Abstract
Mitochondria play a critical role in apoptosis induction in response to myriad stimuli. These organelles release proteins into the cytosol which trigger caspase activation or perform other functions relevant to apoptosis, including cytochrome c (cyt-c), caspases, AIF, and SMAC (Diablo). The mechanisms by which these proteins escape from mitochondria remain enigmatic. Moreover, it is unclear whether release of these proteins versus disturbances in core mitochondrial functions represents the cell death commitment mechanism. In this regard, suppression of apoptosis using broad-spectrum caspase inhibitory compounds has been reported in many circumstances to prevent the morphological and biochemical manifestations of apoptosis, and yet not protect cells from death and not preserve clonigenic survival. Thus, while mitochondrial damage can be coupled to caspase activation pathways, cell death commitment often occurs upstream of caspase activation when mitochondria-dependent cell death pathways are invoked. Here, we review evidence implicating dysregulation of cellular pH as a component of the cell death mechanism involving mitochondria. Cell Death and Differentiation (2000) 7, 1155–1165
Similar content being viewed by others
Introduction
The mitochondria of healthy cells maintain an electrochemical gradient (Δψ) across their inner membrane which is created by pumping protons from the matrix to the inter-membrane space (IMS) of these organelles in conjunction with electron transport through the respiratory chain (reviewed in1). The electrochemical gradient consists of ΔpH and ΔVm components, but fundamentally is caused by a net efflux of H+ ions from matrix to the outer surface of the inner membrane, with the membrane acting like a capacitor that builds up charge and is poised to discharge when conditions are permissive. Influx of protons back into the matrix is thought to be mediated chiefly by the F0F1-ATPase/H+ pump. This multi-subunit protein complex includes a H+ channel coupled to an ATPase.2 Proton accumulation on the external surface of the inner membrane provides the proton-motive force that permits H+ ions to flow through the channel and into the matrix, using an elegant biomechanical process to drive the ATPase, converting ATP from ADP and PO4−3 (Pi). While operating normally in a single direction with H+ entering the matrix for the purpose of generating ATP, the F0F1-ATPase is a bidirectional H+ pump. Under conditions where ΔVm is low, the F0F1-ATPase can operate in reverse direction, effluxing H+ and consuming ATP.1 Also, up to a certain point, the F0F1-ATPase can pump protons against a H+ gradient when ADP or Pi is in limiting supply and ATP is present in relative excess.3,4,5
Additional ion-channels have been discovered in the inner membrane which are capable of modulating mitochondria pH gradients, including a H+/K+ antiporter, Na+/H+ exchanger, Cl−/HCO3− antiporter, uncoupling proteins, and the Pi /OH− exchanger6 (reviewed in7,8,9,10). In contrast to the inner membrane, the outer membrane of mitochondria is completely porous to H+, presumably due to the presence of porin (VDAC), a β-sheet-type transmembrane channel protein which is involved in transporting metabolites, including ADP and ATP, between mitochondria and cytosol. Even in its ‘closed’ confirmation, this family of channel proteins (the best characterized of which is the Voltage-dependent Anion Channel [VDAC]) creates a pore of approximately 1.8 angstroms diameter, which is adequate for passage of H+ and other biologically relevant ions.11
Disturbances in mitochondrial membrane potential during apoptosis
Depolarization of mitochondria and loss of the electrochemical gradient (Δψ) is universally associated with apoptosis and cell death (reviewed in12). Controversy reins however as to mechanisms responsible and the kinetics of Δψ disruption relative to other events involved in mitochondrial apoptosis. Depending on the stimulus employed, for example, release of cytochrome c (cyt-c) can either precede or follow membrane depolarization. The emerging consensus from the literature suggests that excessive Ca2+ influx represents a proto-typical example of a cell death stimulus where mitochondrial membrane depolarization precedes cyt-c release. In response to elevations in cytosolic Ca2+, mitochondria are known to import Ca2+ from the cytosol, where Ca2+ is drawn to the electronegative matrix via the Ca2+ uniporter, creating transient depolarizations of the inner membrane, followed by efflux of H+ and restoration of Δψ.13 Typically, mitochondria can tolerate a few to several pulses of Ca2+, but ultimately their capacity to adapt to Ca2+ loads is overwhelmed and the mitochondria depolarize irreversibly due to a profound change in the inner membrane permeability representing the so-called ‘permeability transition’.14 The molecules responsible for permeability transition (PT) are unknown, but the ability of compounds such as bongekreic acid and atractyloside, which modulate the adenine nucleotide translocator (ANT), has implicated this transporter involved in exchanging ADP and ATP between mitochondria and cytosol (reviewed in14). When PT occurs, the associated ∼1.5 kDa (2.0–2.6 nm diameter) channel opens, protons and ions equilibrate freely across the inner membrane, and osmotic disequilibrium ensues, resulting in swelling of the matrix space (reviewed in15). Because the inner membrane with its folded cristae has a larger surface area than the surrounding outer membrane, eventually matrix swelling causes rupture of the outer membrane, thereby spilling the contents of the IMS into the cytosol, including cyt-c and other proteins normally sequestered in this compartment of mitochondria. This Ca2+-based mechanism for cell death, involving mitochondrial swelling and release of apoptogenic proteins, may be particularly relevant to ischemia-reperfusion injury and neuroexcitotoxicity involving NMDA-family receptors, a family of plasma membrane Ca2+ channels that open in response to glutamate and related neurotransmitters and which have been implicated in cell death during stroke and several other neurological diseases (reviewed in16,17).
In contrast to pathological elevations in Ca2+, most studies suggest that a wide-variety of apoptotic stimuli induce release of cyt-c from mitochondria prior to membrane depolarization, including growth factor or neurotrophin withdrawal, DNA-damaging drugs and radiation, inhibitors of protein kinases such as staurosporine, elevations in pro-apoptotic Bcl-2 family proteins, and various agonists or antagonists of the retinoid/steroid hormone family of nuclear receptors (reviewed in18,19). When cells are insulted with these sorts of stimuli, mitochondrial membrane depolarization typically occurs as a late event, after caspase activation. Indeed, caspase-inhibitors markedly delay or even prevent the ultimate depolarization of mitochondria in these scenarios, revealing that cyt-c release occurs in advance of any detectable fall in (Δψ).20,21,22,23 Up until the event of cyt-c release, mitochondrial functions appear to be largely intact, inasmuch as oxygen consumption continues (respiration) and ATP levels remain near normal.22 After cyt-c release, however, electron chain-transport may become interrupted, if insufficient cyt-c remains docked to its high-affinity sites on cyt-c-oxidase. Thus, loss of (Δψ) represents a rather late event in many models of apoptosis, probably occurring after commitment to cell death. Moreover, using protonophores to pharmacologically depolarize mitochondria in intact cells, it has been possible to create conditions in vitro where cells sustain their survival through anaerobic glycolysis and to demonstrate that depolarization is insufficient for triggering cyt-c release.24,25 Thus, cyt-c release can be uncoupled from membrane depolarization, separating these events mechanistically.
Hyper-polarization of the inner membrane: an early event associated with mitochondria-dependent apoptosis
Not only does depolarization occur after cyt-c release in many models of apoptosis, but it has been suggested that the inner membrane of mitochondria may undergo a transient hyperpolarization during apoptosis. Using cationic dyes that partition across the inner membrane of mitochondria in accordance with the Nernst equation, several groups have reported evidence of an initial rise in Δψ, occurring before or contemporaneously with cyt-c release, and preceding caspase activation and subsequent membrane depolarization.20,22,24,26,27 Moreover, in some of these reports, the mitochondria of the same cells were observed to undergo hyperpolarization in response to staurosporine but depolarization in response to Ca2+ influx, with both types of stimuli triggering cyt-c release.28 Several difficulties however exist with interpreting results derived from use of cationic dyes for monitoring Δψ (reviewed in29,30). For example, the amount of these dyes which accumulate in mitochondria is dependent not only on Δψ but also the volume of the organelles, thus complicating interpretation when organellar swelling occurs. Some of the commonly used dyes also can leak out of mitochondria when PT occurs, due to simple diffusion rather than partitioning in accordance with membrane potential. Other problems include auto-quenching when intra-mitochondrial concentrations of dye are too high and destruction by oxidation, a likely problem in the face of elevated reactive oxygen species production in ailing mitochondria. Also, dye entry into cells is regulated at the plasma membrane, in addition to mitochondrial membranes.
