Abstract
Tamoxifen remains an important adjuvant therapy to reduce the rate of breast cancer recurrence among patients with oestrogen-receptor-positive tumours. Cytochrome P-450 2D6 metabolises tamoxifen to metabolites that more readily bind the oestrogen receptor. This enzyme also metabolises selective serotonin reuptake inhibitors (SSRI), so these widely used drugs – when taken concurrently – may reduce tamoxifen's prevention of breast cancer recurrence. We studied citalopram use in 184 cases of breast cancer recurrence and 184 matched controls without recurrence after equivalent follow-up. Cases and controls were nested in a population of female residents of Northern Denmark with stages I–III oestrogen-receptor-positive breast cancer 1985–2001 and who took tamoxifen for 1, 2, or most often for 5 years. We ascertained prescription histories by linking participants' central personal registry numbers to prescription databases from the National Health Service. Seventeen cases (9%) and 21 controls (11%) received at least one prescription for the SSRI citalopram while taking tamoxifen (adjusted conditional odds ratio=0.85, 95% confidence interval=0.42, 1.7). We also observed no reduction of tamoxifen effectiveness among regular citalopram users (⩾30% overlap with tamoxifen use). These results suggest that concurrent use of citalopram does not reduce tamoxifen's prevention of breast cancer recurrence.
Similar content being viewed by others
Main
Tamoxifen is a selective oestrogen receptor modulator (Jordan and Dowse, 1976) that reduces by half the risk of breast cancer recurrence in early-stage patients whose tumour cells express the oestrogen receptor (Early Breast Cancer Trialists' Collaborative Group, 2005). To be pharmacologically active, tamoxifen must be metabolised to secondary metabolites that bind the oestrogen receptor 100-fold more readily than tamoxifen itself (Malet et al, 1988). Four cytochrome P-450 enzymes (CYPs) catalyse this activation (CYP2D6, CYP3A4, CYP3A5, and CYP2C9) (Malet et al, 1988). CYP2D6 catalyses formation of 4-hydroxytamoxifen from tamoxifen (Coller et al, 2002) and formation of 4-hydroxy-N-desmethyltamoxifen from N-desmethyltamoxifen (Stearns et al, 2003). These two secondary metabolites have the highest binding affinity for the oestrogen receptor, and binding affinity correlates with inhibition of cell growth (Coezy et al, 1982). The secondary metabolites are, therefore, the most important modulators of the oestrogen receptor in the tamoxifen pathway (Lim et al, 2005).
Breast cancer patients treated with tamoxifen may also take other prescription medications that are metabolised by some of the same enzymes that activate tamoxifen. For example, depression is a common comorbidity in breast cancer patients (Massie, 2004), and many selective serotonin reuptake inhibitors (SSRI), which are widely used medications indicated primarily to treat depression (Hansen et al, 2003), are metabolised by CYP2D6 (Zanger et al, 2004). SSRI competition with tamoxifen and N-desmethyltamoxifen for CYP2D6, or direct inhibition of CYP2D6 by SSRI, could reduce the production of the tamoxifen metabolites with high receptor-binding affinity, and thereby reduce tamoxifen's prevention of breast cancer recurrence. Competition between tamoxifen and the SSRI paroxetine reduced the plasma concentration of endoxifen in a cross-over clinical trial (Stearns et al, 2003). Furthermore, the mean plasma concentration of 4-hydroxy-N-desmethyltamoxifen was more than two-fold greater among women who were taking no CYP2D6 competitor drug than among women who were taking such a drug (Jin et al, 2005). In vivo studies thus demonstrate a compelling biological basis for the hypothesis that concomitant use of SSRI would reduce tamoxifen's prevention of breast cancer recurrence.
In the largest study to date of the potential for drug–drug interaction to reduce tamoxifen's protection against breast cancer recurrence, we examined whether Danish breast cancer patients with oestrogen-receptor-positive tumours who were treated with tamoxifen for 1, 2, or most often for 5 years had a higher rate of recurrence if they were concomitantly taking the SSRI citalopram or its S-stereoisomer (‘citalopram’ from here onwards) than if they were not. As described in more detail below, citalopram was the most frequently prescribed SSRI in the study population.
Materials and methods
The study was approved by the Boston University Medical Campus Institutional Review Board and The Regional Committee on Biomedical Research Ethics of Aarhus County.
