Abstract
Introduction:
Ultrasound (US) measures of muscle thickness are used in the management and monitoring of muscle changes during rehabilitation, but it is unknown how this measure compares to magnetic resonance imaging (MRI) measures of muscle cross-sectional area (CSA) in patients with spinal cord injury (SCI).
Case Presentation:
Six participants with incomplete SCI underwent US imaging and MRI of their gastrocnemius and tibialis anterior (TA) muscles.
Discussion:
Significant correlations were found between US muscle thickness and MRI CSA in the gastrocnemius (R=0.91, P<0.001), and TA muscles (R=0.58, P<0.05). US muscle thickness in the gastrocnemius and TA of patients with incomplete SCI may be used as a cheaper alternative measure of CSA as measured using MRI, and this measure may be clinically useful to track progress in muscle gains during rehabilitation.
Similar content being viewed by others
Introduction
Following spinal cord injury (SCI), aside from reductions in muscle recruitment, deficits in motor function may also be secondary to changes in muscle mass and structure below the level of injury.1–4 In rehabilitation research, magnetic resonance imaging (MRI) is often used to quantify reductions in lower limb muscle cross-sectional area (CSA) in SCI,5 but not without excessive and potentially unnecessary costs. Ultrasonography (US) has also been used to study distal skeletal muscle thickness changes in this patient population,6 providing a potentially more practical and cost-effective alternative to MRI. Although evidence has established a moderate level of reliability for US measures of muscle size (thickness and estimated cross-sectional area, and volume) in healthy human populations, there is limited evidence in clinical populations.7 As pathological muscle alterations occur in SCI distal to the injury site, such as increased muscle fat infiltration,4,8 it is unknown whether US measures of skeletal muscle thickness are accurate in this patient population; thus, concurrent validity using MRI should be established. Accordingly, the purpose of this preliminary study was to compare MRI measures of CSA with US measures of lower-extremity muscle thickness in participants with incomplete SCI. We hypothesized that these two measures would be significantly correlated.
Methods
Participant characteristics
Participants were recruited from the Rehabilitation Institute of Chicago’s Spinal Cord Injury database, in accordance with Northwestern University IRB #STU00087983 and the Declaration of Helsinki. Six male individuals with chronic cervical incomplete SCI participated (average age=41±13 years old). Three individuals were classified using the American Spinal Cord Injury Association Impairment Scale (AIS) as AIS C C5-8 level, 1 participant was classified as AIS D C1-4 level and one participant was classified as AIS D C5-8 level. All participants were able to ambulate for at least 10 m using assistive devices. See Table 1 for details on participant characteristics.
Magnetic resonance imaging
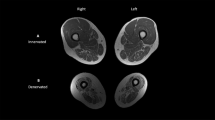
All MRI was performed on a 3.0 Tesla Prisma magnetic resonance scanner (Siemens, Erlangen, Germany). Participants were imaged in supine with knees in extension and ankles at their desired comfortable position. For leg muscle, CSA quantification, scans included both lateral and medial gastrocnemius muscles combined and tibialis anterior (TA) muscles of both legs. The specific MRI parameters included the following: TR=6.59 ms, TE1=2.45 ms TE2=3.675 ms, flip angle=12°, bandwidth 1=510, bandwidth 2=660 Hz/Px and an imaging matrix of 448×266. A read field of view of 320–340 mm was used with a 16-channel body array surface coil, and 60 slices per slab with a slice thickness of 5 mm and a slice oversampling of 6.7% were acquired. The acquisition time was 4:12 min with a rectangular view of 60%, four averages and an in-plane resolution of 0.5 mm. Localizer scans were used to obtain the MRI data in the axial plane perpendicular to the tibia.
Analysis of the lower-extremity MRI data was performed using customized imaging analysis scripts created in MATLAB (Version 2014b, MathWorks, Natick, MA, USA) to determine cross-sectional area by manually defining regions of interest (ROI). ROI were defined in the gastrocnemius and TA by contouring each muscle within its fascial borders. Each outline of the individual muscles yielded a CSA value. To best approximate the peak CSA at the muscle belly, the average individual muscle CSA value was calculated as the CSA average across five slices above and five slices below the slice with the maximum area (see Figure 1). The combined CSA measurement for the medial and lateral gastrocnemius was used for the analyses. The inter-rater reliability using this method has been demonstrated to be high in this patient population in the major leg muscles, with an ICC (2,1) value range of 0.94–0.99.9
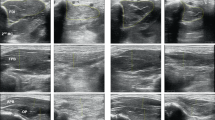
(a) A magnetic resonance image of the tibialis anterior (left outline) and gastrocnemius muscles (right outline) for one representative participant. (b) An ultrasound measure of the muscle thickness of the gastrocnemius muscle for one representative participant. (c) An ultrasound measure of the muscle thickness of the tibialis anterior muscle for one representative participant.
Ultrasound imaging
US images were captured (Aixplorer SuperSonic Imagine, Aix-en-Provence, France) while the participant was instructed to sit in a comfortable position with their knee at 20° extension and ankle in a relaxed position, at a 90°angle. This angle was chosen to standardized testing conditions between participants. It has been previously demonstrated that muscle thickness does not significantly vary at different ankle angles.10 Participants were asked to remain relaxed with the gastrocnemius and TA passive. This position was chosen to mimic the knee and ankle positions in the MRI machine. Image acquisition of the TA and medial, and lateral gastrocnemius was performed for both legs (five images per muscle). The transducer was positioned at the mid-belly region of the respective muscle and aligned with the fascicles as viewed from the B-mode image (see Figure 1). Ultrasound muscle thickness has been shown to be an accurate measure of muscle morphometry.11
A custom-written MATLAB program was used to measure muscle thickness from the US images. Muscle thickness was measured by digitizing the distance between the superficial and deep aponeuroses in the medial and lateral gastrocnemius, as well as in the TA. An average thickness was calculated between trials for each muscle, and the values for the medial and lateral gastrocnemius were averaged to get a gastrocnemius approximation similar to that obtained using MRI.