For these reasons, we recently sought an alternative approach to monitoring pH locally within mitochondria, applying Green Fluorescent Protein (GFP) technology to this question of membrane hyperpolarization. In this regard, Tsien and colleagues have described mutants of GFP which emit light in a pH-dependent fashion.31 These pH-dependent GFPs can be directed to various organellar compartments by expression in cells as fusion proteins, appended with targeting peptides. One such GFP mutant, which produces a near-linear increase in its fluorescence emissions within the pH range 7.5–8.5, was directed to the matrix space of mitochondria by expression with an N-terminal COX-IV leader sequence.24,31 The pH-GFP protein remains in the matrix, regardless of how apoptosis is induced, and appears to retain its near-linear pH-dependent fluorescence emission characteristics within intact cells under a wide variety of conditions.
Using pH-GFP to monitor matrix pH, it was observed that mitochondria-dependent apoptotic stimuli such as Bax over-expression and staurosporine induce rapid alkalinization of the matrix, with concomitant acidification of the cytosol. This change in pH was substantial, estimated at ∼0.5 pH units in most experiments. Thus, the H+ gradient becomes attentuated within minutes after confrontation with these apoptotic stimuli, suggesting that the net efflux of H+ from matrix to IMS has increased. This increase in ΔpH may account for the hyperpolarization noted when using cationic dyes. Though contrary conclusions were reached by others studying the effects of Bcl-2 in isolated rat mitochondria,32 those observations were based on use of protonophores at apparently sub-optimal concentrations.
Importantly, both hyperpolarization (as measured with cationic dyes) and matrix alkalinization are blocked by Bcl-2, an anti-apoptotic protein that localizes to mitochondria and which is known to prevent cyt-c release (reviewed in12,33,34). Also, hyperpolarization and matrix alkalinization are caspase-independent, inasmuch as treatment of cells with broad-spectrum irreversible caspase inhibitors does not prevent these changes in mitochondrial physiology.20,22,24,27 In contrast to mitochondria-dependent cell death stimuli, apoptosis induced by anti-Fas antibody can occur without either hyperpolarization or matrix alkalinization,24 indicating that an alternative pathway which bypasses mitochondria is involved with this Tumor Necrosis Factor Receptor (TNFR) family member (reviewed in35).
As in many other models of apoptosis, eventually hyperpolarization and matrix alkalinization are followed (typically several h later) by mitochondrial depolarization and an equilibration of mitochondrial and cytosolic pH, indicative of the phenomenon of permeability transition (PT).24 This transition is markedly delayed (typically by a day or more) when caspase-inhibitors are included in cultures, suggesting that PT induction in this setting is a post-caspase activation event. Thus, a change in mitochondrial H+ transport resulting in an increase in the H+ gradient represents an early event associated with mitochondria-dependent apoptosis, preceding caspase activation and PT. How these changes in pH regulation relate to the mechanism of cyt-c release remains unclear to date. Experiments using protonophores to dissipate the H+ gradient of mitochondria suggest that cyt-c release induced by staurosporine occurs independently of hyperpolarization or matrix alkalinization, implying that these events are separable mechanistically.24 However, this issue requires further evaluation in the future, given limitations of sustaining survival of animal cells in the presence of protonophores and other potential confounding effects of these agents on cell physiology.
Conservation of mechanisms in yeast
Ectopic expression of mammalian Bax protein in budding (S. cerevisiae) or fission (S. pombe) yeast induces cell death (reviewed in36). Similar to its actions in mammalian cells, when expressed in yeast, the Bax protein targets mitochondria and induces cyt-c release.37,38 Moreover, co-expression of Bcl-2, Bcl-XL or other anti-apoptotic Bcl-2 family members can rescue yeast from the cytotoxic effects of Bax. Studies of the effects of Bax on mitochondria in intact yeast have provided evidence of hyperpolarization (based on cationic dyes) and matrix alkalinization (based on pH-GFP), with concomitant cytosol acidification.24,26,39,40 Furthermore, Bcl-2 or Bcl-XL prevents these effects of Bax on mitochondria and pH regulation.
These observations suggest that the mechanism employed by Bax for triggering mitochondrial alterations leading to cell death are at least partly conserved in yeast and animal cells, and they demonstrate that Bax can profoundly affect the regulation of mitochondrial H+ transport even in simple eukaryotes. Thus, the effects of Bax on mitochondrial H+ regulation may reflect an intrinsic property of this protein and/or indicate conservation of an ancient cell death mechanism. Furthermore, this cell death mechanism is caspase-independent, since no caspases have been identified in the genome of the yeast, S. cerevisiae. Interestingly, ectopic expression of Bax in bacteria has been reported to cause increased efflux of H+, resulting in marked acidification of the extracellular medium and alkalinization of the cell interior,41 thus mimicking Bax's effects upon mitochondria in mammalian cells. This Bax-induced H+ efflux from bacteria is accompanied by increased O2 consumption, implying an intact membrane and electron transport chain. Given the similarities between bacteria and mitochondria, it is therefore tempting to speculate that Bax directly or indirectly triggers a process that results in increase efflux of H+ from mitochondria.
Mutagenesis studies are consistent with, but do not prove, that the lethal effects of Bax in yeast correlate with its ability to either form pores in membranes or at least to insert into membranes.36,42,43 In this regard, Bax is predicted to share structural similarity with other Bcl-2 family proteins which are strikingly similar to the pore-forming domains of bacterial toxins (reviewed in44). The Bax protein inserts in the outer membrane of mitochondria, and may either directly create channels or collaborate with other proteins in the outer membrane such as VDAC to create protein-translocation channels.36,45,46,47,48,49,50,51 It has also been suggested that Bax may translocate to the inner membrane of mitochondria, interacting with inner membrane proteins such as ANT.52
Debate reins as to whether the cytotoxic effects of Bax in yeast require an intact respiratory chain, but the consensus appears to be that Bax can kill yeast regardless of whether grown on fermentable versus non-fermentable substrates – though the kinetics and efficiency of cell death may be superior under conditions where respiration is maintained.53 Regardless of the details of the mechanism, the ability of Bax to confer a lethal phenotype when ectopically expressed in yeast has permitted functional analysis of the relevance of several mitochondrial proteins using genetic approaches in which various yeast genes are ablated. Since yeast can grow anaerobically, it has been possible to approach certain questions in yeast which cannot be addressed in animal cells. The resulting picture however has been confusing, with widely varying results reported. For example, rho-zero cells lacking mitochondrial DNA (and hence containing mitochondria incapable of executing oxidative phosphorylation), ANT-deficient, and VDAC (porin)-deficient yeast have been reported to either retain sensitivity or display resistance to Bax, depending on the nutrients on which cells are grown and the conditions used to induce Bax expression.37,39,40,43,52,53,54,55,56
The F0F1-ATPase and cell death regulation
Classical techniques of yeast genetics have been applied for analysis of mechanisms of Bax induced cell death, revealing a role for the F0F1-ATPase/H+ pump.57 For these experiments, yeast were randomly mutagenized using chemical mutagens, and Bax-resistant strains were identified. Complementation cloning indicated that yeast genes encoding a specific subunit of the F0F1-ATPase (atp4) could restore Bax-sensitivity, implying that the Bax-resistance phenotype was due to a defect in the F0F1-ATPase. Analysis of independently derived mutant strains in which homologous recombination was used to knock-out the same subunit (atp4) or the atpδ subunit of the F0F1-ATPase also revealed a requirement for Bax-induced lethality in yeast. Subsequent studies confirmed that yeast lacking the atp4- or atp2-encoded subunits of the F0F1-ATPase display resistance to Bax-induced lethality.39,40 Moreover, by expressing mitochondria-targeted pH-sensitive GFP in yeast, evidence has been obtained indicating that the F0F1-ATPase is required for Bax-induced mitochondrial matrix alkalinization and cytosol acidification,24 based on use of a mutant strain in which the atp4-encoded subunit of the F0F1-ATPase was ablated by homologous recombination.58
Oligomycin binds subunits comprising the F0 portion of the F0F1-ATPase and potently inhibits this H+ pump.59 Using oligomycin as a pharmacological probe, it was demonstrated that Bax-induced cell death and cyt-c release in both yeast and mammalian cells can be attentuated when the H+ pump is shut-down.24,43,57,60 Inhibition of Bax-induced cyt-c release from isolated mammalian mitochondria was also inhibited.60 Oligomycin also inhibited cell death induced by p53, in a cell line where p53 is known to induce transcription of the endogenous bax gene and to trigger apoptosis through a bax-dependent mechanism.57 Similar conclusions have been reached using oligomycin-treated cell lines in which cyt-c release and apoptosis were induced by DNA-damaging anticancer drugs, or UV-irradiation.21,61 Importantly, while oligomycin attentuates cyt-c release and apoptosis induced by Bax, staurosporine, and DNA damaging agents, it does not interfere with Fas-induced cyt-c release or apoptosis (a mitochondria-independent stimulus),24 suggesting a causal relation between the F0F1-ATPase and execution of the mitochondrial cell death pathway.
