Abstract
Study design:
Prospective observational study of acute spinal cord-injured (SCI) patients.
Objectives:
To determine how effectively mean arterial blood pressure (MAP) and spinal cord perfusion pressure (SCPP) are maintained at target levels in acute SCI patients.
Setting:
Single-institution study at a Canadian level-one trauma center.
Methods:
Twenty-one individuals with cervical or thoracic SCI were enrolled within 48 h of injury. A lumbar intrathecal drain was inserted for monitoring intrathecal cerebrospinal fluid pressure (ITP). The MAP was monitored concurrently with ITP, and the SCPP was calculated. Data was recorded hourly from the time of first assessment until at least the end of the 5th day post injury.
Results:
All subjects had at least one recorded episode with a MAP below 80 mm Hg, and 81% had at least one episode with a MAP below 70 mm Hg. On average, subjects with cervical injuries had 18.4% of their pressure recordings below 80 mm Hg. Subjects with thoracic cord injuries had on average 35.9% of their MAP recordings <80 mm Hg.
Conclusion:
It is common practice to establish MAP targets for optimizing cord perfusion in acute SCI. This study suggests that even in an acute SCI referral center, when prospectively scrutinized, the actual MAP may frequently fall below the intended targets. Such results raise awareness of the vigilance that must be kept in the hemodynamic management of these patients, and the potential discrepancy between routinely setting target MAP according to ‘practice guidelines’ and actually achieving them.
Similar content being viewed by others
Introduction
Acute trauma to the spinal cord causes the primary neurologic injury, after which a series of complex pathophysiologic processes such as inflammation, ischemia, and excitotoxicity contribute to further secondary damage.1 Unfortunately, clinicians have few neuroprotective treatment options to offer such patients in order to minimize this secondary damage. The administration of methylprednisolone, once the standard of care, is now controversial and abandoned in many centers.2 Early surgical decompression has been convincingly shown to be neuroprotective in many animal models of spinal cord injury (SCI),3 and recently has been reported to potentially confer modest neurologic benefit in human patients when performed within 24 h of injury.4 Finally, hemodynamic support to avoid hypotension and ensure spinal cord perfusion may reduce ischemic damage to the injured spinal cord.5, 6
Considerable animal data has supported the important role of ischemia in promoting further secondary injury to the spinal cord surrounding the area of trauma.7 In clinical practice, attempts to provide sufficient perfusion to the injured cord by supporting mean arterial blood pressure (MAP) are generally considered to be beneficial. Strong scientific evidence, however, is lacking with respect to the actual target MAP that should be achieved in acute SCI patients, and for what length of time post-injury. A number of investigators have reported on clinical protocols in which MAP targets of at least 85 or 90 mm Hg have been employed in acute SCI patients for 5–7 days.5, 6 Various reviews of this topic have been conducted over the past decade, which have arrived at the similar conclusion that strong clinical evidence is lacking. The clinical practice guidelines put forth by the Consortium for Spinal Cord Medicine in 2008 acknowledged that ‘the appropriate resuscitation end point and optimal MAP for maintenance of spinal cord perfusion are not known.’8 A recent systematic review by Casha and Christie9 in 2011 has recommended a target MAP of at least 85 mm Hg for up to 7 days post injury, based on weak evidence.
There is widespread appreciation of the need to avoid hypotension, and there is likely moderate agreement at a practical level about the need to maintain a loosely defined ‘desirable’ MAP in acute SCI patients during their early post-injury management. It is one thing, however, to appreciate and acknowledge ‘practice guidelines’ and ‘recommendations’, and quite another to actually translate and implement these effectively in patients. Despite the fact that numerous reviews have been conducted on the topic of MAP support in acute SCI,10, 11, 12 to our knowledge, there are no published reports that specifically evaluate how successfully target MAPs were maintained in acute SCI patients. Therefore, the purpose of this study was to evaluate the observed MAP in a cohort of prospectively evaluated acute SCI patients, in order to determine how closely the intended MAP was actually achieved in these individuals.