Study population
The source population included female residents of four Northern Danish counties (Aarhus, North Jutland, Viborg, and Ringkøbing) aged 35–69 at diagnosis of primary International Union Against Cancer stage I, II, or III breast cancer (UICC, 1997) between 1985 and 2001 and who were reported to the Danish Breast Cancer Cooperative Group (DBCG). The DBCG has enrolled nearly all Danish breast cancer patients younger than age 70 at diagnosis into its clinical database since 1977 (Andersen and Mouridsen, 1988; Jensen et al, 2003). More than 90% of Danish breast cancer cases are reported to the DBCG and more than half of the DBCG patients are enrolled in clinical trials (Andersen and Mouridsen, 1988). The same standardized forms are used to follow all patients reported to the DBCG, regardless of whether they enrol in a trial, so the registry provides the data quality advantage of a clinical trial setting with the generalisability advantage of a population-based setting.
We divided the source population into three groups: (a) group I women whose tumour expressed the oestrogen receptor protein and who were treated with tamoxifen for at least 1 year; (b) group II women whose tumour did not express the oestrogen receptor protein, were not treated with tamoxifen, and who survived for at least one year; and (c) group III women, comprising all others, who were excluded from this analysis. Group I women were assigned to tamoxifen therapy protocols of 1, 2, or 5 years, depending on the guideline extant in Denmark at the time of their diagnoses. We included group II women to estimate the direct association of citalopram prescription with recurrence rate, if any. We further restricted the source population to women diagnosed with breast cancer after the date that their county of residence began to maintain an electronic prescription database (Aarhus=1996, North Jutland=1989, Ringkøbing=1998, Viborg=1998), which were used to ascertain use of prescription medications, including citalopram. Follow-up time began 1 year after the date of breast cancer diagnosis and continued until the date of the first of breast cancer recurrence, death from any cause, loss to follow-up (e.g., emigration), 10 years of follow-up, or 1 September 2006.
Cases were women with local or distant breast cancer recurrence occurring during their follow-up time among the members of groups I and II. We selected one control for each case without replacement from members of the source population who had not had a breast cancer recurrence after the same amount of follow-up time. We matched controls to cases on (a) group membership (group I or II), (b) menopausal status at diagnosis (premenopausal or postmenopausal), (c) date of breast cancer surgery (caliper matched±12 months), (d) county of residence at the time of diagnosis, and (e) UICC stage at diagnosis (stage I, II, or III).
Data collection
We used the Danish Civil Personal Registration (CPR) number assigned to each case and control to link data sets. The CPR is a unique identification number assigned to all Danish residents alive on 1 April 1968, born thereafter, or upon immigration.
We collected demographic information (age, menopausal status, and hospital of diagnosis), tumour characteristics (UICC stage, histological grade, and oestrogen-receptor expression), and therapy characteristics (primary surgical tumour management, receipt of radiation therapy, receipt of chemotherapy, and receipt of tamoxifen therapy) from the DBCG database.
We collected data on receipt of citalopram prescription and other potential CYP2D6 inhibitors (including other SSRI) by linking the CPR number of cases and controls to the prescription databases maintained by each county (see, for example, the description of North Jutland's database (Gaist et al, 1997)).
Analytic variables
Recurrence
We used the DBCG definition of breast cancer recurrence as any type of breast cancer subsequent to the initial course of therapy (Andersen and Mouridsen, 1988). Given the definition of the source population and follow-up time, all cases of recurrence occurred between 1 and 10 years after the primary breast cancer diagnosis.
Prescription status
Prescription medications are coded by the Anatomical Therapeutic Chemical (ATC) classification system (WHO Collaborating Centre for Drug Statistics Methodology, 2007). We defined SSRI antidepressants as all those classified in group N06AB by the ATC. These are the SSRI drugs: zimeldine (N06AB02), fluoxetine (N06AB03), citalopram (N06AB04), paroxetine (N06AB05), sertraline (N06AB06), alaproclate (N06AB07), fluvoxamine (N06AB08), etoperidone (N06AB09), and escitalopram (N06AB10). We defined citalopram exposure as any prescription for citalopram (N06AB04) or its S-stereoisomer escitalopram (N06AB10).
We classified cases and controls as those with no record of a citalopram prescription during their follow-up time (never citalopram) and those with any record of prescription for citalopram during their follow-up time (ever citalopram). We used a similar procedure to classify cases and controls as ever or never users of another SSRI or of another prescription medication that is a CYP2D6 inhibitor or substrate, aside from those indicated to treat breast cancer recurrence or its effects. See the Supplementary online material for a complete list of these medications and the frequency of their use in the study population.