In addition to cyt-c release and apoptosis, a role for the F0F1-ATPase in apoptosis-associated mitochondrial hyperpolarization and mitochondrial matrix alkalinization has also been supported by experiments using oligomycin in mammalian cells. Though oligomycin itself causes some hyperpolarization and matrix alkalinization by precluding H+ re-entry into the matrix, the apoptosis-associated increases in membrane potential and matrix pH are greater than those induced by oligomycin. Moreover, oligomycin reportedly prevents the rise in membrane potential and matrix pH induced by mitochondria-dependent cell death stimuli such as Bax or staurosporine.24 Thus, the F0F1-ATPase is implicated in the changes in mitochondrial H+ transport witnessed in cells stimulated to undergo apoptosis.
Use of any pharmacological agent, including oligomycin, for dissecting cellular mechanisms is subject to caveats, and thus the results reported thus far should be viewed as preliminary. For example, at higher concentrations, oligomycin can also interfere with the Na+-K+ ATPase.62 Unlike oligomycin, however, oubain, a specific Na+-K+/ATPase inhibitor, failed to attentuate Bax-induced cell death when used at sublethal concentrations,57 suggesting that Na+-K+-ATPase suppression is unlikely to explain the effects of oligomycin and cyt-c release. Nevertheless, other complementary approaches are needed to assuage concerns about the specificity of oligomycin, such as conditional ablation of expression of F0F1-ATPase subunits using antisense technology or other approaches.
What role precisely does the F0F1-ATPase play in mitochondria-dependent apoptosis? Given that matrix pH rises while cytosolic pH falls and that F0F1-ATPase inhibitors oppose these changes, the simplest interpretation is that apoptotic stimuli such as Bax and staurosporine cause the H+ pump to run backwards, effluxing H+ and consuming ATP. In this regard, the F0F1 complex can pump protons in reverse from the matrix across the inner membrane into the IMS (which is essentially contiguous with the cytosol where ions are concerned) under circumstances where ΔVm is low or the matrix [ATP]/[ADP][Pi], ratio becomes high such that ADP or Pi is in limited supply.3,4 Under these conditions, the F0F1 complex consumes ATP, resulting in extrusion of protons from mitochondria. Though hyperpolarization of mitochondria would seem incompatible with reverse operation of the F0F1-ATPase, it is actually unknown how high a negative voltage the F0F1 complex can pump against, due to the technical difficulties of working with sub-mitochondrial particles that contain functional F0F1-ATPase (reviewed in1). Also, given the many artifacts associated with use of membrane-permeant cationic dyes for in situ estimation of Vm (reviewed in29,30), the actual Vm of mitochondria in cells stimulated to undergo apoptosis is unclear. Probably all that can be safely said is that a gross depolarization of the inner membrane is not an early event when apoptosis is induced by Bax, staurosporine, or related stimuli, but whether ΔVm is truly elevated from basal conditions is potentially arguable.
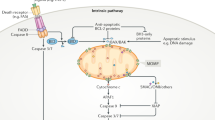
With respect to [ATP]/[ADP] (ï) [Pi] ratios, ATP/ADP exchange between the mitochondrial matrix and cytosol reportedly becomes impaired early in apoptosis (reviewed in19). ATP/ADP exchange is mediated largely by the adenine nucleotide translocator (ANT) in the inner membrane, in collaboration with the voltage-dependent anion channel (VDAC) in the outer-membrane.14 Physical and functional interactions have been reported between anti-and pro-apoptotic Bcl-2 family proteins and the ANT, VDAC or both. If ATP/ADP exchange is shut-off, one would expect matrix ATP/ADP ratios to rise, favoring reverse operation of the F0F1/ATPase. However, if this hypothesis is correct, then other mechanisms must also come into play, since reverse operation of the F0F1/H+-pump should eventually reach a steady-state condition where H+ influx and efflux were equalized. Thus, the changes in pH observed in cells undergoing apoptosis in response to Bax, staurosporine, and related stimuli probably are not entirely attributable to reverse-operation of the F0F1/H+-pump, and could derive from other parallel events such as trapping of organic bases in the mitochondrial matrix, counter-ion movements, increased exchange of OH− for Pi via the mitochondrial OH−/Pi antiporter as a result of ATP hydrolysis, or other processes19 (Figure 1).
