Abstract
Reciprocal interactions between endothelial cells (ECs) and adipocytes are fundamental to maintain white adipose tissue (WAT) homeostasis, as illustrated by the activation of angiogenesis upon WAT expansion, a process that is impaired in obesity. However, the molecular mechanisms underlying the crosstalk between ECs and adipocytes remain poorly understood. Here, we show that local production of polyamines in ECs stimulates adipocyte lipolysis and regulates WAT homeostasis in mice. We promote enhanced cell-autonomous angiogenesis by deleting Pten in the murine endothelium. Endothelial Pten loss leads to a WAT-selective phenotype, characterized by reduced body weight and adiposity in pathophysiological conditions. This phenotype stems from enhanced fatty acid β-oxidation in ECs concomitant with a paracrine lipolytic action on adipocytes, accounting for reduced adiposity. Combined analysis of murine models, isolated ECs and human specimens reveals that WAT lipolysis is mediated by mTORC1-dependent production of polyamines by ECs. Our results indicate that angiocrine metabolic signals are important for WAT homeostasis and organismal metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Image source data and Excel files of all data presented in graphs within the figures and extended data figures have been supplied in Source Data files. Separate files for each figure have been supplied.
References
Kruger-Genge, A., Blocki, A., Franke, R. P. & Jung, F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20184411 (2019).
Graupera, M. & Claret, M. Endothelial cells: new players in obesity and related metabolic disorders. Trends Endocrinol. Metab. 29, 781–794 (2018).
Hasan, S. S. & Fischer, A. The endothelium: an active regulator of lipid and glucose homeostasis. Trends Cell Biol. 31, 37–49 (2021).
Arany, Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451, 1008–1012 (2008).
Fan, Z. et al. Exercise-induced angiogenesis is dependent on metabolically primed ATF3/4(+) endothelial cells. Cell Metab. 33, 1793–1807 (2021).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Eelen, G., Treps, L., Li, X. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis updated. Circ. Res 127, 310–329 (2020).
Kobialka, P. & Graupera, M. Revisiting PI3-kinase signalling in angiogenesis. Vasc. Biol. 1, H125–H134 (2019).
Bilanges, B., Posor, Y. & Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 20, 515–534 (2019).
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012).
Angulo-Urarte, A. et al. Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nat. Commun. 9, 4826 (2018).
Hare, L. M. et al. Heterozygous expression of the oncogenic Pik3ca(H1047R) mutation during murine development results in fatal embryonic and extraembryonic defects. Dev. Biol. 404, 14–26 (2015).
Kobialka, P. S. H. et al. Low dose AKT inhibitor miransertib cures PI3K-related vascular malformations in preclinical models of human disease. Preprint at bioRxiv https://doi.org/10.1101/2021.07.16.452617 (2021).
Serra, H. et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat. Commun. 6, 7935 (2015).
Lesche, R. et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32, 148–149 (2002).
Claxton, S. et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis 46, 74–80 (2008).
Sanz, E. et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl Acad. Sci. USA 106, 13939–13944 (2009).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Rupnick, M. A. et al. Adipose tissue mass can be regulated through the vasculature. Proc. Natl Acad. Sci. USA 99, 10730–10735 (2002).
Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 18, 478–489 (2013).
Herold, J. & Kalucka, J. Angiogenesis in adipose tissue: the interplay between adipose and endothelial cells. Front. Physiol. 11, 624903 (2020).
Robciuc, M. R. et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724 (2016).
Sung, H. K. et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 17, 61–72 (2013).
Elias, I. et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61, 1801–1813 (2012).
Rudnicki, M. et al. Endothelial-specific FoxO1 depletion prevents obesity-related disorders by increasing vascular metabolism and growth. eLife https://doi.org/10.7554/eLife.39780 (2018).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Carracedo, A. et al. A metabolic prosurvival role for PML in breast cancer. J. Clin. Invest 122, 3088–3100 (2012).
