Abstract
Patients with multiple myeloma-bearing translocation t(11;14) have recently been shown to benefit from the apoptosis-inducing drug venetoclax; however, the drug lacks FDA approval in multiple myeloma thus far due to a potential safety signal in the overall patient population. Selinexor is an inhibitor of nuclear export that is FDA-approved for patients with multiple myeloma refractory to multiple lines of therapy. Here, we report that in four patients with multiple myeloma with t(11;14), the concomitant administration of venetoclax and selinexor was safe and associated with disease response. Moreover, the combination was synergistic in t(11;14) multiple myeloma cell lines and caused decreased levels of Cyclin D1 (which is overexpressed due to the CCND1-IGH fusion) when given in combination as compared to single agents. These data suggest that the combination of venetoclax and selinexor is effective and t(11;14) may serve as a therapeutic marker for response and target for future clinical trials.
Similar content being viewed by others
Introduction
In the t(11;14)(q13;q32) translocation, CCND1 at chromosome 11q13 is juxtaposed to the IGH gene at chromosome 14q32, resulting in overexpression of the protein Cyclin D11,2,3,4. Screening of multiple myeloma (MM) cell lines for sensitivity to venetoclax showed that high sensitivity was restricted to cell lines with CCND1 translocations5,6. Venetoclax induces apoptosis by acting as a BH3-mimetic to inhibit the anti-apoptotic factor BCL2 and is approved for use in certain B-cell malignancies and acute myeloid leukemia7. Venetoclax has been studied in combination with bortezomib and dexamethasone (versus placebo with bortezomib and dexamethasone) for relapsed/refractory myeloma; however, the benefit in progression-free survival (PFS) in the venetoclax arm was offset by increased mortality, thus hindering its path to approval8. A subset analysis showed that patients who possessed t(11;14) and/or high BCL2 expression had clinical responses and longer PFS without increased mortality, leading to cautious off-label use of the medication in select patients. Selinexor is an inhibitor of nuclear export (SINE) that blocks the cargo-binding pocket of XPO1. XPO1 recognizes cargo proteins bearing a nuclear export signal and shuttles them out of the nucleus. These cargoes include tumor-suppressor proteins such as p53, as well as proteins binding to select mRNA including BCL29. Cyclin D1 is a known cargo protein of XPO110,11. Cyclin D1 is essential in the regulation of the cell cycle, and its overexpression can result in uncontrolled cell growth, contributing to cancer development and progression12,13,14. Selinexor is approved for patients with myeloma who have received at least four prior lines of therapy and are refractory to two proteasome inhibitors, two immunomodulating therapies, and a CD38-targeted monoclonal antibody15,16.
We report here, four patients with relapsed/refractory t(11;14) MM who had progression of disease after multiple lines of treatment and were considered for venetoclax treatment based on previous data showing efficacy of venetoclax in t(11;14) MM17. All patients responded initially to venetoclax but ultimately developed resistance and progressive disease. The addition of selinexor recaptured responses and lead to clinical benefit, suggesting a synergistic effect of the combination. The combination of venetoclax and selinexor was further studied in MM cell lines with and without t(11;14) translocations and showed enhanced synergy in those cell lines bearing the CCND1-IGH translocation.
Results
Patients with relapsed and refractory multiple myeloma with t(11;14) tolerate and respond to selinexor and venetoclax combination
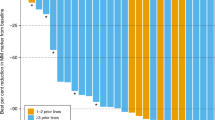
A 55–60-year-old man with free kappa light-chain MM, R-ISS stage 1, and standard risk cytogenetics with t(11;14); (Fig. 1a) was treated with selinexor and venetoclax off-label therapy. The patient had progression of disease (POD) through seven lines of therapy which are depicted in Fig. 1b. The patient’s best response to therapy was a very good partial response (VGPR) with daratumumab, pomalidomide, and dexamethasone (dara-PD). He otherwise had minimal response (MR) to prior regimens. He completed 25 cycles of dara-PD with eventual POD. Due to lack of other therapeutic options, he received venetoclax 400 mg daily and achieved a partial response (PR), then a VGPR with the addition of bortezomib 1.3 mg/m2 every two weeks and dexamethasone 12 mg weekly. The patient had 17 cycles of treatment before POD, at which point bortezomib was substituted for carfilzomib with continued POD. The patient was enrolled onto a clinical trial of a BCMAxCD3 bi-specific T-cell engager with POD. Given prior resistance to venetoclax-based therapy, he was given selinexor 60 mg weekly in combination with venetoclax 400 mg daily and dexamethasone 40 mg weekly with subsequent VGPR. His kappa light-chain levels correlated with response and are shown in Fig. 1c. The patient had a 10-month duration of response. Upon progression, the patient was then treated with commercial chimeric antigen receptor T-cell (CAR-T) therapy.