Model for F0F1-ATPase participation in mitochondria-dependent apoptosis. Normally, the F0F1-ATPase transports H+ into the matrix, producing ATP which is then exchanged for cytosolic ADP via ANT at the inner-membrane and VDAC at the outer-membrane (left). Apoptotic signals such as Bax may somehow inhibit ATP/ADP exchange through effects either on ANT (shown) or on VDAC (not shown), thus causing the [ATP]:[ADP] ratio to rise (second panel). When the ATP:ADP ratio reaches a critical value, F0F1-ATPase theoretically, could reverse direction (third panel). The resulting hyperpolarization or other related events may trigger cyt-c release. For reverse operation of the F0F1-ATPase to be sustained, other unidentified mechanisms must come into play (fourth panel). For example, Pi is normally rapidly exchanged for OH− via an antiporter. Though entirely speculation, depletion of Pi from the matrix could deny the F0F1-ATPase the substrate it requires (in combination with ADP) to produce ATP, thus favoring reverse operation
The F0F1-ATPase is capable of modulating apoptosis responses, but how central is it for cell death induced by stimuli such as Bax? Though debatable,38,39,40 some reports indicate that Bax can kill even rho-zero or rho (−) yeast.37,57 Furthermore, it has been shown that mammalian rho-zero cells are still capable of undergoing apoptosis in response to mitochondria-dependent cell death stimuli and that Bcl-2 retains its ability to suppress cell death in these cells.63,64 Since rho-zero cells lack mitochondrial DNA and since the mitochondrial genome encodes two of the subunits of the F1 portion of the F0F1-ATPase, it would seem that H+ pumping by this multi-subunit protein complex is not critical, implying that proteins comprising the F0F1-ATPase participate in some other way in apoptosis. However, even without these two mitochondrial genome-encoded subunits, the incomplete F0F1-ATPase retains ATPase activity and is required for maintenance of mitochondrial membrane potential in rho-zero cells due to electrogenic ADP3−/ATP4− exchange.5,65 Thus, the incomplete F0F1-ATPase of rho-zero cells could theoretically participate in the mitochondrial membrane hyperpolarization observed in Bax-expressing cells, including Bax-expressing rho-zero yeast.39 Nevertheless, using Bax-resistant yeast in which complementation cloning experiments suggest defects in components of the F0F1-ATPase, we have identified gain-of-function mutations which can be engineered into the Bax protein, allowing it to kill under conditions were wild-type Bax does not.42 These mutations fall into two central α-helices within the Bax protein which are known to be important for pore-formation in vitro,57 removing polar residues in these α-helices, which presumably makes it easier for Bax to insert into membranes.42 This observation implies that the F0F1-ATPase may be expendable, if the function of Bax is enhanced. Because the specific defect in the Bax-resistant mutant strain of yeast used for these studies is undetermined, these findings imply the results should be interpreted with caution. Nevertheless, these findings imply that the role of the F0F1-ATPase in Bax-induced cell death may be indirect, with the F0F1-ATPase perhaps creating a permissive state through effects on pH gradients or Vm which is required for optimal function of wild-type Bax. Also of possible relevance, Bax association with mitochondrial membranes in yeast was reported to be dependent on ATP,43 raising the possibility that the F0F1ATPase might indirectly influence membrane insertion and function of Bax via its effects on mitochondrial ATP levels.
Cytosol acidification during apoptosis
Several reports indicate that acidification of the cytosol occurs in mammalian cells undergoing apoptosis. The extent of the change in pH observed varies among reports, but typically represents a drop of ≈0.3–0.4 pH units. The stimuli which induce cytosol acidification include over-expression of Bax (in both mammalian cells and yeast), staurosporine, UV-irradiation, etoposide, anti-Fas antibodies, growth factor deprivation, somatostatin, and p53.66,67,68,69,70,71,72,73 Studies using pharmacological inhibitors of the plasma membrane Na+/H+ antiporter and vacuolar H+/ATPase suggest that these H+ transporters remain functional during apoptosis, though they do not evidently compensate sufficiently for the decreased pH of the cytosol so as to hold pH near neutral. Nor do these particular H+ transporters appear to be the cause of the observed cytosolic acidification, though they may allow growth factors or other cyto-protective stimuli to oppose cytosol acidification.71,74,75
Cytosol acidification can be either caspase-dependent or caspase-independent.24,69,76,77,78 With death receptors such as Fas, caspase recruitment to the activated receptor complex occurs, resulting in direct activation of these cell death proteases (reviewed in79,80). Fas induces cytosol acidification as a downstream consequence of caspase activation, perhaps due to cleavage of unidentified regulators of cellular pH located at the plasma membrane or other non-mitochondrial sites. In contrast, mitochondria-dependent stimuli, including over-expression of Bax, p53, staurosporine, and growth factor deprivation also induce cytosol acidification, but where tested, appear to do so through a caspase-independent mechanism which is not suppressible by broad-spectrum irreversible inhibitors of these proteases, such as benzoyl-valine-alanine-asparate-fluoromethylketone (zVAD-fmk).24,69,78 Moreover, the decrease in cytosolic pH precedes caspase activation in cells undergoing apoptosis in response to mitochondria-dependent cell death stimuli (Bax, staurosporine, UV-irradiation), whereas the opposite is observed in Fas-induced apoptosis.24,77
While caspase inhibitors fail to prevent cytosol acidification in response to mitochondria-dependent cell death stimuli, over-expression of Bcl-2 does.24,69,73,77 Oncogenic v-Abl also prevents cytosolic acidification and apoptosis in hematopoietic cells deprived of growth factors.70 Furthermore, apoptosis suppression by v-Abl is reportedly lost when cytosol acidification is induced by pharmacological inhibitors of the plasma-membrane Na+/H+ antiporter. Though cause-and-effect data are lacking to implicate pH as the explanation, it may also be of relevance that hematopoietic cells expressing oncogenic Abl can maintain their survival, failing to undergo apoptosis, even though cyt-c release occurs after growth factor withdrawal.81
Is cytosol acidification important for apoptosis? Several lines of evidence suggest it at least contributes. For example, when F0F1-ATPase inhibitors (oligomycin) were employed, cytosol acidification induced by Bax or staurosporine was impaired and apoptosis was reduced.24 Similarly, when using protonophores or membrane-permeant organic bases to equilibrate H+ across biological membranes, thus ‘clamping’ the pH at 7.1–7.4, apoptosis induced by Bax, staurosporine, UV-irradiation, growth factor deprivation, and lovastatin can be reduced.24,68,69,82 Neither F0F1-ATPase inhibitors nor pH clamping completely prevents apoptosis of cells, however, suggesting that while pH changes make contributions, they are not essential for apoptosis in most instances – perhaps having more to do with the kinetics of apoptosis than whether or not it occurs. Thus, rectifying the problem of cytosol acidification does not uniformly prevent apoptosis.71 Nevertheless, it remains possible, though untested, that differences in the kinetics of apoptosis could be important determinants of clonigenic survival in vivo, when death stimuli are transient in nature and survival factors/signals (either endogenous or exogenous) are applied shortly thereafter. In this regard, many growth factors which deliver survival signals have been shown to induce cytosol alkalinization through effects on Na/H+ exchange at the plasma membrane.70,71,72,83 Effects of growth survival factors on the vacuolar H+/ATPase have also been documented, thus opposing acidification and sustaining cell viability.68,74 Thus, it may be speculated that one of the mechanisms by which growth factors promote cell survival is by maintaining cytosolic pH in the alkaline range.
In contrast to mitochondria-dependent cell death stimuli, H+ pump inhibitors and protonophores did not inhibit Fas-induced apoptosis in Type I cell lines in which Bcl-2 does not rescue from Fas-induced apoptosis.24 Thus, the changes in pH observed in Type I cells treated with Fas are a downstream event, which does not make major contributions to apoptosis. In some types of cells (so-called Type II), death receptors such as Fas trigger apoptosis through a pathway in which mitochondria participate, probably as a result of caspase-mediated cleavage and activation of Bid, a pro-apoptotic member of the Bcl-2 family that targets mitochondria and activates Bax.34 In a Type II cell line where apoptosis was induced by anti-Fas antibody, it was reported that clamping pH in the neutral range partially suppressed apoptosis.68 These differences in Type I and II cells with respect to the effects of H+ pump inhibitors and protonophores on Fas-induced apoptosis also argue for the existence of two mechanisms of cytosol acidification and for two apoptosis pathways–one in which cytosol acidification makes measurable contributions to apoptosis (mitochondria-dependent) and another in which cytosol acidification is entirely expendable (mitochondria-independent/death receptor).