Materials and methods
The acute SCI patients who were evaluated in this study were part of an ongoing prospective observational trial at our institution, in which lumbar catheters were inserted to monitor intrathecal pressure and then simultaneous monitoring of MAP and intrathecal pressure was conducted for 3–5 days post injury. Previously, we have reported on the use of lumbar intrathecal catheters to monitor cerebrospinal fluid (CSF) pressure and to drain CSF to improve spinal cord perfusion pressure (SCPP) in acute SCI.13 While we eliminated from our clinical protocol the aspect of actively draining CSF to improve SCPP, we continued to enrol patients to monitor MAP and CSF pressure, and to support further studies of CSF biomarkers.14
Acute SCI patients with ASIA Impairment Scale (AIS) A, B, or C cervical or thoracic injuries were enrolled if it was possible to obtain a valid baseline neurologic examination from them, obtain consent, and insert a lumbar catheter within 48 h from the time of injury. Patients suffering from concomitant head injuries, intoxication/sedation or other major injuries that precluded a reliable neurologic examination were excluded. Additional inclusion and exclusion criteria are specified in Table 1.
As described in our previous studies,13, 14 intrathecal catheter insertion was performed preoperatively under the supervision of a spine surgeon. Under sterile conditions, a lumbar intrathecal catheter (PERIFIX Custom Epidural Anesthesia Kit, B. Braun Medical Inc., Bethleham, PA, USA) was inserted at L2/3 or L3/4 and advanced 15–20 cm from its entry point. The catheter was then connected to a Becker External Drainage and Monitoring System (Medtronic, Inc., Minneapolis, MN, USA). The monitoring system’s pressure transducer was zeroed at the level of the patient’s right atrium, approximated clinically at the patient’s midaxillary line. The transducer was recalibrated whenever there was a change to the patient’s bed height or inclination. For MAP monitoring, a radial catheter was placed. The transducers for the intrathecal pressure and arterial line were connected to a multichannel monitoring system (SpaceLabs Healthcare, Issaquah, WA, USA) to allow for simultaneous display of intrathecal cerebrospinal fluid pressure (ITP) and MAP.
Whenever clinically possible, MAP and ITP were recorded on at least an hourly basis. The SCPP was calculated to be the difference between MAP and ITP. For the purposes of this trial, we established a target MAP of at least 80 mm Hg. Patients were monitored in either the intensive care unit or a spine step-down nursing unit with a nurse:patient ratio of either 1:1 or 1:2. In both settings, clinical care was directed by an attending critical care physician. The MAP was managed at the critical care physician’s discretion with a combination of volume resuscitation and vasopressors based on objective assessments of effective intravascular volume and adequacy of tissue perfusion. Intrathecal catheters were removed 5 days post insertion.
All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. The clinical trial protocol received approval from our university’s ethics review board (Protocol H08-00673).
Results
A total of 21 patients were enrolled between April 2009 and September 2011 and included in this analysis. The cohort included 10 subjects with cervical injuries and 11 with thoracic injuries. All but one was male. There were 13 patients presenting as AIS A, 4 as AIS B, and 4 as AIS C. The mean age was 45±18 years (mean±s.d.) and ranged from 19–78 years. Demographic details of patients enrolled in the study are shown in Table 2.
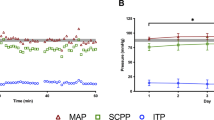
From the 21 patients included in the analysis, we collected a total of 1426 MAP recordings with a mean of 83.7±9.0 mm Hg, 1416 ITP recordings with a mean of 16.7±6.7 mm Hg and 1416 SCPP recordings with a mean of 67.0±11.2 mm Hg (Figures 1a–c).
While the ‘target’ MAP in our study was 80 mm Hg, all 21 subjects had at least one MAP recording below 80 mm Hg. Seventeen patients (81%) had at least one MAP recording below 70 mm Hg. The ITP recordings ranged from 0 mm Hg to 40 mm Hg, as we had previously reported.13 By calculating the SCPP as the difference between MAP and ITP, we observed that 20 patients (95%) had at least one SCPP recording <60 mm Hg, and 17 (81%) had at least one recording <50 mm Hg.