Histopathologic findings in bone marrow biopsy at diagnosis showed extensive involvement by plasma cell myeloma. Neoplastic plasma cells were small and mature in appearance and present in a diffuse interstitial distribution comprising ~50–60% of overall marrow cellularity. Hematoxylin and eosin stain, ×400 magnification (a, left). Immunohistochemistry (IHC) for CD138 highlights neoplastic plasma cells present in abnormal clusters, ×400 magnification (a, middle). Fluorescence in situ hybridization (FISH) studies showed CCND1-IGH fusion in 95% of cells (yellow fusion signals indicated by arrow; a, right). Timeline of prior treatments and free light-chain response for Patient 1 (b, c), Patient 2 (d, e), Patient 3 (f, g) and Patient 4 (h, i). V bortezomib, C cyclophosphamide, D dexamethasone, R lenalidomide, P pomalidomide, K carfilzomib, HDC high dose cyclophosphamide, Dara daratumumab, Ven venetoclax, Sel selinexor, Ixa ixazomib, Elo elotuzumab, ACST autologous stem cell transplant, Benda bendamustine.
An additional three patients with t(11;14) MM were treated with the combination of selinexor and venetoclax. The summary of their treatment history and responses to therapy are outlined in Fig. 1. Patient 2 had a PR to therapy with a duration of 4 months; patient 3 had a MR to therapy with a duration of 3 months; and patient 4 had a VGPR to therapy with a duration of 6 months. Patient 3 enrolled on clinical trial after progression on selinexor and venetoclax. Patients 2 and 4 are now being considered for CAR-T therapy due to the progression of disease on the combination. We have treated a total of four patients with the combination of venetoclax and selinexor, and three patients had a PR or better.
Overall, the combination of selinexor and venetoclax was well tolerated. Selinexor was prescribed at 60 mg or 80 mg weekly, and venetoclax was prescribed at either 400 mg or 800 mg daily based upon tolerance and efficacy. One patient had dose interruption of selinexor due to fatigue and dyspnea, however she was able to resume therapy without further issues. No other dose interruptions or reductions were required. Leukopenia, neutropenia, and thrombocytopenia were observed, but there was no incidence of bleeding or febrile neutropenia. Hyponatremia was also observed, however, did not require intervention and resolved.
Multiple myeloma cell lines with t(11;14) show high levels of synergism between selinexor and venetoclax in vitro
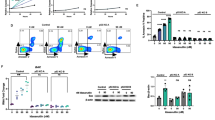
To further test our hypothesis of the preferential effects of selinexor and venetoclax in patients bearing the t(11;14) translocation, we tested the synergy of the combination on human MM cell lines at various concentrations and then examined cell viability 72 h after treatment. MM cell lines with and without the t(11;14) translocation are shown in Supplementary Table 1. The combination of selinexor and venetoclax showed synergy in all multiple myeloma cell lines tested (Fig. 2a–e and Supplementary Fig. 1a–e). U266-B1, bearing the t(11;14) translocation, showed a higher level of synergy in comparison to the non-t(11;14) translocation cell line RPMI-8226 (Fig. 2c). We tested two additional cell lines and saw similar results of higher level of synergy in the t(11;14) translocation cell line in comparison to the non-t(11;14) cell line (Supplementary Fig. 1c).
RPMI-8226 and U266-B1 were treated with increasing doses of selinexor and venetoclax for 72 h, and cell viability was measured using CellTiter Glo. Contour plots were calculated using the Bliss Independence model and were generated using the Synergy Finder web application. Red indicates synergism and green indicates antagonism (a, b). The synergy of RPMI-8226 and U266-B1 was compared using Combination Index (CI) values. The difference was measured using Student’s t test (c). RPMI-8226 and U266-B1 synergy was also calculated using the CompuSyn software. Combination Index values >1 indicates antagonism, =1 indicates additivity, <1 indicates synergy (d, e). RPMI-8226 and U266-B1 were treated with selinexor (200 nM) and venetoclax (1 µM) for 24 h and subjected to a western blot using various antibodies as indicated (f). The normalized protein levels of Cyclin D1, XPO1, MCL-1, p65, and p53 was calculated by the intensity of the western blot bands using ImageJ software (g, h).