How does cytosol acidification facilitate apoptosis? The answer appears to lie in the effects of pH on some caspase activation mechanisms and perhaps on the activity of some caspase-family proteases. For example, cyt-c induces caspase activation by binding the protein Apaf-1, inducing its dATP/ATP-dependent oligomerization, resulting in recruitment of pro-caspase-9 to the complex followed by activation of this cell death protease (reviewed in12,84,85). pH has a profound influence on caspase activation induced in cytosolic extracts in vitro by addition of exogenous cyt-c and dATP. The pH optimum for this reaction was reported to be ∼pH 6.4.24 In contrast, at pH 7.4, cyt-c-mediated caspase activation occurred at only 25% of maximal, suggesting that preventing cytosol acidification may suppress apoptosis induced by mitochondria-dependent stimuli at least in part by interfering with cyt-c-induced assembly of the Apaf-1 apoptosome or activation of caspases in this pathway (Figure 2). Indeed, kinetic analysis of the effects of pH on cyt-c-triggered caspase activation favor the hypothesis that pH affects the rate of assembly of the Apaf-1 apoptosome, though this remains to be directly tested. It is also possible that cytosolic pH could indirectly affect the measured activity of caspases by modulating the activity of endogenous caspase inhibitory proteins, particularly the IAPs, which in humans bind and suppress the apical caspase in the Apaf-1/cyt-c pathway, caspase-9, as well as the next caspase downstream, caspase-3.86,87 In this regard, IAPs are suppressed by SMAC/Diablo, a protein having a pI of ∼5.3.88,69 In addition, caspase-9 has been reported to have a pH optimum of ≈pH 5.5 when measured against a non-ideal substrate (DEVD), in contrast to an optimal substrate (LEDH) where the pH optimal is ∼pH 7.90,91 However, those studies were performed with a recombinant version of caspase-9 missing its N-terminal prodomain (which is not usually cleaved off in vivo) and in the absence of Apaf-1, an important cofactor for this protease in vivo which is necessary to form the holoenzyme complex.92 In contrast to cyt-c, activation of caspases in cytosolic extracts by granzyme-B or caspase-8 occurs optimally at pH 7.1–7.4 (‘neutral’ pH).93
pH and apoptosis regulation. Bax and related mitochondria-dependent apoptosis stimuli induce mitochondrial matrix alkalinization with concomitant cytosol acidication via a Bcl-2 suppressible mechanism. The apparent net efflux of H+ from mitochondria may be regulated by the mitochondrial F0F1-ATPase of the inner membrane. Additional H+ transporters such as the plasma-membrane Na+/H+ exchanger and vacuolar H+/ ATPase also make contributions to regulation of cytosolic pH, as well as lactate production via glycolysis. Acidification of the cytosol promotes cyt-c/ Apaf-1-mediated activation of caspases and may also increase the activity of some processed caspases against some substrates. Growth and survival factors oppose cytosol acidication, often through effects on the Na+/H+ exchanger or vacuolar H+/ATPase (not shown), decreasing caspase activation and apoptosis
Why was this pH-dependence of the cyt-c reaction not previously detected? One possibility is that typically investigators add not only cyt-c to their cytosolic extracts when interrogating this caspase-activation mechanism, but also 1 mM dATP (or ATP) because Apaf-1 binds nucleotides and requires them for oligomerization. However, at these concentrations, these nucleic acids can induce a 0.3–0.4 pH unit drop in the pH of extracts unless counter-measures are taken, thereby bringing pH toward the optimum required for cyt-c-mediated activation of caspases.
These influences of pH on the activation and activity of caspases offer a possible explanation for the effects of pH on apoptosis. They also suggest a possible mechanism for the phenomenon of cyt-c resistance, where introduction of cyt-c into cells by microinjection or electroporation fails to activate caspases in some cellular context but not others.94,95,96 Again, it is intriguing to speculate that cytosol alkalinization induced by growth factors or neurotrophins plays a role here. However, multiple alternative mechanisms for interfering with cyt-c-induced apoptosis have been elucidated (reviewed in97) and thus the relative importance of pH remains to be established.
Ischemia pre-conditioning and pH
Resistance to infarction can be induced in myocardial tissue by sublethal exposure to ischemia-reperfusion injury, apparently through a signal transduction pathway involving protein kinases. This resistance phenomenon is known as ‘preconditioning’ (reviewed in98,99). Ischemia results in cytosol acidification, probably in part from rapid conversion to anaerobic glycolysis. Preconditioning is associated with changes in pH regulation that oppose ischemia-induced cytosolic acidification, correlating with reduced cell death due to either necrosis or apoptosis.100,101,102 The mechanisms responsible for the diminished decline in cytosolic pH in preconditioned myocardial tissue are unclear, but increased activity of the vacuolar H+ / ATPase has been proposed as at least one contributor.102 The role, if any, of mitochondria in the phenomenon of preconditioning remains to be explored. However, any mechanism that tends to maintain cytosolic pH toward the alkaline range seems likely to reduce apoptosis, given that acidification of the cytosol enhances cyt-c-mediated activation of caspases.24
A paradox of ischemia-reperfusion injury relevant to the issue of pH concerns the observation that Ca2+-induced PT pore opening is more easily achieved under alkaline than acidic conditions.14 Thus, after cytosolic acidification is induced by ischemia, subsequent reperfusion tends to restore pH toward neutral, making it easier for the PT pore to open if excess Ca2+ remains (reviewed in103). This enhancing effect of alkalinization on PT pore opening has been associated with necrosis in the context of ischemia-reperfusion injury, rather than apoptosis.
Effects of pH on Bcl-2 family proteins
In addition to pH effects on activation and activity of caspases, changes in pH may modulate the functions of Bcl-2 family proteins. For instance, many Bcl-2 family proteins interact with themselves and each other, forming homo- or heterodimers. Dimerization of these proteins in vitro is enhanced at lower pH, with optima probably in the pH 4–5 range.104 Moreover, channel formation by pore-forming Bcl-2 family proteins in synthetic membranes (including anti-apoptotic Bcl-2, Bcl-XL and pro-apoptotic Bax and Bid) is also markedly enhanced by low pH, again with optima in the pH 4–5 range.50,105,106,107,108 However, since extraordinarily low pH's of this magnitude are unlikely to be encountered in vivo, the significance of these observations is unclear. Nevertheless, since the channels formed by Bcl-2 family proteins in planar bilayers at neutral pH are voltage-dependent, it is conceivable that the contributions of the FoF1-ATPase to ΔVm are required to create a permissive environment for either channel formation by Bcl-2 family proteins and/or for the cyt-c release mechanism.
Another way that pH may influence Bcl-2 family proteins is by affecting their intracellular targeting. In this regard, the Bax protein is often found in the cytosol in a latent state. Translocation of Bax to mitochondria is induced by various apoptotic stimuli, though the mechanism remains unclear.45,109 Recently, it has been reported that alkaline pH (approximately pH=8.1) can induce Bax to assume a membrane-inserting (‘active’) conformation in vitro.110 Further, evidence was presented indicating that lymphokine-deprivation in pro-lymphocytes induces cytosolic alkalinization, correlating with Bax translocation to and insertion into mitochondrial membranes. However, since growth factors treatment (not deprivation) generally promotes cytosol alkalinization in concert with protection from apoptosis,70,71,72,83 the generality of these observations is unclear.
Mitochondria volume regulation, cytochrome c release, and the role of pH
The question of whether the release of cyt-c from mitochondria requires swelling and rupture of mitochondria remains hotly debated and will not be reviewed in detail here.12,18,34 Rather, we will focus on some of the ways that pH might influence mitochondrial volume and the cyt-c release mechanism. Suffice it to say, however, that swelling requires a change in inner membrane permeability or ion-transport that would result in a net gain of water into the matrix space. In contrast, if outer membrane permeability to proteins such as cyt-c is selectively altered, then inner membrane function and mitochondrial volume need not change.