For group I women who ever had a citalopram prescription, we calculated the percentage of time on tamoxifen when they were simultaneously taking citalopram. We created categories of (a) intermittent citalopram use, defined as citalopram use overlapping tamoxifen use for more than 0% but less than 30% of the time on tamoxifen and (b) regular citalopram use, defined as citalopram use overlapping tamoxifen use for 30% or more of the time on tamoxifen. We chose 30% as the overlap boundary to allow sufficient sample size in the regular citalopram subgroup, while also investigating a substantial period of SSRI and tamoxifen comedication.
Covariates
We defined the following set of covariates: (a) time period of breast cancer diagnosis (1985–1993, 1994–1996, and 1997–2001), (b) age at diagnosis (35–44 years, 45–54 years, 55–64 years, and 65–70 years), (c) menopausal status at diagnosis (premenopausal and postmenopausal), (d) county of residence at diagnosis (Aarhus, North Jutland, Viborg, and Ringkøbing), (e) UICC stage at diagnosis (stages I, II, and III), histological grade (grade I, II, III, and missing), surgery type (breast conserving surgery and mastectomy), and receipt of systemic adjuvant chemotherapy (yes and no), and (f) receipt of a prescription for another medication that is a CYP2D6 inhibitor or substrate, including other SSRI, while taking tamoxifen.
Analytic strategy
All analyses were conducted within strata of the two groups (oestrogen-receptor positive and treated with tamoxifen or oestrogen-receptor negative and not treated with tamoxifen). We computed the frequency and proportion of cases and controls within categories of assigned protocol of tamoxifen duration, of citalopram use, of use of other CYP2D6 inhibitors or substrates, and of the covariates. We calculated the number of cases and controls ever receiving citalopram, the number of total prescriptions for citalopram summed over all cases or controls, and the range of the number of prescriptions for citalopram received by each individual case or control.
We estimated the rate ratio associating citalopram prescription with breast cancer recurrence as the odds ratio (OR) in a conditional logistic regression including only citalopram use as the exposure variable and conditioned on the matched factors. By design, this ratio adjusts for confounding by the matched factors (Greenland, 2008). We examined whether the effect of citalopram use was modified by duration of tamoxifen therapy in a stratified analysis. Finally, we adjusted for residual confounding by the covariates that were not included in the matching by including them as independent variables in the conditional logistic regression. We retained in the final model any covariate that affected the log OR from the conditional logistic regression model associating citalopram use with breast cancer recurrence rate by more than 10% (Greenland, 1989). All estimates are accompanied by a 95% confidence interval (CI) calculated by the profile likelihood method. All analyses were performed using SAS version 9.
Results
Table 1 shows the frequency and proportion of cases and controls, within strata of group, in the categories of the covariates. About two-thirds of cases and controls in both groups were diagnosed with primary breast cancer during the period 1997–2001, and the majority was resident in Aarhus or North Jutland counties, because the prescription registries began first in these two counties. A large majority had mastectomy as their primary surgical intervention, which is consistent with the clinical practice pattern previously reported in this region during this time period (Ahern et al, 2008). Group I women (positive oestrogen-receptor expression and treated with tamoxifen) were more likely to be post-menopausal (87%) than were group II women (66%; negative oestrogen-receptor expression and not treated with tamoxifen). Group I women were also less likely to receive systemic adjuvant chemotherapy (11 and 13% of cases and controls, respectively) than were group II women (80 and 70% of cases and controls, respectively); reflecting the preference for hormonal therapy over systemic adjuvant chemotherapy in women whose tumours expressed the oestrogen receptor. Between 3 and 11% of cases and controls ever used citalopram while taking tamoxifen (group I) or during their follow-up period (group II).
Table 2 depicts the pattern of SSRI prescriptions received by cases and controls. In both groups, SSRI prescriptions were primarily written for citalopram or its S-stereoisomer, escitalopram. For example, 17 of 23 group I cases (74%) ever prescribed an SSRI had at least one prescription for citalopram, accounting for 86% of the total number of prescriptions. Similarly, 22 of 30 group I controls (73%) ever prescribed an SSRI had at least one prescription for citalopram, accounting for 64% of their prescriptions. Sertraline accounted for the majority of the remaining prescriptions (11% of the total for cases and 23% for controls).
