Abstract
Glomeruloid microvascular proliferation (GMP) in breast cancer independently adversely affected survival (relative risk 1.9, 95% CI: 1.2–3.0), particularly among women who received adjuvant chemotherapy (10-year survival 27 vs 69%, P=0.0003), and was significantly associated with p53 overexpression and BRCA1 germline mutations. The presence of GMP may influence treatment decisions.
Similar content being viewed by others
Main
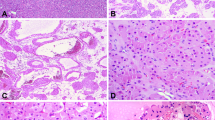
Angiogenesis is under intense study both for its prognostic value and for the potential therapeutic value of interfering with angiogenic pathways. In breast cancer, both higher vascular endothelial growth factor (VEGF) levels (Blackwood and Weber, 1998; Linderholm et al, 2000) and increased microvessel density (MVD) (Weidner et al, 1991, 1992; de Jong et al, 2000) are associated with a poorer prognosis. Recent microarray studies have also identified an association between the expression of genes involved in angiogenesis, such as VEGF, and poor prognosis following breast cancer (van't Veer et al, 2002). Furthermore, the proangiogenic genes COL4A1 and ECGF1 are overexpressed in breast cancers found in BRCA1 mutation carriers, although their prognostic value in such women is unknown (van't Veer et al, 2002). Here, we describe our results with a morphological marker of prognosis of potential importance in the management of women who carry germline BRCA1 mutations. Glomeruloid microvascular proliferations (GMPs) (Figure 1) are focal proliferative buddings of vascular endothelial cells resembling a renal glomerulus. Glomerular microvascular proliferation has been predominantly associated with glioblastoma multiforme (Wesseling et al, 1998), the most aggressive form of glioma. They can be produced in athymic mice by overexpressing VEGF using an adenovirus vector (Sundberg et al, 2001). Recent evidence suggests that these structures may be present and prognostically useful in other tumour types, including breast cancer (Straume et al, 2002). The work presented here is an expansion of the breast cancer data presented by Straume et al, and focuses on two new aspects: (1) the inter-relationship between GMP, p53 overexpression and BRCA1/2 status and (2) the effect of GMP on prognosis in the presence or absence of adjuvant chemotherapy.
Materials and methods
A total of 292 consecutive Ashkenazi Jewish women aged 65 years or less with primary nonmetastatic breast cancer diagnosed at one Montreal institution between 1980 and 1995 were assessed. Sufficient follow-up and tissue were available for 251 subjects. Following ethics committee approval, specimens were evaluated by one pathologist (LR Bégin) using conventional methods. Accumulation of p53 protein was detected by immunohistochemistry as previously described (Yuan et al, 1999). Pathology blocks from all women were tested for founder BRCA1 mutations (185delAG, n=18; 5382insC, n=10) and BRCA2 mutation (6174delT, n=8) that are common in this population, using established techniques (Foulkes et al, 1997).
Staining of endothelial cells by Factor-VIII (A-0082, Dako, Copenhagen) was performed on formalin-fixed and paraffin-embedded archival material as previously published (Straume and Akslen, 2001). The presence of GMP was recorded by the finding of focal glomerulus-like aggregates of closely associated and multilayered factor-VIII positive endothelial cells. Glomerular microvascular proliferations consisted of 15–100 cells. Lumen formation was not necessary for the aggregates to be counted as GMPs. Tangentially sectioned normal vessels, or nonspecific Factor-VIII positivity in stromal components, were excluded. Glomerular microvascular proliferations were categorised as being absent (group 0), rare (not more than one per high-power field (HPF), group 1), or greater than 1 per HPF (group 2). Microvascular density was calculated as the mean number of stained vessels in 10 high-power fields (× 400).
Molecular, pathological and clinical assessments were collected in a mutually blinded manner in a retrospective cohort approach. Subject characteristics were compared using Wilcoxon, t-test, and Fisher's exact testing, with trends in increasing odds ratios being assessed by Cochran–Armitage's test. Differences in breast cancer-specific survival were calculated using the method of Kaplan and Meier. The Cox proportional hazards model was used to assess prognostic factors.
Results
In all, 43 breast cancers (17%) had one or more GMP, with 36 tumours in group 1 and seven in group 2. Their presence was associated with higher nuclear grade (P for trend <0.0001), oestrogen receptor (ER) negativity (OR 4.7, 95% CI: 2.3–9.6), p53 immunohistochemical positivity (OR 4.1, 95% CI: 2.0–8.2), and germline BRCA1 mutations (odds ratio (OR) 2.6, 95% CI: 1.1–6.3), but not tumour size, axillary nodal status, microvascular density (MVD) or germline BRCA2 mutations (Table 1). There was no relationship between higher GMP grouping and higher MVD or BRCA1 mutation type.