Statistical analysis
All statistical analyses of the data were performed using IBM SPSS (Version 21, Armonk, NY, USA). Linear regression analyses were conducted to evaluate correlations between MRI cross-sectional area and US muscle thickness. Significance was set at P⩽0.05.
Discussion
Our results suggest that US measures of muscle thickness in the gastrocnemius and tibialis anterior of patients with incomplete SCI may be used as a surrogate measure of CSA as measured using MRI. These results are in accordance with similar findings comparing ultrasound muscle thickness to MRI CSA in the quadriceps muscles of healthy young adults12 and patients with patellofemoral pain.13 This is beneficial for situations where MRI measures cannot be obtained due to a lack of access to a MRI machine, or if the status of a patient is not conducive for undergoing MRI.
Interestingly, we found relatively weaker correlation in the TA compared with the gastrocnemius. We believe this may be due to a relatively smaller range of values in this muscle. Moreover, it is important to consider the morphology differences of the two muscles. Although the gastrocnemius muscles are much larger than the TA in cross-sectional area,14 the thickness of the TA is greater. Thus, the shape of the two muscles likely has a role in explaining the differences in the strength of correlation between thickness and CSA.
As a preliminary study, one inherent limitation is our relatively smaller sample size. This study is part of a project containing a larger heterogeneous participant pool, but in favor of consistency, we chose participants with a more homogeneous incomplete SCI clinical presentation for this current study.
Conclusion
In participants with incomplete SCI, US measures of gastrocnemius and TA muscle thickness are comparable to measures obtained from MRI. Measures of muscle thickness with US may be clinically useful toward making an informed prognosis of functional recovery, informing management and monitoring gains in muscle mass during rehabilitative intervention. The cost-effectiveness of such needs to be trialed before definitive conclusions toward impacting practice can be made.
References
Giangregorio L, McCartney N . Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006; 29: 489–500.
Biering-Sørensen B, Kristensen IB, Kjaer M, Biering-Sørensen F . Muscle after spinal cord injury. Muscle Nerve 2009; 40: 499–519.
Jayaraman A, Gregory C, Bowden M, Stevens J, Shah P, Behrman A . Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord 2006; 44: 680–687.
Gorgey AS, Dudley GA . Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007; 45: 304–309.
Shah P, Stevens J, Gregory C, Pathare NC, Jayaraman A, Bickel SC et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 2006; 87: 772–778.
Yaeshima K, Negishi D, Yamamoto S, Ogata T, Nakazawa K, Kawashima N . Mechanical and neural changes in plantar-flexor muscles after spinal cord injury in humans. Spinal Cord 2015; 53: 526–533.
English C, Fisher L, Thoirs K . Reliability of real-time ultrasound for measuring skeletal muscle size in human limbs in vivo: a systematic review. Clin Rehabil 2012; 26: 934–944.
Smith AC, Parrish TB, Hoggarth MA, McPherson JG, Tysseling VM, Wasielewski M et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases 2015; 2: 15024.
Smith AC, Knikou M, Yelick K, Alexander AR, Mumane MM, Kritsellis AA et al. MRI measures of fat infiltration in the lower extremities following motor incomplete spinal cord injury: reliability and potential implications for muscle activation. Conf Proc IEEE Eng Med Biol Soc 2016; 5451–5456.
Akagi R, Chino K, Dohi M, Takahashi H . Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiol 2012; 53: 307–311.
Miyatani M, Kanehisa H, Ito M, Kawakami Y, Fukunaga T . The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol 2004; 91: 264–272.
Worsley PR, Kitsell F, Samuel D, Stokes M . Validity of measuring distal vastus medialis muscle using rehabilitative ultrasound imaging versus magnetic resonance imaging. Man Ther 2014; 19: 259–263.
Giles LS, Webster KE, McClelland JA, Cook J . Can ultrasound measurements of muscle thickness be used to measure the size of individual quadriceps muscles in people with patellofemoral pain? Phys Ther Sport 2015; 16: 45–52.
Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res 1992; 10: 928–934.
Acknowledgements
We thank all participants for taking part in this study. Funding for this study was provided by the NIH National Institute of Child Health and Human Development Grant T32 HD057845. ACS is supported by the NIH Extramural Loan Repayment Program for Clinical Researchers and by the Foundation for Physical Therapy Promotion of Doctoral Studies programs. SSML is supported by the NIH National Institute of Child Health and Human Development Grant K12HD073945. JME is supported by the NIH National Institute of Child and Health Development Grant 5R01HD079076-03.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JME—Relevant activities outside the submitted work: board membership, consultancy, other (35% ownership/investment interest in medical consultancy start-up: Pain ID LLC), payment for lectures. The remaining authors declare no conflict of interest.
Additional information
This research was conceptualized and carried out at Northwestern University
Rights and permissions
About this article
Cite this article
Smith, A., Jakubowski, K., Wasielewski, M. et al. Lower extremity muscle structure in incomplete spinal cord injury: a comparison between ultrasonography and magnetic resonance imaging. Spinal Cord Ser Cases 3, 17004 (2017). https://doi.org/10.1038/scsandc.2017.4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.4