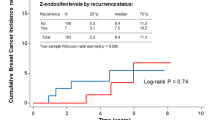
Group I women who ever used citalopram while taking tamoxifen did not have a higher rate of breast cancer recurrence than women who never used citalopram while taking tamoxifen (Table 3; OR=0.79, 95% CI=0.40, 1.6). This OR was not substantially modified by duration of tamoxifen therapy (P=0.23 for test of homogeneity; data not shown). The approximately null effect persisted with adjustment for age category and ever/never use of another CYP2D6 inhibitor or SSRI (OR=0.85, 95% CI 0.42, 1.7). The effects were likewise approximately null within cumulative citalopram prescription categories (intermittent use OR=0.72, 95% CI 0.30, 1.7; regular use OR=1.1, 95% CI 0.37, 3.3). Citalopram use also had no substantial effect on recurrence in group II women (adjusted OR=0.78, 95% CI 0.17, 3.6), suggesting that citalopram does not directly affect the risk of breast cancer recurrence.
Discussion
The results of this study do not support the hypothesis that citalopram, taken concurrently with tamoxifen, reduces tamoxifen's protective effect against breast cancer recurrence in early-stage patients whose tumour cells express the oestrogen receptor.
Our results extend the findings from an earlier study of 28 stage II and III breast cancer patients with recurrence and their matched controls at a single United States oncology centre, which also reported no substantial modification of tamoxifen effectiveness by concomitant use of SSRI inhibitors of CYP2D6 (Lehmann et al, 2004). These results may seem at odds with the strong biological rationale and in vivo evidence that support the hypothesis that CYP2D6 inhibition would reduce tamoxifen's prevention of breast cancer recurrence. It is possible, however, that SSRI medications could reduce the plasma concentration of tamoxifen's secondary metabolites without reducing its anti-tumorigenicity (Ponzone et al, 2004; Ratliff et al, 2004; Stearns et al, 2004). Tamoxifen doses as much as 20-fold lower than the typical US dose of 20 mg day−1 affect biomarkers of cardiovascular, bone, and tumour end points (Decensi et al, 1998, 2003), so the approximately three-fold reduction in the plasma concentration of tamoxifen's secondary metabolites associated with concomitant receipt of the SSRI paroxetine (Jin et al, 2005) may have little consequence.
The key mechanistic question may be whether reduced concentrations of active tamoxifen metabolites result in substantially reduced occupancy of the oestrogen receptor. Dowsett and Haynes (2003) estimated that, in postmenopausal women on a daily dose of 20 mg tamoxifen, tamoxifen and its metabolites occupy 9994 of 10 000 oestrogen receptors. Replicating their calculation using the plasma concentrations of tamoxifen and its metabolites in women with no CYP2D6 variant allele (Jin et al, 2005), tamoxifen and its metabolites would occupy 9999 of 10 000 receptors in women not taking any SSRI and 9997 of 10 000 receptors in women taking the strong CYP2D6-inhibiting SSRI paroxetine. Steady-state concentrations of tamoxifen and its metabolites may be sufficient to manifest fully tamoxifen's antitumorigenic effect in postmenopausal women regardless of whether CYP2D6 inhibition reduces the concentration of some tamoxifen metabolites.
Nonetheless, our results should be considered with the following limitations in mind. First, the majority of SSRI prescriptions in our study were for citalopram or its S-stereoisomer, both originally manufactured by Lundbeck, a company headquartered in Denmark. Citalopram is a modest inhibitor of CYP2D6 compared with some other SSRI medications (Jeppesen et al, 1996). These more potent inhibitors may reduce tamoxifen's protection against breast cancer recurrence, but their interaction with tamoxifen would not have been well measured by this study.