There were 65 breast cancer deaths in this series of women at 10 years follow-up. Kaplan–Meier survival analysis showed that 50.3% of women with GMP died of breast cancer over this period, whereas the mortality was 25.7% for those with no identified GMP (P=0.0003). Microvascular density was not significantly associated with a worse prognosis (P=0.47). In a Cox proportional hazards model, the presence of GMP (defined continuously) was associated with a poor prognosis (relative risk (RR) 1.9, 95% CI: 1.2–3.0) as was positive lymph node status (RR 2.3). Nuclear grade (RR 1.6) and negative ER status (RR 1.7) were of borderline significance, while tumour size, p53 positivity and carrier status did not achieve significance (Table 2). Among women treated with adjuvant chemotherapy, the presence of GMP was an indicator of poor prognosis (10-year survival 27 vs 69%, P=0.0003), while among women not treated with chemotherapy, no statistically significant difference in survival was seen on the basis of GMP status (10-year survival 75 vs 79%, P=0.4).
Discussion
This study is the first to demonstrate that GMP is associated with p53 expression and the presence of germline BRCA1 mutations and it suggests that the presence of GMP is an independent risk factor for death from breast cancer comparable in magnitude to conventional prognostic factors (RR 1.9).
Notably, GMP was not associated with a higher MVD, and the latter was not prognostic for poor survival in our cohort of patients. Vascular endothelial growth factor is implicated in the genesis of both GMP (Sundberg et al, 2001) and increased MVD (De Paola et al, 2002), but the lack of association between GMP and MVD suggests that their developmental pathways may differ. In our cohort, p53 expression was associated with the presence of GMP, but not with increased MVD (P=0.8), the latter being consistent with the literature (Tas et al, 2000). Functional p53 impedes angiogenesis through the regulation of VEGF transcriptional factors Sp1 (Mandlekar and Kong, 2001) and the HIF-1α subunit (Ravi et al, 2000), as well as by upregulating thrombospondin-1 expression (Dameron et al, 1994). Mutated p53 may be one pathway by which a neovascular phenotype associated with GMP (but not MVD) formation is promoted.
Our data suggest that GMP is associated with p53 expression and BRCA1 germline mutations and that all three of these factors may be associated with a worse survival (for details of the p53-BRCA1 relationship, see Goffin et al, 2003). Interestingly, patients who were treated with adjuvant chemotherapy had a poorer outcome if their tumours demonstrated GMP. Glomerular microvascular proliferation was highly significantly associated with p53 expression (P=0.0001), a protein partly responsible for inducing apoptosis in chemotherapy-treated cells and thus potentially responsible for diminished responsiveness to chemotherapy (Fisher, 2001). Opposed to this is the association of GMP with BRCA1 mutations and higher nuclear grade, both of which appear to increase tumour responsiveness to chemotherapy (Chappuis et al, 2002;Wang et al, 2002). In the present study, higher nuclear grade (RR 1.9, P=0.02) and age < 50 years (RR 5.6, P=0.0001) were associated with an increased likelihood of a woman receiving adjuvant chemotherapy, while other factors were not significant on multivariate analysis. The apparent contradiction in associations and chemoresponsiveness is likely a product of the interplay of several response mitigating pathways and the incomplete association between measured factors.
There is evidence that BRCA1-related breast cancers have a distinct profile on microarray analysis (van't Veer et al, 2002) and that these cancers have a distinctive spectrum of TP53 mutations (Greenblatt et al, 2001). Along with evidence that BRCA1 is important in global nucleotide excision repair (Hartman and Ford, 2002), these data hint that BRCA1 mutations induce a genetic profile of which p53 expression and GMP are but two manifestations, with several factors influencing both prognosis and response to treatment. However, the role of BRCA1 mutations in the genesis of such a phenotype requires further investigation.