Enguix, N. et al. Mice lacking PGC-1β in adipose tissues reveal a dissociation between mitochondrial dysfunction and insulin resistance. Mol. Metab. 2, 215–226 (2013).
Collins, S. & Surwit, R. S. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog. Horm. Res 56, 309–328 (2001).
Lee, M. J., Wu, Y. & Fried, S. K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 34, 1–11 (2013).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science https://doi.org/10.1126/science.aal2379 (2017).
Cleaver, O. & Melton, D. A. Endothelial signaling during development. Nat. Med. 9, 661–668 (2003).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing m2-like macrophage polarization. Cell Metab. 31, 1136–1153 (2020).
Crewe, C. et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708 (2018).
Casero, R. A. Jr., Murray Stewart, T. & Pegg, A. E. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat. Rev. Cancer 18, 681–695 (2018).
Arruabarrena-Aristorena, A., Zabala-Letona, A. & Carracedo, A. Oil for the cancer engine: the cross-talk between oncogenic signaling and polyamine metabolism. Sci. Adv. 4, eaar2606 (2018).
Pegg, A. E. Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912 (2016).
Kraus, D. et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508, 258–262 (2014).
Ramos-Molina, B., Queipo-Ortuno, M. I., Lambertos, A., Tinahones, F. J. & Penafiel, R. Dietary and gut microbiota polyamines in obesity- and age-related diseases. Front Nutr. 6, 24 (2019).
Pirinen, E. et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol. Cell Biol. 27, 4953–4967 (2007).
Sadasivan, S. K. et al. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharm. 729, 94–99 (2014).
Gao, M. et al. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem. Biophys. Res. Commun. 505, 93–98 (2018).
Rajeeve, V., Pearce, W., Cascante, M., Vanhaesebroeck, B. & Cutillas, P. R. Polyamine production is downstream and upstream of oncogenic PI3K signalling and contributes to tumour cell growth. Biochem. J. 450, 619–628 (2013).
Zabala-Letona, A. et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 547, 109–113 (2017).
Meana, C. et al. Functional effects of polyamines via activation of human β1- and β2-adrenoceptors stably expressed in CHO cells. Pharm. Rep. 62, 696–706 (2010).
Gomez-Salinero, J. M., Itkin, T. & Rafii, S. Developmental angiocrine diversification of endothelial cells for organotypic regeneration. Dev. Cell 56, 3042–3051 (2021).
Ding, B. S. et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 (2011).
Ding, B. S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102 (2014).
Cao, Z. et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–365 (2014).
Ottone, C. et al. Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 16, 1045–1056 (2014).
Lorenz, L. et al. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature 562, 128–132 (2018).
Singhal, M. et al. Temporal multi-omics identifies LRG1 as a vascular niche instructor of metastasis. Sci. Transl. Med. 13, eabe6805 (2021).
Liu, Z. et al. Glycolysis links reciprocal activation of myeloid cells and endothelial cells in the retinal angiogenic niche. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay1371 (2020).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Schoors, S. et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520, 192–197 (2015).
Wong, B. W. et al. The role of fatty acid β-oxidation in lymphangiogenesis. Nature 542, 49–54 (2017).
Chen, J. et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci. Adv. https://doi.org/10.1126/sciadv.abd7819 (2021).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science https://doi.org/10.1126/science.abc8479 (2021).
Bordallo, C. et al. Putrescine modulation of acute activation of the β-adrenergic system in the left atrium of rat. Eur. J. Pharm. 598, 68–74 (2008).
Sanchez, M. et al. Role of beta-adrenoceptors, cAMP phosphodiesterase and external Ca2+ on polyamine-induced relaxation in isolated bovine tracheal strips. Pharm. Rep. 62, 1127–1138 (2010).
Ma, L. et al. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci. 265, 118739 (2021).
