin P (2002) Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 3: 932–942
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820
Behl C and Moosmann B (2002) Oxidative nerve cell death in Alzheimer's disease and stroke: antioxidants as neuroprotective compounds. Biol. Chem. 383: 521–536
Kolch W. (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351 (Part 2): 289–305
Johnson GL and Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912
Cuda G, Paterno R, Ceravolo R, Candigliota M, Perrotti N, Perticone F, Faniello MC, Schepis F, Ruocco A, Mele E, Cassano S, Bifulco M, Santillo M and Avvedimento EV (2002) Protection of human endothelial cells from oxidative stress: role of Ras-ERK1/2 signaling. Circulation 105: 968–974
Longo L, Vanegas OC, Patel M, Rosti V, Li H, Waka J, Merghoub T, Pandolfi PP, Notaro R, Manova K and Luzzatto L (2002) Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 21: 4229–4239
Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F and Luzzatto L (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14: 5209–5215
Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM and Martini G (2003) Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 370: 935–943
Kosower NS and Kosower EM (1995) Diamide: an oxidant probe for thiols. Methods Enzymol. 251: 123–133
Zehavi-Willner T, Kosower EM, Hunt T and Kosower NS. (1971) Glutathione. V. The effects of the thiol-oxidizing agent diamide on initiation and translation in rabbit reticulocytes. Biochim. Biophys. Acta 228: 245–251
Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y and Lichtenstein A (1997) Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 22: 73–83
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL and Korsmeyer SJ (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75: 241–251
Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X and Stanton RC (1999) Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am. J. Physiol. 276: C1121–1131
Gordon GB, Shantz LM and Talalay P (1987) Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv. Enzyme Regul. 26: 355–382
Salvemini F, Franze A, Iervolino A, Filosa S, Salzano S and Ursini MV (1999) Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 274: 2750–2757
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB and Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15: 1406–1418
Plas DR, Talapatra S, Edinger AL, Rathmell JC and Thompson CB (2001) Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276: 12041–12048
Le Goffe C, Vallette G, Jarry A, Bou-Hanna C and Laboisse CL (1999) The in vitro manipulation of carbohydrate metabolism: a new strategy for deciphering the cellular defence mechanisms against nitric oxide attack. Biochem. J. 344 (Part 3): 643–648
Flohe L, Brigelius-Flohe R, Saliou C, Traber MG and Packer L (1997) Redox regulation of NF-kappa B activation. Free Radic. Biol. Med. 22: 1115–1126
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389: 300–305
Schreck R, Rieber P and Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 10: 2247–2258
Blanchetot C, Tertoolen LG and den Hertog J (2002) Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J. 21: 493–503
Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A and Ghezzi P (2002) Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. USA 99: 9745–9749
Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E and Ghezzi P (2002) Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. USA 99: 3505–3510
Beutler E (1991) Glucose-6-phosphate dehydrogenase deficiency. N. Engl. J. Med. 324: 169–174
Luzzatto L. (1975) Inherited haemolytic states: glucose-6-phosphate dehydrogenase deficiency. Clin. Haematol. 4: 83–108
Robertson EJ (1987) Embryo-derived stem cell lines. In Teratocarcinomes and Embryonic Stem Cells: A Practical Approach, Robertson EJ (ed) (Oxford, UK: IRL Press) pp. 71–112
Acknowledgements
We thank J Downward and D Hancock for their critical review of the paper, D De Cesare and L Casalino for support and advice, C Rallo, M Petrillo and S Cossu for help with the computer work and C Sole, M Terracciano and R Vito for their skilful laboratory assistance. This work was supported by the Italian Telethon Foundation, Grant No. 324/bi and Progetto MURST-CNR Legge 488/92 (Cluster C02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
About this article
Cite this article
Fico, A., Paglialunga, F., Cigliano, L. et al. RETRACTED ARTICLE: Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ 11, 823–831 (2004). https://doi.org/10.1038/sj.cdd.4401420
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401420
Keywords
This article is cited by
-
RNA sequencing profiles reveal dynamic signaling and glucose metabolic features during bone marrow mesenchymal stem cell senescence
Cell & Bioscience (2022)
-
Combination treatment of docetaxel with caffeic acid phenethyl ester suppresses the survival and the proliferation of docetaxel-resistant prostate cancer cells via induction of apoptosis and metabolism interference
Journal of Biomedical Science (2022)
-
Mitochondrial dysfunction is a key pathological driver of early stage Parkinson’s
Acta Neuropathologica Communications (2022)
-
Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: a friend or foe?
Acta Pharmacologica Sinica (2022)
-
In vitro production of desired sex ovine embryos modulating polarity of oocytes for sex-specific sperm binding during fertilization
Scientific Reports (2022)