The opening of the PT-pore in response to Ca2+ is known to be affected by pH with lower pH favoring maintenance of a closed state (reviewed in103). Since sustained PT-pore opening leads to osmotic swelling of mitochondria, pH alterations are potentially relevant to volume regulation of mitochondria in cases where the PT-pore plays a primary role. In contrast to Ca2+ or oxidant-induced PT-pore opening, less is known about the role of pH when apoptosis is induced via mitochondria-dependent pathways where PT-pore opening is a later event and where apparent hyperpolarization rather than depolarization is observed (e.g Bax; staurosporine; anticancer drugs; irradiation; growth factor deprivation). Some studies support the idea of an osmotic imbalance occurring in association with hyperpolarization.19 However, others have failed to reveal evidence of gross mitochondrial swelling despite hyperpolarization of the inner membrane, at least when caspases are prevented from circling back and damaging mitochondria.20,23,24,111,112 Since ΔpH makes major contributions to ΔVm across the inner membrane of mitochondria, pH may indirectly alter the transport characteristics of a variety of voltage-dependent ion-channels in the inner membrane, thus affecting volume regulation (reviewed in7,10,113). Among the potentially relevant molecules of the inner membrane are the K+/H+ antiporter, Na+/H+ exchanger, H+/Pi and H+/pyruvate symotransporters, and the electrogenic K+ uniporter. Hyperpolarization of mitochondria due to an increase in ΔpH would drive more anions such as pyruvate and Pi into the matrix and more K+ due to the electrogenic K+ uniporter, consistent with models which envision swelling.19 In contrast, increases in ΔpH and ΔVm, would be expected to drive K+ and Na+ from the matrix into the IMS via K+/H+ and Na+/H+ exchangers,6 (reviewed in10), which would theoretically favor osmotic shrinking rather than swelling of mitochondria. In this regard, Martinou and colleagues have noted what appears to be shrinkage of mitochondria in a model of apoptosis induced by NGF deprivation of sympathetic neurons.23,114 However, whether this is a common occurrence in apoptosis induced by growth factor-deprivation has not been explored. Clearly, therefore, much remains to be understood about the effects of hyperpolarization on mitochondrial volume regulation.
Because the outer membrane of mitochondria is typically in very close apposition with the inner membrane, it is plausible that the influence of the proton-electrochemical gradient can be translated to outer membrane proteins as well. In this regard, VDAC (porin) is a voltage-gated channel which represents the most abundant protein in the outer membrane of mitochondria. VDAC has been implicated in the cyt-c release mechanism. Evidence has been presented suggesting that both pro- and anti-apoptotic Bcl-2 family proteins directly interact with VDAC and that in collaboration, VDAC plus pro-apoptotic Bax or Bak can create channels in synthetic liposomes that are large enough for passage of cyt-c.51 The formation of collaborative channels by VDAC and pro-apoptotic Bcl-2 family protein is pH-dependent, occurring more efficiently at lower pH, at least in vitro.51 In addition to speculations of a direct role for VDAC in formation of the cyt-c release channel, VDAC has been implicated in a more indirect way in mitochondria-dependent apoptosis through its effects on ATP/ADP exchange between mitochondria and cytosol. The transfer of high energy equivalents from respiring mitochondria to the cytosol is mediated by VDAC, either by direct exchange of ATP and ADP or by transport of creatine-phosphate from the IMS across the outer membrane and into the cytosol where it can be used by creatine kinase to convert ADP to ATP.19 By measuring the ratios of creatine phosphate: creatine in mitochondria, it has been suggested that VDAC-mediated transport may be shut-down during apoptosis. Over-expression of Bcl-XL, in contrast, restores VDAC-mediated transport. How closure of the VDAC channel and impaired net ATP/ADP exchange induces apoptosis is unclear, though several hypotheses have been offered.19,24
Conclusions
Emerging evidence suggests that an alteration in cellular pH regulation represents an early event associated with apoptosis induction via the mitochondria-dependent pathway. Though many details remain unclear, some types of physiologically-relevant apoptotic stimuli appear to induce mitochondrial matrix alkalinization, with attendant hyperpolarization of these organelles. Further, at least under some circumstances, these changes in pH regulation and hyperpolarization can precede cyt-c release, caspase activation and PT pore opening. Genetic and biochemical data also suggest that a change in mitochondrial pH regulation lies close to the mechanism of action of Bcl-2 family proteins. Finally, changes in cytosolic pH in response to cell death and cell survival stimuli can influence caspase activation pathways in cells. It will be interesting in the future to explore whether pharmacological agents that modulate pH in cells through their effects on specific membrane transporters can be employed for therapeutic purposes in the modulation of apoptosis pathways in vivo.
Abbreviations
- ANT:
-
adenine nucleotide translocator
- GFP:
-
green fluorescent protein
- IMS:
-
inter-membrane space
- PT:
-
permeability transition
- TNFR:
-
tumor necrosis factor receptor
- VDAC:
-
voltage-dependent anion channel
References
Hatefi Y . 1985 The mitochondrial electron transport and oxidative phosphorylation system. Ann Rev of Biochem 54: 1015–1069
Konforti B . 1999 Picture story. How proton pumps make ATP. Nature Structural Biology 6: 1090
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J . 1994 In Molecular Biology of The Cell. (3rd ed.) (New York London: Garland Publishing, Inc.)
Nicholls D and Budd S . 2000 Mitochondria and neuronal survival. Physiol Review 80: 315–360
Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, Wei Y-H and Murphy MP . 1999 Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur. J. Biochem. 262: 108–116
Numata M, Petrecca K, Lake N and Orlowski J . 1998 Identification of a mitochondrial Na+/H+ exchanger. J. Biol. Chem. 273: 6951–6959
Halestrap AP . 1989 The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim. Biophys. Acta 973: 355–382
Garlid KD, Jaburek M and Jezek P . 1998 The mechanism of proton transport mediated by mitochondrial uncoupling proteins. FEBS Lett 438: 10–14
Garlid KD . 1994 Mitochondrial cation transport: A progress report. J Bioenerg Biomembr 26: 537–542
Bernardi P . 1999 Mitochondrial transport of cations: channels, exchangers and permeability transition. Physiol Rev. 79: 1127–1155
Mannella CA, Forte M and Colombini M . 1992 Toward the molecular structure of the mitochondrial channel, VDAC. J. Bioenerg. Biomembr. 24: 7–19
Kroemer G and Reed JC . 2000 Mitochondrial control of cell death. Nature Medicine 6: 513–519
Loew LM, Carrington W, Tuft RA and Fay FS . 1994 Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc. Natl. Acad. Sci. USA 91: 12579–12583
Bernardi P, Colonna R, Constantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronnilli V and Scorrano I . 1998 The Mitochondrial permeability transition. Biofactors 8: 273–281
Crompton M . 1999 The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341: 233–249
Mattson MP . 2000 Apoptotic and anti-apoptotic synaptic signaling mechanisms. Brain. Pathol. 10: 300–312
Nicholls D and Budd S . 1998 Mitochondria and neuronal glutamate excitotoxicity. Biochim. Biophys. Acta. 1366: 97–111
Green DR and Reed JC . 1998 Mitochondria and apoptosis. Science 281: 1309–1312
Vander Heiden MG and Thompson CB . 1999 Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell. Biol. 1: E209–E216
Bossy-Wetzel E, Newmeyer D and Green D . 1998 Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17: 37–49
Goldstein J, Waterhouse N, Juin P, Evan G and Green D . 2000 The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell. Biol. 2: 156–162