Zhang, L., Gong, H., Sun, Q., Zhao, R. & Jia, Y. Spermidine-activated satellite cells are associated with hypoacetylation in ACVR2B and Smad3 binding to myogenic genes in mice. J. Agric Food Chem. 66, 540–550 (2018).
Eilken, H. M. et al. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun. 8, 1574 (2017).
Czyzyk, T. A. et al. κ-Opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J. 24, 1151–1159 (2010).
Nogueiras, R. et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J. Neurosci. 29, 5916–5925 (2009).
Czyzyk, T. A. et al. Mice lacking delta-opioid receptors resist the development of diet-induced obesity. FASEB J. 26, 3483–3492 (2012).
Canto, C. & Garcia-Roves, P. M. High-resolution respirometry for mitochondrial characterization of ex vivo mouse tissues. Curr. Protoc. Mouse Biol. 5, 135–153 (2015).
Deberardinis, R. J., Lum, J. J. & Thompson, C. B. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J. Biol. Chem. 281, 37372–37380 (2006).
Acknowledgements
We thank members of the Endothelial Pathobiology and Microenvironment Group for helpful discussions. We thank the CERCA Program/Generalitat de Catalunya and the Josep Carreras Foundation for institutional support. The research leading to these results has received funding from la Fundación BBVA (Ayuda Fundacion BBVA a Equipos de Investigación Científica 2019, PR19BIOMET0061) and from SAF2017-82072-ERC from Ministerio de Ciencia, Innovación y Universidades (MCIU) (Spain). The laboratory of M.G. is also supported by the research grants SAF2017-89116R-P (FEDER/EU) co-funded by European Regional Developmental Fund (ERDF), a Way to Build Europe and PID2020-116184RB-I00 from MCEI; by the Catalan Government through the project 2017-SGR; PTEN Research Foundation (BRR-17-001); La Caixa Foundation (HR19-00120 and HR21-00046); by la Asociación Española contra el Cancer-Grupos Traslacionales (GCTRA18006CARR, also to A.C.); European Foundation for the Study of Diabetes/Lilly research grant, also to M.C.); and by the People Programme (Marie Curie Actions; grant agreement 317250) of the European Union’s Seventh Framework Programme FP7/2007-2013 and the Marie Skłodowska-Curie (grant agreement 675392) of the European Union’s Horizon 2020 research. The laboratory of A.C. is supported by the Basque Department of Industry, Tourism and Trade (Elkartek) and the department of education (IKERTALDE IT1106-16), the MCIU (PID2019-108787RB-I00 (FEDER/EU); Severo Ochoa Excellence Accreditation SEV-2016-0644; Excellence Networks SAF2016-81975-REDT), La Caixa Foundation (ID 100010434), under the agreement LCF/PR/HR17, the Vencer el Cancer foundation and the European Research Council (ERC) (consolidator grant 819242). CIBERONC was co-funded with FEDER funds and funded by Instituto de Salud Carlos III (ISCIII). The laboratory of M.C. is supported by the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement 725004) and CERCA Programme/Generalitat de Catalunya (M.C.). The laboratory of D.S. is supported by research grants from MINECO (SAF2017-83813-C3-1-R, also to L.H., cofounded by the ERDF), CIBEROBN (CB06/03/0001), Government of Catalonia (2017SGR278) and Fundació La Marató de TV3 (201627-30). The laboratory of R.N. is supported by FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (RTI2018-099413-B-I00 and and RED2018-102379-T), Xunta de Galicia (2016-PG057 and 2020-PG015), ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement 810331), Fundación BBVA, Fundacion Atresmedia and CIBEROBN, which is an initiative of the ISCIII of Spain, which is supported by FEDER funds. The laboratory of J.A.V. is supported by research grants from MICINN (RTI2018-099250-B100) and by La Caixa Foundation (ID 100010434, LCF/PR/HR17/52150009). P.M.G.-R. is supported by ISCIII grant PI15/00701 cofinanced by the ERDF, A Way to Build Europe. Personal support was from Marie Curie ITN Actions (E.M.), Juan de la Cierva (IJCI-2015-23455, P.V.), CONICYT fellowship from Chile (S.Z.), Vetenskapsradet (Swedish Research Council, 2018-06591, L.G.) and NCI K99/R00 Pathway to Independence Award (K99CA245122, P. Castel).