To determine how frequently patients were observed to fall below the target MAP, we looked at the total number of MAP recordings for a particular individual and then calculated the percentage of his/her recordings that were below 80 mm Hg and below 70 mm Hg. Similarly, we assessed how often, per patient, the calculated SCPP values were below 60 mm Hg and 50 mm Hg. The results stratified between cervical and thoracic SCI patients are summarized in Table 3 and illustrated in Figure 2. For cervical SCI (n=10), each patient had an average of 18.4% of all MAP measurements fall below 80 mm Hg, and an average of 3.2% below 70 mm Hg. For thoracic SCI (n=11), each patient had, on average, 35.9% of all MAP measurements fall below 80 mm Hg, and an average of 8.2% below 70 mm Hg.
The results, stratified by severity of neurologic impairment (AIS A versus AIS B/C) are summarized in Table 4 and illustrated in Figure 3. For AIS A (n=13), each patient had an average of 35.8% of all MAP measurements fall below 80 mm Hg, and an average of 7.6% below 70 mm Hg. For AIS B or C (n=8) each patient had, on average, 14.1% of all MAP measurements fall below 80 mm Hg, and an average of 3.0% below 70 mm Hg.
Discussion
The purpose of this study was to determine how successful we really were at maintaining the intended MAP in the acute SCI patients enrolled in our clinical trial. To our knowledge, while a body of literature exists that describes the need for optimizing perfusion and maintaining MAP, a formal assessment of how well the target MAP is actually achieved has not been reported. Here, we had the luxury of prospectively evaluating MAP, SCPP and ITP in this clinical protocol. And although we have previously reported on the surprising changes in ITP after surgical decompression and in the acute post-injury period,13 we had not carefully evaluated how successful we were at maintaining the intended MAP. In this study, it appears that although we successfully achieved an average MAP above 80 mm Hg across all subjects, we were unable to consistently maintain any one subject’s MAP at this level for the duration of the observation period.
A number of studies, including oft-cited reports by Vale et al.6 and Levi et al.5 have prompted many centers to impose a target MAP of 85–90 mm Hg for acute SCI patients.5, 6 Systematic reviews of this literature, including that conducted by the Consortium for Spinal Cord Medicine in their generation of clinical practice guidelines in 2008, have reflected the importance of avoiding hypotension.8 However, the ability to conclusively determine what the optimal MAP should be for maximizing neurologic recovery is hampered by the lack of strong clinical evidence, as pointed out by Casha and Christie9 in their recent systematic review. When we established the research protocol for this initiative at our institution, the uncertainties in determining the optimal MAP from the available clinical literature were recognized, although we acknowledged that a target MAP of at least 85–90 mm Hg had been adopted by many. In discussion with our intensive care physicians, we agreed upon a minimal MAP of 80 mm Hg for 5–7 days. Concerns had been raised around the potential risks of maintaining a MAP of 85–90 mm Hg, with the primary concern being the aggressive use of vasopressors, particularly in elderly individuals with cervical SCI. Hence, this slightly lower target of 80 mm Hg was agreed upon.
Our results are, therefore, quite revealing. In essence, we found that almost all patients had MAP recordings below 80 mm Hg at some point in time during the study, and ∼80% had a MAP recorded below 70 mm Hg. One response to this data would be to say ‘well, we are much more strict about maintaining a MAP target of 90 mm Hg, and so these drops in MAP do not occur in our patients.’ That may well be true, but our note of caution is that we held a similar contention, albeit around an intentionally lower target MAP. Regardless of whether an institution sets the target MAP to 70, 75, 80, 85 or 90 mm Hg for acute SCI patients, the message from our somewhat humbling data is that what the patients actually experience (with respect to MAP) may not be what the clinical team is expecting. Vigilance around this issue will differ from center to center, but we would not have believed that such drops in MAP could happen so frequently if we had not looked this carefully at our own data.