Angiogenesis is a complex process and its full understanding will require analysis at the level of morphology and gene expression. Here, we describe the poor prognosis associated with GMP, which is a highly characteristic lesion resulting from a gene expression profile that is as yet undefined. As antiangiogenic therapy is currently under intense investigation, it will be important to establish whether the presence of GMP alters the effectiveness of such therapies.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Blackwood MA, Weber BL (1998) BRCA1 and BRCA2: from molecular genetics to clinical medicine. J Clin Oncol 16: 1969–1977
Chappuis PO, Goffin J, Wong N, Perret C, Ghadirian P, Tonin PN, Foulkes WD (2002) A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J Med Genet 39: 608–610
Dameron KM, Volpert OV, Tainsky MA, Bouck N (1994) Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265: 1582–1584
de Jong JS, van Diest PJ, Baak JP (2000) Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology 36: 306–312
De Paola F, Granato AM, Scarpi E, Monti F, Medri L, Bianchi S, Amadori D, Volpi A (2002) Vascular endothelial growth factor and prognosis in patients with node-negative breast cancer. Int J Cancer 98: 228–233
Fisher DE (2001) The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis 6: 7–15
Foulkes WD, Wong N, Brunet JS, Bégin LR, Zhang JC, Martinez JJ, Rozen F, Tonin PN, Narod SA, Karp SE, Pollak MN (1997) Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res 3: 2465–2469
Goffin JR, Chappuis PO, Bégin LR, Wong N, Brunet JS, Hamel N, Paradis AJ, Boyd J (2003) The impact of germ-line BRCA1 mutations and over-expression of p53 on prognosis and response to treatment following breast cancer: 10 year follow up data. Cancer 97: 527–536
Greenblatt MS, Chappuis PO, Bond JP, Hamel N, Foulkes WD (2001) TP53 mutations in breast cancer associated with BRCA1 or BRCA2 germ-line mutations: distinctive spectrum and structural distribution. Cancer Res 61: 4092–4097
Hartman AR, Ford JM (2002) BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet 32: 180–184
Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R (2000) Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 18: 1423–1431
Mandlekar S, Kong AN (2001) Mechanisms of tamoxifen-induced apoptosis. Apoptosis 6: 469–477
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A (2000) Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 14: 34–44
Straume O, Akslen LA (2001) Expression of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol 159: 223–235
Straume O, Chappuis PO, Salvesen HB, Halvorsen OJ, Haukaas SA, Goffin JR, Bégin LR, Foulkes WD, Akslen LA (2002) Prognostic importance of glomeruloid microvascular proliferation indicates an aggressive angiogenic phenotype in human cancers. Cancer Res 62: 6808–6811
Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF (2001) Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol 158: 1145–1160
Tas F, Yavuz E, Aydiner A, Saip P, Disci R, Iplikci A, Topuz E (2000) Angiogenesis and p53 protein expression in breast cancer: prognostic roles and interrelationships. Am J Clin Oncol 23: 546–553
van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der KK, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–536
Wang J, Buchholz TA, Middleton LP, Allred DC, Tucker SL, Kuerer HM, Esteva FJ, Hortobagyi GN, Sahin AA (2002) Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer 94: 3107–3114
Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G (1992) Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84: 1875–1887
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324: 1–8
Wesseling P, van der Laak JA, Link M, Teepen HL, Ruiter DJ (1998) Quantitative analysis of microvascular changes in diffuse astrocytic neoplasms with increasing grade of malignancy. Hum Pathol 29: 352–358
Yuan ZQ, Bégin LR, Wong N, Brunet JS, Trifiro M, Gordon PH, Pinsky L, Foulkes WD (1999) The effect of the I1307 K APC polymorphism on the clinicopathological features and natural history of breast cancer. Br J Cancer 81: 850–854
Acknowledgements
We thank Ann-Josée Paradis for assistance with mutation identification. Grants to WDF from the Canadian Genetic Diseases Network, the Fonds de la Recherche en Santé du Québec (FRSQ) Cancer Network-Breast and Ovarian Tumour Bank Axis and the Susan G Komen Foundation, and grants to LAA from the Norwegian Cancer Society and the Norwegian Research Council are acknowledged. JRG is a recipient of the Canadian Association of Medical Oncologists/Canadian Institutes of Health Research Fellowship. WDF is a Chercheur Clinicien Boursier of the FRSQ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Goffin, J., Straume, O., Chappuis, P. et al. Glomeruloid microvascular proliferation is associated with p53 expression, germline BRCA1 mutations and an adverse outcome following breast cancer. Br J Cancer 89, 1031–1034 (2003). https://doi.org/10.1038/sj.bjc.6601195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601195
Keywords
This article is cited by
-
Vascular remodeling in cancer
Oncogene (2014)
-
Vascular proliferation is a prognostic factor in breast cancer
Breast Cancer Research and Treatment (2012)
-
Vascular proliferation is increased in basal-like breast cancer
Breast Cancer Research and Treatment (2011)
-
Triple-negative breast cancer
Breast Cancer Research (2010)
-
Identification of the first case of germline duplication of BRCA1 exon 13 in an Italian family
Familial Cancer (2010)