Author information
Authors and Affiliations
Contributions
E.M., M.C., A.C. and M.G. conceived the project. E.M., P.V., L.G., A.Z.-L., A.M.-R., J.L.L., D.B., L.G., I.C., S.Z., P.G.-P. O.O.-C., L.M., A.M.-G., S.D.C., N.M.-M, P. Castel, L.V.-J., I.G.-G. and S.F.-R. performed experiments and analyzed data with the supervision of J.V., D.S., L.H., R.B., P.G.-R., R.N., P. Cohen, M.C., A.C. and M.G. E.M., P.V., L.G., S.D.C., A.C. and M.G. wrote the manuscript and designed the Figures. L.H., D.B., R.N., P.G.-R., M.C., A.C. and M.G. provided funding.
Corresponding author
Ethics declarations
Competing interests
M.G. has a research agreement with Merck and Venthera. None of those have a relationship with adipose tissue vasculature. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editor: Christoph Schmitt.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
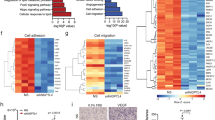
Extended Data Fig. 1 Validation of Pten deletion in PteniΔEC mice.
a) Enrichment ratios of expression of VE-cadherin (Cdh5) and tissue-specific gene in endothelial cell-specific mRNA preparations from eWAT, BAT, muscle and liver after ribosome immunoprecipitation from RibotagiHAEC mice (eWAT n = 3, BAT, muscle and liver n = 2). (b) Representative images of flat-mounted eWAT from 5-week-old control RibotagiHAEC mice stained with HA (white), ICAM2 (green) and isolectin (IB4) (blue). White islets show high magnification of selected regions shown below. (c) Quantification of Pten mRNA levels in WAT, BAT liver and muscle endothelium from RibotagiHAEC and PteniΔEC- RibotagiHAEC at 5 weeks of age (n = 6 per group). (d-g) Western blot analysis of Pten expression in (d) adipose-EC obtained from control (n = 5) and PteniΔEC mice (n = 6), (e) lung-EC obtained from control (n = 6) and PteniΔEC mice (n = 6), (f) muscle-EC obtained from control (n = 4) and PteniΔEC mice (n = 5) and (g) BAT-EC obtained from control (n = 3) and PteniΔEC mice (n = 6). Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 1c).
Extended Data Fig. 2 Endothelial Pten loss leads to reduced fat mass, and it is not associated with fibrosis, altered differentiation or cell death.
(a) Tissue weight in control and PteniΔEC mice at 12 weeks of age (control n = 8, PteniΔEC n = 6). (b and c) Body composition analysis of control and PteniΔEC mice at 14 weeks (control n = 7, PteniΔEC n = 5) by MRI. (d and e) Gene expression analysis of fibrosis markers in whole tissue extracts from (d) eWAT (control n = 4, PteniΔEC n = 6) and (e) iWAT (control n = 5, PteniΔEC n = 6) from control and PteniΔEC mice at 12 weeks of age. (f) Representative microscopic images showing in vitro adipocyte differentiation from preadipocytes (contained in the stromal vascular fraction (SVF) obtained from iWAT depots from 6-week-old control and PteniΔEC mice. A representative image of cultured SVF prior to differentiation is shown to the left. (g and h) Gene expression analysis of adipocyte differentiation markers in whole tissue extracts from (g) eWAT (control n = 5, PteniΔEC n = 4) and (h) iWAT (control n = 4, PteniΔEC n = 5) from control and PteniΔEC mice at 12 weeks of age. (i and j) Representative confocal images of (i) eWAT and (j) iWAT sections stained with cleaved caspase-3 (red) and DAPI (white) from 12-week-old control and PteniΔEC mice. (k) Levels of leptin in serum of control and PteniΔEC mice at 14 weeks of age (control n = 5, PteniΔEC n = 4). Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 2b, c, d, e, g, k).