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB . 1997 Bcl-XL regulates the membrane protential and volume homeostasis of mitochondria. Cell 91: 627–637
Martinou I, Desagher S, Eskes R, Antonsson B, André E, Fakan S and Martinou J-C . 1999 The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell. Biol. 144: 883–889
Matsuyama S, Llopi J, Deveraux Q, Tsien R and Reed J . 2000 Changes in intramitochondrial and cytosolic pH: Early events that modulate caspase activation during apoptosis. Nat. Cell. Biol. 2: 318–325
Minamikawa T, Williams DA, Bowser DN and Nagley P . 1999 Mitochondrial permeability transition and swelling can occur reversibly without inducing cell death in intact human cells. Exp. Cell. Res. 246: 26–37
Minn J, Kettlun C, Liang H, Kelekar A, Vander Heiden M, Chang B, Fesik S, FIll M and Thompson C . 1999 BcI-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J 18: 632–643
Krohn AJ, Wahlbrink T and Prehn JH . 1999 Mitochondrial depolarization is not required for neuronal apoptosis. J. Neurosci. 19: 7394–7404
Budd SL, Tenneti L, Lishnak T and Lipton SA . 2000 Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc. Natl. Acad. Sci. USA 97: 6161–6166
Petronilli V, Miotto G, Canton M, Colonna R, Bernardi P and Di Lisa F . 1998 Imaging the mitochondrial permeability transition pore in intact cells. Biofactors 8: 263–272
Rottenberg H and Wu S . 1998 Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochimica et Biophysica Acta 1404: 393–404
Llopis J, McCaffery JM, Miyawaki A, Farquhar MG and Tsien RY . 1998 Measurement of cytosolic, mitochondrial and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 95: 6803–6808
Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacroniqe V, Matsuda H and Tsujimoto Y . 1998 Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. USA 95: 1455–1459
Reed J . 1998 Bcl-2 family proteins. Oncogene 17: 3225–3236
Gross A, McDonnell J and Korsmeyer S . 1999 BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911
Vaux DL, Haecker G and Strasser A . 1994 An evolutionary perspective on apoptosis. Cell 76: 777–779
Matsuyama S, Nouraini S and Reed J . 1999 Yeast as a tool for apoptosis research. Curr. Opin. Microbiol. 2: 618–623
Zha H, Fisk HA, Yaffe MP, Mahajan N, Herman B and Reed JC . 1996 Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell. Biol. 16: 6494–6508
Manon S, Chaudhuri B and BuÈrin M . 1997 Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells and prevention of these effects by coexpression of Bcl-XL. FEBS Lett. 415: 29–32
Gross A, Pilcher K, Blachly-Dyson E, Basso E, Jockel J, Bassik MC, Korsmeyer SJ and Forte M . 2000 Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-XL . Mol. Cell. Biol. 20: 3125–3136
Harris MH, Vander Heiden MG, Kron SJ and Thompson CB . 2000 Role of oxidative phosphorylation in bax toxicity. Mol. Cell. Biol. 20: 3590–3596
Asoh S, Nishimaki K, Nanbu-Wakao R and Ohta S . 1998 A trace amount of the human pro-apoptotic factor Bax induces bacterial death accompanied by damage of DNA. J. Biol. Chem. 273: 11384–11391
Nouraini S, Six E, Matsuyama S, Krajewski S and Reed J . 2000 The putative pore forming-domain of bax regulates mitochondrial localization and interaction with bcl-xL. Mol. Cell. Biol. 20: 1604–1615
Priault M, Chaudhuri B, Clow A, Camougrand N and Manon S . 1999 Investigation of bax-induced release of cytochrome c from yeast mitochondria: Permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur. J. Biochem. 260: 684–691
Schendel S, Montal M and Reed JC . 1998 Bcl-2 family proteins as ion-channels. Cell Death Differ. 5: 372–380
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG and Youle RJ . 1997 Movement of bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139: 1281–1292
Goping I, Gross A, Lavoie J, Nguyen M, Jemmerson R, Roth K, Korsmeyer S and Shore G . 1998 Regulated targeting of BAX to mitochodria. J. Cell Bio. 143: 207–215
Gross T, Jockel J, Wei MC and Korsmeyer SJ . 1998 Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17: 3878–3885
Jrgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D and Reed JC . 1998 Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. U S A 95: 4997–5002
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou J-C . 1997 Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372
Schlesinger P, Gross A, Yin X-M, Yamamoto K, Saito M, Waksman G and Korsmeyer S . 1997 Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA 94: 11357–11362
Shimizu S, Narita M and Tsujimoto Y . 1999 Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487
Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HLA, Preevost M-C, Xie Z, Matsuyama S, Reed JC and Kroemer G . 1998 Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281: 2027–2031
Priault M, Camougrand N, Chaudhuri B, Schaeffer J and Manon S . 1999 Comparison of the effects of bax-expression in yeast under fermentative and respiratory conditions: investigation of the role of adenine nucleotides carrier and cytochrome c. FEBS Lett. 456: 232–238
Greenhalf W, Stephan C and Chaudhuri B . 1996 Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 380: 169–175
Clow A, Greenhalf W and Chaudhuri B . 1998 Under respiratory growth conditions, Bcl-X(L) and Bcl-2 are unable to overcome yeast cell death triggered by a mutant BAX protein lacking the membrane anchor. Eur. J. Biochem. 258: 19–28
Kissova I, Polcic P, Kempna P, Zeman I, Sabova L and Kolarov J . 2000 The cytotoxic action of Bax on yeast cells does not require mitochondrial ADP/ATP carrier but may be related to its import to the mitochondria. FEBS Lett. 471: 113–118
Matsuyama S, Xu Q, Velours J and Reed JC . 1998 Mitochondrial F0F1-ATPase proton-pump is required for function of pro-apoptotic protein bax in yeast and mammalian cells. Mol. Cell 1: 327–336
Velours J, Arselin G, Paul MF, Galante M, Durrens P, Aigle M and Guerin B . 1989 The yeast ATP synthase subunit 4: structure and function. Biochimie. 71: 903–915
Walker JE, Lutter R, Dupuis A and Runswick MJ . 1991 Identification of the subunits of F1F0-ATPase from bovine heart mitochondria. Biochemistry 30: 5369–5378
Narita M, Shimizu S, Ito T, Chittenden T, Lutz R, Matsuda H and Tsujimoto Y . 1998 Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 95: 14681–14686
Ikemoto H, Tani E, Ozaki I, Kitagawa H and Arita N . 2000 Calphostin C-mediated translocation and integration of bax into mitochondria induces cytochrome c release before mitochondrial dysfunction. Cell Death Differ. 7: 511–520
Arato-Oshima T, Matsui H, Wakizaka A and Homareda H . 1996 Mechanism responsible for oligomycin-induced occlusion of Na+ within Na/K-ATPase. J. Biol. Chem. 271: 25604–25610
Jacobson M, Burne J, King M, Miyashita T, Reed J and Raff M . 1995 Apoptosis and Bcl-2 protein in cells without mitochondrial DNA. Nature 361: 365–368
Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H and Tsujimoto Y . 1995 Prevention of hypoxia-induced cell death by Bcl-2 and BclxL. Nature 374: 811–813
Buchet K and Godinot C . 1998 Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted ρ° cells. J. Biol. Chem. 273: 22983–22989
Ishaque A and Al-Rubeai M . 1998 Use of intracellular pH and annexin-V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J. Immunol. Methods 221: 43–57
Barry MA, Reynolds JE and Eastman A . 1993 Etoposide-induced apoptosis in human HL-60 cells is associated with intracellular acidification. Cancer Res. 53: 2349–2357
Gottlieb R, Nordberg J, Skowronski E and Babior B . 1996 Apoptosis induced in Jurkat cells by agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA 93: 654–658
Furlong IJ, Ascaso R, Lopez Rivas A and Collins MK . 1997 Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. J. Cell. Sci. 110: 653–661
Chen Q, Benson RS, Whetton AD, Brant SR, Donowitz M, Montrose MH, Dive C and Watson AJ . 1997 Role of acid/base homeostasis in the suppression of apoptosis in haemopoietic cells by v-Abl protein tyrosine kinase. J. Cell. Sci. 110: 379–387