Second, we have not collected genotype data to characterize functional CYP2D6 variants (Hayhurst et al, 2001) that affect the metabolism of tamoxifen (Jin et al, 2005). The combination of genotype and receipt of CYP2D6-inhibiting medications has been related to tamoxifen effectiveness in a previous study (Goetz et al, 2007). We do not, however, expect ever-receipt of citalopram while taking tamoxifen to be related to CYP2D6 genotype, as this genotype would be unknown to the patient and provider at the first citalopram prescription. This study's results therefore pertain to the usual clinical setting. In addition, CYP2D6 genotype is unlikely to cause citalopram prescription, or to share a common causal ancestor, so CYP2D6 genotype does not satisfy the requisite causal structure of a confounder (Greenland et al, 1999). It may be possible that CYP2D6 genotype is related to adherence to citalopram prescription or to long-term maintenance of the prescription, resulting from differences in the occurrence of adverse drug reactions in women with the different alleles. Such a relation could confound the association between breast cancer recurrence and duration of citalopram prescription while taking tamoxifen. Some non-randomized studies suggest such a relation between genotype and SSRI adherence (Rau et al, 2004; Zourková et al, 2007), whereas others suggest no such relation (Stedman et al, 2002; Gerstenberg et al, 2003; Roberts et al, 2004; Hedenmalm et al, 2006; Sugai et al, 2006; Suzuki et al, 2006). In the only randomized trial, CYP2D6 genotype was not related to either the occurrence of adverse events or to adherence to paroxetine prescription (Murphy et al, 2003). Paroxetine is the most potent CYP2D6 inhibitor of tamoxifen metabolism among the SSRI class (Jin et al, 2005). If CYP2D6 genotype does not affect receipt or adherence to SSRI prescription, then it cannot confound the association we have reported.
Last, we do not know the indications for which citalopram was prescribed to the study participants, although ordinarily it would be prescribed primarily to treat depression. SSRI may also be prescribed to treat hot flushes (Stearns, 2006), but such prescriptions are rare in Danish breast cancer patients.
Weighing against these limitations are the strengths of the data quality. This study relied upon the Danish Breast Cancer Cooperative Group's registry of breast cancer patients, which provides clinical trial quality data in a population-wide setting in the four Northern Danish Counties. For example, the positive predictive value of breast cancer recurrence recorded by the DBCG equaled 99.4% in a validation study (Hansen et al, 1997), showing that there are few false-positive recurrences registered in the DBCG. In addition, of 1888 local and distant recurrences identified by medical record review among 4455 breast cancer patients assigned to a DBCG protocol, 1813 (96%) were correctly registered as recurrences in the DBCG database, 74 (3.9%) were identified as breast cancer deaths, and only 1 (0.05%) was not identified as either a recurrence or breast cancer death.
The prescription databases are generated by a computerised pharmacy accounting system that sends data to the Danish National Health Service, which refunds part of the costs associated with prescribed drugs. Given the direct connection between receipt of prescription medications and the pharmacy accounting system of the Danish National Health Service, we expect the prescription records to have excellent validity. The prescriptions from the four counties are merged into a research database at Aarhus University. In Denmark, antidepressants are available only at pharmacies and the patient must have a prescription from a medical doctor. Therefore, the county prescription databases are expected to have high sensitivity and specificity for ascertainment of citalopram prescriptions in the source population. Furthermore, because the prescription records antecede the date of breast cancer recurrence, they are a prospective data source presumably immune to differential classification bias (Rothman et al, 2008).
Despite these advantages, the study yielded only 17 cases of breast cancer recurrence among tamoxifen-treated women who had used citalopram while taking tamoxifen. The study was designed with 80% power to detect an OR of 1.6, and ultimately had 90% power to detect an OR of 2.3.