Extended Data Fig. 3 Molecular and histological vascular characterization in several tissues of control and PteniΔEC mice.
(a) Representative images of CD31-stained blood vessels (white) in sections from brain, muscle, liver and heart and IB4-stained blood vessels (white) in cryosections of BAT and from control (upper panels) and PteniΔEC mice (lower panels) at 12 weeks of age. (b) Quantification of vessel density expressed as percentage of total area in brain, muscle, liver, heart, and BAT sections from control and PteniΔEC mice at 12 weeks of age (brain n = 8, muscle, liver and heart control n = 4, PteniΔEC n = 3, BAT n = 4). (c) Gene expression analysis of endothelial markers Pecam1 (left graph) and Cdh5 (right graph) in whole tissue extracts from control and PteniΔEC mice at 5 weeks of age (BAT n = 5, muscle n = 4, liver n = 8, heart n = 4, lung n = 9, intestine n = 5, eWAT=5, iWAT=5). (d) VegfA gene expression analysis in whole tissue extracts from eWAT and iWAT from control (n = 5) and PteniΔEC (n = 9) mice at 12 weeks of age. (e) Representative images of CD31 (white) and Ter119 (red) stained sections of control and PteniΔEC eWAT at 12 weeks of age. (h) Representative hematoxylin and eosin staining of BAT sections from control and PteniΔEC mice at 12 weeks of age. Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 3b, c, d).
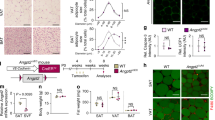
Extended Data Fig. 4 In vivo characterization upon endothelial Pten loss.
(a,i) Scheme illustrating the experimental protocol. (b) Representative images of eWAT from control and PteniΔEC mice at 14 and 19 weeks of age. (c) eWAT pad weight at 14 (control n = 15, PteniΔEC n = 16) and 19 (control n = 13, PteniΔEC n = 10) weeks of age, (d) Quantification of adipocyte area of eWAT at 14 (control n = 8, PteniΔEC n = 10) and 19 (control n = 7, PteniΔEC n = 8) weeks of age, (e) iWAT pad weight at 14 (control n = 14, PteniΔEC n = 16) and 19 (control n = 13, PteniΔEC n = 10) weeks of age from control and PteniΔEC mice. (f) Tissue weight in control and PteniΔEC mice at 14 weeks of age (n = 11). (g) Confocal images of eWAT whole-mount staining with IB4 (red) from control and PteniΔEC mice at 14 and 19 weeks of age. (h) Quantification of IB4-positive area in eWAT depots from control and PteniΔEC mice at 14 (n = 6 per group) and 19 weeks of age (n = 7 per group). (j) Representative images of eWAT from control and PteniΔEC mice at 5 and 12 weeks of age. (k) eWAT pad weight in control and PteniΔEC mice at 5 (control n = 9, PteniΔEC n = 10) and 12 (control n = 7, PteniΔEC n = 5) weeks of age. (l) Quantification of adipocyte area of eWAT from control and PteniΔEC mice at 5 (n = 4) and 12 (n = 4) weeks of age. (m) iWAT pad weight in control and PteniΔEC mice at 5 (control n = 9, PteniΔEC n = 10) and 12 (control n = 7, PteniΔEC n = 5) weeks of age. (n) Tissue weight in control and PteniΔEC mice at 12 weeks of age (control n = 7, PteniΔEC n = 5). (o) Confocal images of eWAT whole-mount staining with IB4 (red) from control and PteniΔEC mice at 5 and 12 weeks of age. (p) Quantification of IB4-positive area in eWAT depots from control and PteniΔEC mice at 5 and 12 weeks of age (control n = 7, PteniΔEC n = 5). Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 4c, d, e, f, h, k, l, m, n, p).