One limitation of this study was that the MAP was recorded typically each hour, and so it is not possible to know exactly how long patients experienced these periods during which the MAP fell below the intended target. Currently, we are developing methods of capturing data electronically directly from the pressure transducers such that MAP measurements can be recorded continuously throughout the course of the study. Nonetheless, despite the fact that there is some potential ‘sampling error’ inherent in recording MAP each hour through the course of a patient’s stay in our ICU or step-down unit, we observed that many of those ‘samples’ fell below what was intended. Owing to the inclusion criteria of the study, the patients who were enrolled generally had isolated SCI and no other major injuries. While our results, therefore, may not be applicable to multitrauma patients, it could be argued that it should have been easier in our isolated SCI patients to maintain their MAP above target levels.
With our current method of collecting data, we were unable to determine the length of time a patients’ MAP might be below 80 or 70 mm Hg, and the clinical significance of having a transient drop in MAP below such levels can be debated. We are, however, conscious of the fact that the spinal cord is very sensitive to changes in blood pressure and perfusion. Hence, even transient drops in perfusion to an already compromised cord may be clinically relevant. Perhaps this is no better illustrated than in human spinal surgery where, in response to a sudden drop in motor or sensory evoked potentials, the anaesthesiologist’s first response is to typically increase the patient’s MAP, and this often quickly restores the electrophysiologic recordings back to normal.15
Obviously, with a mixed cohort of 21 patients with cervical and thoracic injuries of AIS A, B and C severity, we do not have the statistical power to determine whether maintaining or failing to maintain MAP at 80 mm Hg related to long-term outcome.16 The intention of this study was not to try and link MAP or periods of relative hypotension with eventual neurologic recovery. This would have most certainly required a larger cohort of patients with a more homogeneous injury severity. Rather, this was, to some degree, a knowledge translation exercise, to determine how well the ‘knowledge’ (that being a recommended target MAP in acute SCI patients) was actually being implemented in ‘real life’.
It was surprising to us that more of the thoracic SCI patients had MAP recordings that fell below the target than the cervical SCI patients. We expected that due to neurogenic shock, it would have been more challenging to keep the cervical SCI patients above 80 mm Hg. Our observations might be explained by greater clinical vigilance and awareness around maintaining the MAP in cervical SCI patients. Additionally, the majority (8 of 11) thoracic SCI patients suffered AIS A injuries while half of the cervical SCI patients (5 of 10) had AIS A injuries; this may have also manifested in greater difficulties in maintaining the target MAP in the thoracic patients. As Figure 3 illustrates, individuals with AIS A injuries were more likely than those with AIS B or C injuries to experience MAP levels below 80 mm Hg. Furthermore, because many patients with isolated thoracic paraplegia often appear very stable post injury (as compared with the cervical SCI who are often intubated and require more round-the-clock care), we speculate that there is more complacency around their MAP management and less urgency to aggressively drive it up when it drops below 80 mm Hg. The more stable thoracic SCI patients are also likely to be managed in our spine step-down nursing unit where the nurse:patient ratio is 1:2, as compared with the main ICU where it is 1:1. Our impression is that while there is a heightened awareness around neurogenic shock causing a drop in systemic blood pressure, the decreases in MAP below expected levels may also be related to the patients’ level of sedation, analgesia and consciousness.
As ITP is not typically measured in SCI, it is more difficult to interpret the SCPP data. Past traumatic brain injury literature has historically suggested that cerebral perfusion pressures below 60 mm Hg are related to poor outcome.17 While it is recognized that a single cerebral perfusion pressure threshold of 60 mm Hg may be less meaningful than a determination of an individual’s ‘optimal’ cerebral perfusion pressure,18 we decided to use this simply as a cutoff for our analysis of SCPP. All of the patients had calculated SCPP recordings below 60 mm Hg at some point in time, with the vast majority experiencing some moments below 50 mm Hg. Whether ITP monitoring would provide more useful data around cord perfusion than the recorded MAP has yet to be determined.