Li J and Eastman A . 1995 Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. Role of the Na(+)/H(+)-antiport. J. Biol. Chem. 270: 3203–3211
Rebollo A, Gomez J, Martinez de Aragon A, Lasters P, Silva A and Perez-Sala D . 1995 Apoptosis induced by IL-2 withdrawal is associated with an intracellular acidification. Exp. Cell. Res. 218: 581–585
Thangaraju M, Sharma K, Leber B Andrews DW, Shen S-H and Srikant CB . 1999 Regulation of acidification and apoptosis by SHP-1 and Bcl-2. J. Biol. Chem. 274: 29549–29557
Niessen H, Meisenholder GW and Li HL, et al. 1997 Granulocyte colony-stimulating factor upregulates the vacuolar proton ATPase in human neutrophils. Blood 90: 4598–4601
Pèrez-Sala D, Collado-Escobar D and Mollinedo F . 1995 Intracellular alkalinization suppresses Lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem. 270: 6235–6242
Wolf CM and Eastman A . 1999 Intracellular acidification during apoptosis can occur in the absence of a nucleus. Biochem. Biophys. Res. Commun. 254: 821–827
Meisenholder GW, Martin SJ, Green DR, Nordberg J, Babior BM and Gottlieb RA . 1996 Events in apoptosis: Acidification is downstream of protease activation and bcl-2 protection. J. Biol. Chem. 271: 16260–16262
Zanke BW, Lee C, Arab S and Tannock IF . 1998 Death of tumor cells after intracellular acidification is dependent on stress-activated protein kinases (SAPK/JNK) pathway activation and cannot be inhibited by Bcl-2 expression or interleukin 1beta-converting enzyme inhibition. Cancer Res. 58: 2801–2808
Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV and Boldin MP . 1999 Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17: 331–367
Ashkenazi A and Dixit V . 1998 Death receptors: signaling and modulation. Science 281: 1305–1308
Chen Q, Takeyama N, Brady G, Watson AJM and Dive C . 1998 Blood cells with reduced mitochondrial membrane potential and cytosolic cytochrome c can survive and maintain clonogenicity given appropriate signals to suppress apoptosis. Blood 92: 4545–4553
Perez-Sala D, Collado-Escobar D and Mollinedo F . 1995 Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem. 270: 6235–6242
Moolenaar WH, Tsien RY, van der Saag PT and de Laat SW . 1983 Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature 304: 645–648
Reed JC . 1997 Cytochrome C: Can't live with it; Can't live without it. Cell 91: 559–562
Green DR . 2000 Apoptotic pathways: Paper wraps stone blunts scissors. Cell 102: 1–4
Deveraux QL, Takahashi R, Salvesen GS and Reed JC . 1997 X-linked IAP is a direct inhibitor of cell death proteases. Nature 388: 300–304
Deveraux Q and Reed J . 1999 IAP family proteins: Suppressors of apoptosis. Genes Dev. 13: 239–252
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson R and Vaux DL . 2000 Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43–53
Du C and Fang M . 2000 Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X . 1997 Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413
Garcia-Calvo M, Peterson EP, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW and Thornberry NA . 1999 Purification and catalytic properties of human caspase family members. Cell Death Differ. 6: 362–369
Stennicke HR, Jurgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ and Salvesen GS . 1998 Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273: 27084–27090
Stennicke HR and Salvesen GS . 1997 Biochemical characteristics of caspase-3, -6, -7 and -8. J. Biol. Chem. 272: 25719–25723
Deshmukh M and Johnson JE . 1998 Evidence of a Novel Event during Neuronal Death: Development of Competence-to-Die in Response to Cytoplasmic Cytochrome c. Neuron 21: 695–705
Garland J and Rudin C . 1998 Cytochrome c induces caspase-dependent apoptosis in intact hematopoietic cells and overrides apoptosis suppression mediated by bcl-2, growth factor signaling, MAP-kinase-kinase and malignant change. Blood 92: 1235–1246
Burgess D, Svensson M, Dandrea T, Gröulund K, Hammarquist F, Orrenius S and Cotgreave I . 1999 Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ. 6: 256–261
Reed J and Paternostro G . 1999 Post-mitochondrial regulation of apoptosis during heart failure. Proc. Natl. Acad. Sci. USA 96: 7614–7616
Yellon D, Alkhulaifi A and Pugsley W . 1993 Preconditioning the human myocardium. Lancet. 342: 276–277
Steenbergen C, Perlman ME, London RE and Murphy E . 1993 Mechanism of preconditioning. Ionic alterations. Circulation Research 72: 112–125
Asimakis GK, Inners-McBride K, Medellin G and Conti VR . 1992 Ischemic preconditioning attenuates acidosis and postischemic dysfunction in isolated rat heart. American Journal of Physiology 263: H887–H894
deAlbuquerque CP, Gerstenblith G and Weiss RG . 1994 Importance of metabolic inhibition and cellular pH in mediating preconditioning comtractile and metabolic effects in rat hearts. Circ. Res. 74: 139–150
Gottlieb RA, Gruol DL, Zhu JY and Engler RL . 1996 Preconditioning in Rabbit Cardiomyocytes Role of pH, vacuolar proton ATPase and apoptosis. J. Clin. Invest. 97: 2391–2398
Lemasters J, Nieminen A-L, Qian T, Trost L, Elmore S, Nishimura Y, Crowe R, Cascio W, Bradham C, Brenner D and Herman B . 1998 The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366: 177–195
Xie Z, Schendel S, Matsuyama S and Reed JC . 1998 Acidic pH promotes dimerization of Bcl-2 family proteins. Biochemistry 37: 6410–6418
Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC . 1997 Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA 94: 5113–5118
Matsuyama S, Schendel S, Xie Z and Reed J . 1998 Cytoprotection by Bcl-2 requires the pore-forming α5 and α6 helices. J. Biol. Chem. 273: 30995–31001
Schendel S, Azimov R, Pawlowski K, Godzik A, Kagan B and Reed J . 1999 Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol. Chem. 274: 21932–21936
Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik S-W, FIll M and Thompson CB . 1997 Bcl-xL forms an ion channel in synthetic lipid membranes. Nature 385: 353–357
Nechushtan A, Smith C, Hsu Y-T and Youle R . 1999 conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341
Khaled AR, Kim K, Hofmeister R, Muegge K and Durum SK . 1999 Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc. Nat. Acad. Sci. USA 96: 14476–14481
Scarletta JL, Sheardb PW, Hughesa G, Ledgerwooda EC, Kuc H and Murphya MP . 2000 Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in jurkat cells. FEBS Lett. 475: 267–272
Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR and Newmeyer DD . 1999 The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell. Biol. 147: 809–822
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson JD . 1994 Energy Conversion: Mitochondria and Chloroplasts. In: Molecular Biology of the Cell. Third Edition ed. Robertson M, ed. (New York, NY: Garland Publishing, Inc.) pp. 653–720.vol 14).
Rodriguez J and Lazebnik Y . 1999 Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 13: 3179–3184
Acknowledgements
We thank R Cornell for manuscript preparation, and acknowledge the generous support of the NIH (GM60554) and the American Heart Association (9920070Y).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Matsuyama, S., Reed, J. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ 7, 1155–1165 (2000). https://doi.org/10.1038/sj.cdd.4400779
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400779
Keywords
This article is cited by
-
m6A-Induced lncRNA MEG3 Promotes Cerebral Ischemia-Reperfusion Injury Via Modulating Oxidative Stress and Mitochondrial Dysfunction by hnRNPA1/Sirt2 Axis
Molecular Neurobiology (2024)
-
Caspase-8 activation by cigarette smoke induces pro-inflammatory cell death of human macrophages exposed to lipopolysaccharide
Cell Death & Disease (2023)
-
Surface-functionalized luteolin-loaded nanocarriers successfully delayed lung cancer progress in rats
Journal of Materials Science (2023)
-
Inhibition of PRMT6 reduces neomycin-induced inner ear hair cell injury through the restraint of FoxG1 arginine methylation
Inflammation Research (2022)
-
SESN2 protects against denervated muscle atrophy through unfolded protein response and mitophagy
Cell Death & Disease (2021)