Extended Data Fig. 5 Low endothelial Pten expression correlates with high vascular content in mouse and human white adipose tissue.
(a) Representative confocal images of wild-type eWAT whole-mount staining with IB4 (red) after 12 weeks of chow diet or HFD. (b) Quantification of IB4 positive area in eWAT from wild-type mice exposed to chow diet or HFD for 12 weeks (chow diet n = 4, HFD n = 5). (c) In vivo Pten mRNA expression levels in adipose endothelial cells from wild-type RibotagiHAEC mice after exposure to chow diet or HFD for 12 weeks (chow diet n = 5, HFD n = 4). (d) Representative confocal images of visceral adipose tissue (VAT) whole-mount stained with CD31 (red) from non-obese and patients with obesity. (e) Quantification of vessel branches in VAT whole-mount stained with CD31 from non-obese and patients with obesity (non-obese n = 6, obese n = 10). (f) Pten mRNA expression levels in VAT tissue extracts from non-obese and patients with obesity (non-obese n = 5, obese n = 6). Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 5b, c, e, f).
Extended Data Fig. 6 Low endothelial Pten expression correlates with improved glucose systemic metabolism in mice.
(a) Tissue weight in control and PteniΔEC mice exposed to HFD for 10 weeks (control n = 9, PteniΔEC n = 4). (b) Insulin tolerance test (ITT) performed in control and PteniΔEC mice fed with HFD for 10 weeks (control n = 13, PteniΔEC n = 10). Bars to the right show AUC quantification of blood glucose monitoring during ITT (control n = 13, PteniΔEC n = 10). (c) Basal insulin levels and (d) HOMA-IR index in control and PteniΔEC mice fed with HFD for 10 weeks (control n = 6, PteniΔEC n = 4). (e) Masson trichrome fibrosis staining in eWAT and iWAT sections from control and PteniΔEC mice exposed to HFD for 10 weeks. (f) Gene expression analysis of fibrosis markers in whole tissue extracts from eWAT (control n = 7, PteniΔEC n = 4) from control and PteniΔEC mice exposed to HFD for 10 weeks. (g) Tissue weight in control and PteniΔEC mice exposed to HFD for 34 weeks (control n = 9, PteniΔEC n = 8). (h) ITT performed in control and PteniΔEC mice fed with HFD for 30 weeks (control n = 10, PteniΔEC n = 9). Bars to the right show AUC quantification of blood glucose monitoring during ITT. (i) Basal insulin levels and (j) HOMA-IR index in control and PteniΔEC mice fed with HFD for 34 weeks (control n = 9, PteniΔEC n = 8). (k) Masson trichrome fibrosis staining in eWAT and iWAT sections from control and PteniΔEC mice exposed to HFD for 34 weeks. Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 6a, c, d, f, i, j) and 2-way ANOVA with Bonferroni correction (Extended Data Fig. 6b, h).
Extended Data Fig. 7 Pten-null ECs proliferation depends on lipid oxidation.
