Abstract
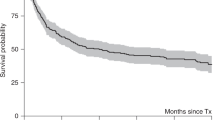
A single cycle of high-dose chemotherapy with stem cell support (HDC) in women with responsive metastatic breast cancer (BC) consistently achieves over 50% complete and near complete response (CR/nCR). This significant cytoreduction results in a median event-free survival (EFS) of 8 months, and approximately 20% 3-year and 16% 5-year EFS in selected patients. To improve long-term outcomes, new strategies to treat minimal residual tumor burden are needed. Increased total dose delivered can be achieved with two cycles of HDC. Critical design issues include shortening induction chemotherapy to avoid development of drug resistance and the use of different agents for each HDC cycle. We have determined the maximum tolerated dose (MTD) for paclitaxel combined with high-dose melphalan in the context of a double transplant and explored the impact of a short induction phase. Between June 1994 and August 1996, we enrolled 32 women with metastatic BC on to this phase I double transplant trial. Induction consisted of doxorubicin 30 mg/m2/day days 1–3 given for 2 cycles every 14 days with G-CSF 5 μg/kg s.c. days 4–12. Stem cell collection was performed by leukapheresis in each cycle when the WBC recovered to above 1000/μl. Patients with stable disease or better response to induction were eligible to proceed with HDC. HDC regimen I (TxM) included paclitaxel with dose escalation from 0 to 300 mg/m2 given on day 1 and melphalan 180 mg/m2 in two divided doses given on day 3. HDC regimen II was CTCb (cyclophosphamide 6 g/m2, thiotepa 500 mg/m2, and carboplatin 800 mg/m2 total doses) delivered by 96-h continuous infusion. At the first dose level of 150 mg/m2 paclitaxel by 3 h infusion, four of five patients developed dose-limiting toxicity consisting of diffuse skin erythema and capillary leak syndrome. Only two of these five completed the second transplant. Subsequently, paclitaxel was delivered by 24-h continuous infusion together with 96 h of dexamethasone and histamine receptor blockade. This particular toxicity was not observed again. No toxic deaths occurred and dose-limiting toxicity was not encountered. Three patients were removed from study prior to transplant: one for insurance refusal and two for disease progression. All others completed both cycles of transplant. Complete and near complete response (CR/nCR) after completion of therapy was achieved in 23 (72%) of 32 patients. The median EFS is 26 months. The median overall survival has not yet been reached. At a median follow-up of 58 months, EFS and overall survival are 41% and 53%, respectively. This double transplant approach is feasible, safe, and tolerable. Treatment duration is only 14 weeks and eliminates lengthy induction chemotherapy. The observed event-free and overall survivals are promising and are better than expected following a single transplant. Whilst selection biases may in part contribute to this effect, a much larger phase II double transplant trial is warranted in preparation for a potential randomized comparison of standard therapy vs single vs double transplant. Bone Marrow Transplantation (2001) 27, 269–278.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mick R, Begg CB, Antman K et al. Diverse prognosis in metastatic breast cancer: who should be offered alternative initial therapies? Breast Cancer Res Treat 1989 13: 33–38
Greenberg PAC, Hortobagyi GN, Smith TL et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer J Clin Oncol 1996 14: 2197–2205
Antman KH, Rowlings PA, Vaughan WP et al. High-dose chemotherapy with autologous hematopoietic stem-cell support for breast cancer in North America J Clin Oncol 1997 15: 1870–1879
Ayash LJ, Wheeler C, Fairclough D et al. Prognostic factors for prolonged progression-free survival with high-dose chemotherapy with autologous stem cell support for advanced breast cancer J Clin Oncol 1995 13: 2043–2049
Stadtmauer EA, O'Neill A, Goldstein LJ et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer New Engl J Med 2000 342: 1069–1076
Lotz JP, Cure H, Janvier M et al. High-dose chemotherapy (HD-CT) with hematopoietic stem cells transplantation (HSCT) for metastatic breast cancer (MBC): results of the French protocol PEGASE 04 Proc ASCO 1999 19: 43a (A-161)
Peters WP, Rosner G, Vredenburgh J et al. A prospective, randomized comparison of two doses of combination alkylating agents (AA) as consolidation after CAF in high-risk primary breast cancer involving ten or more axillary lymph nodes (LN): preliminary results of CALGB 9082/SWOG 9114/NCIC MA-13 Proc ASCO 1999 18: 1a