The results presented herein are, nonetheless, important and timely. A United States Food and Drug Administration advisory committee recently recommended relabelling tamoxifen with information on gene–drug and drug–drug interactions mediated by CYP2D6 (American Cancer Society, 2007). Furthermore, the current practice guidelines of the United States National Comprehensive Cancer Network note that some SSRI reduce the formation of active tamoxifen metabolites, that citalopram and venlaflaxine appear to have minimal impact on tamoxifen metabolism, and that ‘the clinical impact of these observations is not known’ (National Comprehensive Cancer Network, 2008). Breast cancer patients taking tamoxifen and their physicians may therefore be concerned about SSRI comedication, even when antidepressants are strongly indicated. Our results suggest that citalopram prescription does not reduce tamoxifen's prevention of breast cancer recurrence.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahern TP, Larsson H, Garne JP, Cronin-Fenton DP, Sørensen HT, Lash TL (2008) Trends in breast-conserving surgery in Denmark, 1982–2002. Eur J Epidemiol 23: 109–114
American Cancer Society (2007) Tamoxifen: some women don't get full benefit. American Cancer Society News Center http://www.cancer.org/docroot/NWS/content/NWS_1_1x_Tamoxifen_Some_Women_Dont_Get_Full_Benefit.asp. Last accessed 13 June 2007
Andersen KW, Mouridsen HT (1988) Danish Breast Cancer Cooperative Group (DBCG). A description of the register of the nation-wide programme for primary breast cancer. Acta Oncologica 27: 627–647
Coezy E, Borgna JL, Rochefort H (1982) Tamoxifen and metabolites in MCF-7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res 42: 317–323
Coller JK, Krebsfaenger N, Klein K, Endrizzi K, Wolbold R, Lang T, Nüssler A, Neuhaus P, Zanger UM, Eichelbaum M, Mürdter TE (2002) The influence of CYP2B6 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol 54: 157–167
Decensi A, Bonanni B, Guerrieri-Gonzaga A, Gandini S, Robertson C, Johansson H, Travaglini R, Sandri MT, Tessadrelli A, Farante G, Salinaro F, Bettega D, Barreca A, Boyle P, Costa A, Veronesi U (1998) Biologic activity of tamoxifen at low doses in healthy women. J Natl Cancer Inst 90: 1461–1467
Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, Veronesi P, Torrisi R, Cazzaniga M, Mora S, Sandri MT, Pelosi G, Luini A, Goldhirsch A, Lien EA, Veronesi U (2003) A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic markers. J Natl Cancer Inst 95: 779–790
Dowsett M, Haynes BP (2003) Hormonal effects of aromatase inhibitors: focus on premenopausal effects and interaction with tamoxifen. J Steroid Biochem Mol Biol 86: 255–263
Early Breast Cancer Trialists' Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet 365: 1687–1717
Gaist D, Sørensen HT, Hallas J (1997) The Danish prescription registries. Dan Med Bull 44: 445–448
Gerstenberg G, Aoshima T, Fukasawa T, Yoshida K, Takahashi H, Higuchi H, Murata Y, Shimoyama R, Ohkubo T, Shimizu T, Otani K (2003) Relationship between clinical effects of fluvoxamine and the steady-state plasma concentrations of fluvoxamine and its major metabolite fluvoxamino acid in Japanese depressed patients. Psychopharmacol 167: 443–448
Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Weinshilboum RM, Fritcher EG, Nibbe AM, Desta Z, Nguyen A, Flockhart DA, Perez EA, Ingle JN (2007) The impact of cytochrome P-450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101: 113–121
Greenland S (1989) Modeling and variable selection in epidemiologic analysis. Am J Public Health 79: 340–349
Greenland S (2008) Introduction to regression modeling. In Modern Epidemiology, 3rd edn, Rothman KJ, Greenland S, Lash TL (eds) pp 418–458. Philadelphia: Lippincott Williams & Wilkins
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10: 37–48
Hansen DG, Søndergaard J, Vach W, Gram LF, Rosholm JU, Kragstrup J (2003) Antidepressant drug use in general practice: inter-practice variation and association with practice characteristics. Eur J Clin Pharmacol 59: 143–149
Hansen PS, Andersen E, Andersen KW, Mouridsen HT (1997) Quality control of end results in a Danish adjuvant breast cancer multi-center study. Acta Oncologica 36: 711–714
Hayhurst GP, Harlow J, Chowdry J, Gross E, Hilton E, Lennard MS, Tucker GT, Ellis SW (2001) Influence of phenylalanine-481 subsitutions on the catalytic activity of cytochrome P-450 2D6. Biochem J 355: 373–379
Hedenmalm K, Güzey C, Dahl ML, Yue QY, Spigset O (2006) Risk factors for extrapyramidal symptoms during treatment with selective serotonin reuptake inhibitors, including cytochrome P-450 enzyme, and serotonin and dopamine transporter and receptor polymorphisms. J Clin Psychopharmacol 26: 192–197
Jensen AR, Ewertz M, Cold S, Storm HH, Overgaard J (2003) Time trends and regional differences in registration, stage distribution, surgical management, and survival of breast cancer in Denmark. Eur J Cancer 39: 1783–1793
Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brøsen K (1996) Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol 51: 73–78