(a-c) Endothelial expression of lipid catabolic genes analysed by qPCR and normalized by mL32 gene expression in (a) BAT (control RibotagiHAEC n = 5, PteniΔEC- RibotagiHAEC n = 4), (b) muscle (control RibotagiHAEC n = 5, PteniΔEC- RibotagiHAEC n = 5), (c) and liver (control RibotagiHAEC n = 5, PteniΔEC n = 4) from control RibotagiHAEC and PteniΔEC- RibotagiHAEC mice at 5 weeks of age. (d) Glucose uptake measurement in control and PteniΔEC endothelial cells (control n = 6, PteniΔEC n = 8). (e and f) In vitro expression analysis of (e) lipid catabolic genes and (f) Cd36 by qPCR in control and PteniΔEC lung-derived primary endothelial cells (lung-EC) (control n = 4, PteniΔEC n = 6). (g) Fatty Acid β-Oxidation (FAO) in lung-EC from control and PteniΔEC mice (n = 8 per group). (h) OCR in control and PteniΔEC lung-EC in lipid-enriched medium. (i) Bars show OCR quantification from control and PteniΔEC lung-EC in lipid-enriched medium (control n = 8, PteniΔEC n = 10). (j) Tissue weight in control and PteniΔEC mice treated with vehicle or etomoxir for 5 weeks (control - vehicle n = 15, control - etomoxir n = 9, PteniΔEC - vehicle n = 11, PteniΔEC - etomoxir n = 10). (k) Representative images of IB4 stained blood vessels (red) in flat-mounted eWAT from control, PteniΔEC, PteniΔEC-Pgc1βiΔEC and Pgc1βiΔEC mice at 10 weeks of age. (l) Quantification of IB4 positive area in eWAT from control, PteniΔEC, PteniΔEC-Pgc1βiΔEC and Pgc1βiΔEC mice at 10 weeks of age (control n = 9, PteniΔEC n = 6, PteniΔEC-Pgc1βiΔEC n = 8, Pgc1βiΔEC n = 5). (m) eWAT pad weight of control PteniΔEC, PteniΔEC-Pgc1βiΔEC and Pgc1βiΔEC mice at 10 weeks of age (control n = 18, PteniΔEC n = 5, PteniΔEC - Pgc1βiΔEC n = 10, Pgc1βiΔEC n = 10). Data represent mean ± SEM (error bars). Samples represent biological replicates. All statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 7a, b, c, d, e, f, e, i, j) and one-way ANOVA with Bonferroni correction (Extended Data Fig. 7l, m).
Extended Data Fig. 8 Loss of endothelial Pten results in increased polyamine levels.
(a) Schematic representation of control and PteniΔEC eWAT explant experiment. eWAT from 5 weeks of age control and PteniΔEC mice were cut in ~25 mg pieces, placed in 500ul of DMEM low glucose + 0,5% FFA free BSA, and incubated for the indicated time points. (b) Experimental design of conditioned media collection and fractioning. A 3 kDa cut off filter retained the protein and vesicle fraction, whereas the metabolite-rich fraction was collected as the flow-through. The lipolytic effect of both fractions was tested on eWAT explants of wild-type mice. (c) Label-free 13C fraction of metabolites related to polyamines biosynthesis in adipose-ECs from control and PteniΔEC mice (n = 6 per group) from Fig. 6g. MET: Methionine; SAM: S-Adenosylmethionine; dcSAM: Decarboxylated S-adenosylmethionine; MTA: 5´ Methylthioadenosine. Data represent mean ± SEM (error bars). Statistical analysis was performed by t-test (Extended Data Fig. 8c).
Extended Data Fig. 9 Loss of endothelial Pten results in increased label-free polyamine levels.
(a, b) Schematic representation of the polyamine metabolic pathway and expression of the indicated metabolites in (d) adipose endothelial cells (control n = 6, PteniΔEC n = 6) and (e) eWAT (control n = 8, PteniΔEC n = 7) measured by LCMS. Data represent mean ± SEM (error bars). Statistical analysis was performed by t-test (Extended Data Fig. 9a,b).
Extended Data Fig. 10 Spermidine supplementation in mice exposed to HFD ameliorates glucose systemic metabolism.