The Scandinavian Breast Cancer Study Group 9401 . Results from a randomized adjuvant breast cancer study with high dose chemotherapy with CTCb supported by autologous bone marrow stem cells versus dose escalated and tailored FEC therapy Proc ASCO 1999 18: 2a
Holden S, Teicher B, Ayash L, Frei E III . A preclinical model for sequential high dose chemotherapy Cancer Chemother Pharmacol 1995 36: 61–64
Elias AD, Wheeler C, Richardson P et al. A phase I trial of double transplant for metastatic breast cancer: sequence and interval considerations ASBMT March, 1998, abstr
Ayash L, Elias A, Wheeler C et al. Double dose-intensive chemotherapy with autologous marrow and peripheral blood progenitor cell support for metastatic breast cancer: a feasibility study J Clin Oncol 1994 12: 37–44
Ayash LJ, Elias A, Schwartz G et al. Double dose-intensive chemotherapy with autologous stem cell support for metastatic breast cancer: no improvement in PFS by the sequence of high-dose melphalan followed by CTCb J Clin Oncol 1996 14: 2984–2992
Holmes FA, Walters RS, Theriault RL et al. Phase II trial of paclitaxel: an active drug in metastatic breast cancer J Natl Cancer Inst 1991 83: 1797–1805
Reichman BS, Seidman AD, Crown JPA et al. Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer J Clin Oncol 1993 11: 1943–1951
Henderson IC, Berry D, Demetri G et al. Improved disease-free (DFS) and overall survival (OS) from the addition of sequential paclitaxel (T) but not from the escalation of doxorubicin (A) dose level in the adjuvant chemotherapy of patients (PTS) with node-positive primary breast cancer (BC) Proc ASCO 1998 17: 101a (A-390A)
Parker R, Lee KB, Dabholkar M et al. Influence of paclitaxel:cisplatin sequencing on cisplatin-DNA adduct repair in human ovarian cancer cells Proc AACR 1993 34: A2122
Parness J, Horwitz SB . Paclitaxel binds to polymerized microtubules in vitro J Cell Biol 1991 91: 479–487
Rowinsky EK, Donehower RC, Jones RJ et al. Microtubule changes and cytotoxicity in leukemic cell lines treated with paclitaxel Cancer Res 1988 48: 4093–4100
Cabral F, Wible L, Brenner S et al. Paclitaxel-requiring mutant of Chinese hamster ovary cells with impaired mitotic spindle activity J Cell Biol 1983 97: 30–39
Roy SN, Horwitz SB . A phosphoglycoprotein with paclitaxel resistance in J774.2 cells Cancer Res 1985 45: 3856–3963
Rahman Z, Kavanagh J, Champlin R et al. Chemotherapy immediately following autologous stem-cell transplantation in patients with advanced breast cancer Clin Cancer Res 1998 4: 2717–2721
Winer E, Berry D, Duggan D et al. Failure of higher dose paclitaxel to improve outcome in patients with metastatic breast cancer – results from CALGB 9342 Proc ASCO 1998 17: 101a
Elias A, Mazanet R, Wheeler C et al. GM-CSF potentiated peripheral blood progenitor cell (PBPC) collection with or without bone marrow as hematologic support of high-dose chemotherapy: Two protocols Breast Cancer Treat Res 1991 20: S25–29
George SL, Desu MM . Planning the size and duration of a clinical trial studying the time to some critical event J Chronic Disease 1974 27: 15–24
O'Brien PC, Fleming TR . A multiple testing procedure for clinical trials Biometrics 1979 35: 549–556
Cox DF . Analysis of Binary Data Chapman and Hall: London 1970
Kaplan EL, Meier R . Nonparametric estimation from incomplete observation J Am Stat Assoc 1958 53: 457–481
Antman K, Ayash L, Elias A et al. A phase II study of high dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard dose therapy J Clin Oncol 1992 10: 102–110
Rahman ZU, Frye DK, Buzdar AU et al. Impact of selection process on response rate and long-term survival of potential high-dose chemotherapy candidates treated with standard-dose doxorubicin-containing chemotherapy in patients with metastatic breast cancer J Clin Oncol 1997 15: 3171–3177
Sledge GW, Neuberg D, Ingle J et al. Phase III trial of doxorubicin versus paclitaxel versus doxorubicin plus paclitaxel as first-line therapy for metastatic breast cancer: an intergroup trial Proc ASCO 1997 16: 1a (A-2)
Frei E III, Elias A, Wheeler C et al. The relationship between high dose treatment and combination chemotherapy: the concept of summation dose intensity Cancer Res 1998 4: 2027–2037
Teicher BA, Holden SA, Herman TS et al. Characteristics of five human tumor cell lines and sublines resistant to cis-diamminedichloroplatinum (II) Int J Cancer 1991 47: 252–260
Teicher BA, Ara G, Keyes SR et al. Acute in vivo resistance in high-dose therapy Clin Cancer Res 1998 4: 483–491