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97: 30–39
Jordan VC, Dowse LJ (1976) Tamoxifen as an anti-tumour agent: effect on oestrogen binding. J Endocrinol 68: 297–303
Lehmann D, Nelsen J, Ramanath V, Newman N, Duggan D, Smith A (2004) Lack of attenuation in the antitumor effect of tamoxifen by chronic CYP isoform inhibition. J Clin Pharmacol 44: 861–865
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55: 471–478
Malet C, Gompel A, Spritzer P, Bricout N, Yaneva H, Mowszowicz I, Kuttenn F, Mauvais-Jarvis P (1988) Tamoxifen and hydroxyl-tamoxifen isomers vs estradiol effects on normal human breast cells in culture. Cancer Res 48: 7193–7199
Massie MJ (2004) Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 32: 57–71
Murphy Jr GM, Kremer C, Rodrigues HE, Schatzberg AF (2003) Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 160: 1830–1835
National Comprehensive Cancer Network (2008) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer v.2.2008. pp. 37 http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf
Ponzone R, Biglia N, Sismondi P (2004) Re: Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. Letter. J Natl Cancer Inst 96: 883–884
Ratliff B, Dietze EC, Bean GR, Moore C, Wanko S, Seewaldt VL (2004) Re: Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. Letter. J Natl Cancer Inst 96: 883
Rau T, Wohlleben G, Wuttke H, Thuerauf N, Lunkenheimer J, Lanczik M, Eschenhagen T (2004) CYP2D6 genotype: impact on adverse effects and nonresponse during treatment with antidepressants-a pilot study. Clin Pharmacol Ther 75: 386–393
Roberts RL, Mulder RT, Joyce PR, Luty SE, Kennedy MA (2004) No evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 19: 17–23
Rothman KJ, Greenland S, Lash TL (2008) Types of epidemiologic studies. In Modern Epidemiology, 3rd edn, Rothman KJ, Greenland S, Lash TL (eds) pp 95–97; Philadelphia: Lippincott, Williams & Wilkins
Stearns V (2006) Serotonergic agents as an alternative to hormonal therapy for the treatment of menopausal vasomotor symptoms. Treat Endocrinol 5: 83–87
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95: 1758–1764
Stearns V, Johnson MD, Rae JM, Novielli A, Bhargava P, Hayes DF, Desta A, Flockhart DA (2004) Re: active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. Letter. J Natl Cancer Inst 96: 884–885
Stedman CA, Begg EJ, Kennedy MA, Roberts R, Wilkinson TJ (2002) Cytochrome P450 2D6 genotype does not predict SSRI (fluoxetine or paroxetine) induced hyponatraemia. Hum Psychopharmacol 17: 187–190
Sugai T, Suzuki Y, Sawamura K, Fukui N, Inoue Y, Someya T (2006) The effect of 5-hydroxytryptamine 3A and 3B receptor genes on nausea induced by paroxetine. Pharmacogenomics J 6: 351–356
Suzuki Y, Sawamura K, Someya T (2006) Polymorphisms in the 5-hydroxytryptamine 2A receptor and cytochromeP4502D6 genes synergistically predict fluvoxamine-induced side effects in Japanese depressed patients. Neuropsychopharmacology 31: 825–831
UICC (1997) TNM Classification of Malignant Tumours, 5th edn. Switzerland: Springer
WHO Collaborating Centre for Drug Statistics Methodology (2007) About the centre. http://www.whocc.no/atcddd/. Last accessed 31 May 2007
Zanger UM, Raimundo S, Eichelbaum M (2004) Cytochrome P-450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn-Schmiedeberg's Arch Pharmacol 369: 23–37
Zourková A, Cesková E, Hadasová E, Ravcuková B (2007) Links among paroxetine-induced sexual dysfunctions, gender, and CYP2D6 activity. Sex Marital Ther 33: 343–355
Acknowledgements
This research was supported by grants from the US National Cancer Institute (R01 CA118708), Danish Cancer Society (DP06117), and the Karen Elise Jensen Foundation. The prescription databases are supported by the Western Danish Research Forum for Health Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lash, T., Pedersen, L., Cronin-Fenton, D. et al. Tamoxifen's protection against breast cancer recurrence is not reduced by concurrent use of the SSRI citalopram. Br J Cancer 99, 616–621 (2008). https://doi.org/10.1038/sj.bjc.6604533
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604533
Keywords
This article is cited by
-
Breast cancer recurrence in relation to antidepressant use
Cancer Causes & Control (2016)
-
Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen
Breast Cancer Research and Treatment (2013)
-
CYP2D6 genotyping and use of antidepressants in breast cancer patients: test development for clinical application
Metabolic Brain Disease (2012)
-
Pharmacogenetic Testing: Time for Clinical Practice Guidelines
Clinical Pharmacology & Therapeutics (2011)
-
Concurrent use of tamoxifen with CYP2D6 inhibitors and the risk of breast cancer recurrence
Breast Cancer Research and Treatment (2011)