(a, b) Positive control of lipolysis in (a) WAT tissue explants and (b) primary adipocytes. (c) Positive control of cAMP production in primary adipocytes. (d) Epinephrine and norepinephrine level in control eWAT measured by LCMS (n = 5). Data represent mean ± SEM (error bars). (e) Tissue weight of wild-type mice fed with HFD and treated with vehicle or spermidine for 6 weeks (vehicle n = 9, spermidine n = 10). (f) GTT performed in wild-type fed with HFD and treated with vehicle or spermidine for 6 weeks (vehicle n = 9, spermidine n = 7). (g) AUC quantification of blood glucose monitoring during GTT. (h) Food intake measurement in wild-type mice fed with HFD and treated with vehicle or spermidine for 6 weeks (n = 7). (i) Body weight curve of wild-type mice fed with HFD and treated with vehicle or spermidine with pair-feeding conditions after week 10. PF, Pair feeding applied to control mice (n = 7). (j) Body weight at sacrifice values of chow and HFD wild-type mice used for experiments represented in Fig. 7k (n = 12 per group). (k) Amd1 mRNA expression levels analysed by qPCR normalized by mL32 gene expression in eWAT endothelium from control RibotagiHAEC and PteniΔEC- RibotagiHAEC (control RibotagiHAEC n = 7, PteniΔEC- RibotagiHAEC n = 8). (l) Western blot analysis of Amd1, S6K (phosphorylated and total) in adipose-derived primary endothelial cells from PteniΔEC mice after treatment with vehicle (DMSO) or mTOR inhibitor (rapamycin, 1 μm) (n = 6). Red arrowhead shows residual phosphorylated Akt signal. (c) Quantification of Amd1 signal obtained in immunoblots showed in (m) (vehicle n = 6, rapamycin n = 5). Data represent mean ± SEM (error bars). Samples represent biological replicates. Statistical analysis was performed by the two-sided Mann-Whitney test (Extended Data Fig. 10a, b, c, e, g, h, j, m) and ANOVA with Bonferroni correction (Extended Data Fig. 10f, i).
Supplementary information
Supplementary Information
This file contains four sets of data: (1) List of reagents; (2) List of primers; (3) List of antibodies; and (4) Information about the human cohorts.
Source data
Figure 1
Source data Fig. 1
Figure 2
Source data Fig. 2
Figure 3
Source data Fig. 3
Figure 4
Source data Fig. 4
Figure 5
Source data Fig. 5
Figure 6
Source data Fig. 6
Figure 7
Source data Fig. 7
Figure 8
Source data Fig. 8
Source data Extended Data Fig. 1
Source data Extended Data Fig. 1.
Source data Extended Data Fig. 2
Source data Extended Data Fig. 2.
Source data Extended Data Fig. 3
Source data Extended Data Fig. 3.
Source data Extended Data Fig. 4
Source data Extended Data Fig. 4.
Source data Extended Data Fig. 5
Source data Extended Data Fig. 5.
Source data Extended Data Fig. 6
Source data Extended Data Fig. 6.
Source data Extended Data Fig. 7
Source data Extended Data Fig. 7.
Source data Extended Data Fig. 8
Source data Extended Data Fig. 8.
Source data Extended Data Fig. 9
Source data Extended Data Fig. 9.
Source data Extended Data Fig. 10
Source data Extended Data Fig. 10.
Rights and permissions
About this article
Cite this article
Monelli, E., Villacampa, P., Zabala-Letona, A. et al. Angiocrine polyamine production regulates adiposity. Nat Metab 4, 327–343 (2022). https://doi.org/10.1038/s42255-022-00544-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00544-6
This article is cited by
-
Hepatic nutrient and hormone signaling to mTORC1 instructs the postnatal metabolic zonation of the liver
Nature Communications (2024)
-
The evolving functions of the vasculature in regulating adipose tissue biology in health and obesity
Nature Reviews Endocrinology (2023)
-
Endothelial cell-derived stem cell factor promotes lipid accumulation through c-Kit-mediated increase of lipogenic enzymes in brown adipocytes
Nature Communications (2023)
-
Deletion of endothelial leptin receptors in mice promotes diet-induced obesity
Scientific Reports (2023)
-
A new signal that shrinks fat
Nature Metabolism (2022)