In summary, we found that our intended maintenance of blood pressure in acute SCI patients was not nearly as consistent as we would have expected, even in the context of a controlled research protocol. We are a level-1 trauma center with considerable experience in the management of acute SCI patients. Therefore, this ‘real-world variability’ is very revealing to us, and may be very informative to other centers that look after this patient population with similar well-intended clinical practice guidelines. Interestingly, this similar variability has been previously recognized in the clinical management of comparable physiologic variables in traumatic brain injury patients. In an analysis of the five largest recruiting centers in a national clinical trial of hypothermia for traumatic brain injury, Clifton et al.19 revealed significant inter-center differences in the management of MAP and cerebral perfusion pressure, again, in a rigorous clinical trial setting among very experienced institutions. These authors contend that such variability in implementing guidelines around MAP control may deleteriously affect the outcome of a traumatic brain injury clinical trial, and it is reasonable to assume that such would be the case for any SCI clinical trial. Clearly, having explicit targets for maintaining MAP are important, but being conscious of the challenges in achieving those targets longitudinally during the early post-injury period is needed to optimize care for the acute SCI patient.
Data archiving
There were no data to deposit.
References
Kwon B . Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004; 4: 451–464.
Felleiter P, Müller N, Schumann F, Felix O, Lierz P . Changes in the use of the methylprednisolone protocol for traumatic spinal cord injury in Switzerland. Spine 2012; 37: 953–956.
Furlan JC, Noonan V, Cadotte DW, Fehlings MG . Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma 2011; 28: 1371–1399.
Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, Harrop JS et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 2012; 7: e32037.
Levi L, Wolf A, Belzberg H . Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery 1993; 33: 1007–1016 (discussion 1016–7).
Vale FL, Burns J, Jackson AB, Hadley MN . Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J. Neurosurg 1997; 87: 239–246.
Rivlin AS, TATOR CH . Regional spinal cord blood flow in rats after severe cord trauma. J Neurosurg 1978; 49: 844–853.
Consortium for Spinal Cord Medicine. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2008; 31: 403–479.
Casha S, Christie S . A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28: 1479–1495.
Bernhard M, Gries A, Kremer P, Böttiger BW . Spinal cord injury (SCI)—prehospital management. Resuscitation 2005; 66: 127–139.
Blood pressure management after acute spinal cord injury. Neurosurgery 2002; 50: S58–S62.
Ploumis A, Yadlapalli N, Fehlings MG, Kwon BK, Vaccaro AR . A systematic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal Cord 2009; 48: 356–362.
Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine 2009; 10: 181–193.
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg 2007; 89: 2440–2449.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2006; 45: 190–205.
Clifton GL, Miller ER, Choi SC, Levin HS . Fluid thresholds and outcome from severe brain injury. Crit Care Med 2002; 30: 739–745.
Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury*. Crit Care Med 2012; 40: 2456–2463.
Clifton GL, Choi SC, Miller ER, Levin HS, Smith KR Jr, Muizelaar JP et al. Intercenter variance in clinical trials of head trauma--experience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg 2001; 95: 751–755.
Acknowledgements
We wish to acknowledge support from the Michael Smith Foundation for Health Research, the Rick Hansen Man in Motion Research Fund, the VGH/UBC Hospital Foundation and the Vancouver Coastal Health Research Institute. Dr Dvorak is the Paetzold Chair in Spinal Cord Injury Research. Dr Kwon is the Canada Research Chair in Spinal Cord Injury and a Scholar of the Michael Smith Foundation for Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kong, C., Hosseini, A., Belanger, L. et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord 51, 466–471 (2013). https://doi.org/10.1038/sc.2013.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.32
Keywords
This article is cited by
-
All over the MAP: describing pressure variability in acute spinal cord injury
Spinal Cord (2022)
-
Neurorestorative interventions involving bioelectronic implants after spinal cord injury
Bioelectronic Medicine (2019)
-
Monitoring spinal cord hemodynamics and tissue oxygenation: a review of the literature with special focus on the near-infrared spectroscopy technique
Spinal Cord (2019)
-
The differential effects of norepinephrine and dopamine on cerebrospinal fluid pressure and spinal cord perfusion pressure after acute human spinal cord injury
Spinal Cord (2017)
-
Topological data analysis for discovery in preclinical spinal cord injury and traumatic brain injury
Nature Communications (2015)