Brown R, Clugston C, Burns P et al. Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines Int J Cancer 1993 55: 678–684
Weinert TA, Hartwell LH . Characterization of RAD9 of saccharomyces cerevisiae and evidence that its function acts post-translationally in cell cycle arrest after DNA damage Mol Cell Biol 1990 10: 6554–6564
Thiebaut FB, Enns RB, Howell SB . Genes involved in the G2 check point modulate the cell sensitivity to cisplatin Proc AACR 1993 34: 40 (A238)
Seyschab H, Sun Y, Friedl R et al. G2 phase cell cycle disturbance as a manifestation of genetic cell damage Hum Genet 1993 92: 61–68
O'Connor PM, Ferris DK, Pagano M et al. G2 delay induced by nitrogen mustard in human cells affects cyclin A/cdk2 and cyclin B1/cdc2–kinase complexes differently J Biol Chem 1993 268: 8298–8308
Surbone A, Gilewski TA, Norton L . Cytokinetics. In Holland JF, Bast RC Jr, Morton DL et al (eds) Cancer Medicine 4th edn Williams & Wilkins: Baltimore 1997 pp 778–789
Henderson IC, Gelman RS, Harris JR, Canellos GP . Duration of therapy in adjuvant chemotherapy trials Natl Cancer Inst Monograph 1986 1: 95
Wood WC, Budman DR, Korzun AH et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node positive breast cancer New Engl J Med 1994 330: 1253–1259
Bonadonna G, Zambetti M, Valagussa P . Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results JAMA 1995 273: 542–547
Peters WP, Jones RB, Vredenberg J et al. A large, prospective, randomized trial of high-dose combination alkylating agents (CPB) with autologous cellular support (ABMS) as consolidation for patients with metastatic breast cancer achieving complete remission after intensive doxorubicin-based induction therapy (AFM) Proc ASCO 1996 15: 121 (149)
Norton L . Verbal presentation: commentary on reference 43 ASCO May 1996
Dunphy FR, Spitzer G, Buzdar AU et al. Treatment of estrogen receptor-negative or hormonally refractory breast cancer with double high-dose chemotherapy intensification and bone marrow support J Clin Oncol 1990 8: 1207–1216
Broun ER, Sridhara R, Sledge GW et al. Tandem autotransplantation for the treatment of metastatic breast cancer J Clin Oncol 1995 13: 2050–2055
Bezwoda WR, Seymour L, Dansey RD . High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial J Clin Oncol 1995 13: 2483–2489
Vahdat L, Raptis G, Fennelly D et al. Rapidly cycled courses of high-dose alkylating agents supported by filgrastim and peripheral blood progenitor cells in patients with metastatic breast cancer Clin Cancer Res 1995 1: 1267–1273
Crown J, Vahdat L, Raptis G et al. Rapidly cycled courses of high-dose chemotherapy supported by filgrastim and peripheral blood progenitors in patients with metastatic breast cancer Proc ASCO 1994 13: 110 (A-243)
Ghalie R, Williams SF, Valentino LA et al. Tandem peripheral blood progenitor cell transplants as initial therapy for metastatic breast cancer Biol Blood Marrow Transplant 1995 1: 40–46
Bitran JD, Klein L, Samuels B et al. Tandem high-dose chemotherapy supported by hematopoietic progenitor cells yields prolonged survival in stage IV breast cancer Bone Marrow Transplant 1996 17: 157–162
Vahdat LT, Papadopoulos K, Balmaceda C et al. Phase I trial of sequential high-dose chemotherapy with escalating dose paclitaxel, melphalan, and cyclophosphamide, thiotepa, and carboplatin with peripheral blood progenitor support in women with responding metastatic breast cancer Clin Cancer Res 1998 4: 1689–1695
Acknowledgements
This work was supported in part by a grant from the Public Health Service Grant CA13849 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elias, A., Richardson, P., Avigan, D. et al. A short course of induction chemotherapy followed by two cycles of high-dose chemotherapy with stem cell rescue for chemotherapy naive metastatic breast cancer. Bone Marrow Transplant 27, 269–278 (2001). https://doi.org/10.1038/sj.bmt.1702780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702780
Keywords
This article is cited by
-
Capillary leak syndrome caused by cytostatics
Annals of Hematology (2005)
-
High-dose epirubicin, preceded by dexrazoxane, given in combination with paclitaxel plus filgrastim provides an effective mobilizing regimen to support three courses of high-dose dense chemotherapy in patients with high-risk stage II–IIIA breast cancer
Bone Marrow Transplantation (2003)
-
A short course of induction chemotherapy followed by two cycles of high-dose chemotherapy with stem cell rescue for chemotherapy naive metastatic breast cancer: sequential phase I/II studies
Bone Marrow Transplantation (2001)