We performed a western blot to determine the key protein changes with treatment in RPMI-8226 and U266-B1 (Fig. 2f). We found that the t(11;14) cell line, U266-B1, was far more sensitive to the combination treatment of selinexor and venetoclax than RPMI-8226. Given that Cyclin D1 is known to be overexpressed in t(11;14), we measured Cyclin D1 levels and found that in RPMI-8226 (non-t(11;14)) Cyclin D1 was not expressed with any of the treatments; however, in U266-B1 (t(11;14)) Cyclin D1 was overexpressed but was decreased with selinexor, and the reduction was enhanced with the combination treatment (Fig. 2g). We then measured XPO1 protein levels because it is inhibited by selinexor and prior studies show decreased XPO1 expression after XPO1 inhibition18. As expected, with selinexor we saw a reduction in XPO1 protein levels that was further reduced with the combination in U266-B1 but no difference in selinexor and combination was seen in RPMI-8226. We then assessed the effects of treatment on cargo proteins that are known to be regulated by XPO1 and found an increase in tumor-suppressor p53 levels and a decrease in p65 levels with the treatment of selinexor and combination in both cell lines (Fig. 2h). Treatment of the multiple myeloma cell with venetoclax showed an upregulation of MCL-1 but was mitigated with the combination of selinexor and venetoclax. This effect was greater in U266-B1 and could explain the increased synergy alongside the combinatorial effects on Cyclin D1. An additional western blot was performed with treatment in non-t(11;14) cell line, OPM2, and t(11;14) cell line, KMS12BM (Supplementary Fig. 1f). Cyclin D1, MCL-1, and p65 levels all significantly decreased with the combination treatment (Supplementary Fig. 1g–h).
Combination selinexor and venetoclax therapy leads to decreased tumor volume and increased survival in an in vivo xenograft mice model
The effect of the combination of selinexor and venetoclax therapy in patients with multiple myeloma with t(11;14) we described above may have been due to either single drug alone; however, the in vitro synergy led us to hypothesize that the combination effect would be greater than the individual agents in vivo as well. We therefore utilized a xenograft multiple myeloma mouse model of the t(11;14) myeloma cell line KMS12BM. After tumor engraftment, mice were randomized to receive vehicle, selinexor, venetoclax or the combination of selinexor and venetoclax. Overall, the treatments were well tolerated, and the mice received continuous treatment until the vehicle recipients were euthanized due to advanced tumor growth at 17 days. At this timepoint, the combination-treated group showed a significant decrease in tumor volume when compared to the other groups (P < 0.0001). There was a significant increase in overall survival of the mice in the combination treatment group compared to the other groups (P = 0.0004; Fig. 3).
Tumor volume measurements from mice randomized to receive treatment with vehicle, selinexor, venetoclax or the combination of selinexor and venetoclax (a). The combination-treated group showed a significant decrease in tumor volume when compared to the other groups (P = < 0.0001). Mice were treated with selinexor (5 mg/kg) three times per week, venetoclax (100 mg/kg) daily, or the combination of the drugs on the same dose and schedule. Both drugs were given by oral gavage and vehicle-treated mice were treated with both vehicles on the same schedule as the treated groups. The overall survival by Kaplan–Meier estimator is shown in (b). There was a significant increase in the survival of the combination-treated group when compared to the other groups (P = 0.0004). Log-rank test was used to compare survival.
Discussion
In the past decade, the treatment landscape for MM has dramatically changed with proteasome inhibitors, immunomodulatory agents, and antibody therapies becoming the mainstays of therapy. Despite these innovations, cures for multiple myeloma remain elusive. However, successful management of the disease requires multiple lines of therapy and new drugs with novel mechanisms of action. Selinexor inhibits nuclear export with activity in penta-refractory MM. Venetoclax is approved in other hematologic malignancies and shows promise in a subset of MM patients with t(11;14). The combination of selinexor and venetoclax has shown preclinical synergy in other cancer types19,20 and is in Phase 1b clinical trials for relapsed, refractory Non-Hodgkin’s lymphoma or acute myeloid leukemia (NCT03955783; NCT04607772). To our knowledge, this is the first report of patients with MM treated with the combination of selinexor and venetoclax.
Four patients with t(11;14) MM with CCND1-IGH fusion confirmed by FISH who were relapsed or refractory to prior therapies were able to achieve responses with the combination of selinexor and venetoclax, with two patients achieving a VGPR. Importantly, both patients progressed through prior venetoclax-based regimens, yet they still responded to a selinexor and venetoclax combination. Neither patient received single-agent selinexor, so we do not know whether the combination was required for the response. However, the doses of selinexor used were lower than that on the FDA label for use in refractory MM. Alongside the in vitro and in vivo synergy findings shown here, this suggests that there is an effect of the combination allowing for lower dosing of selinexor. Since adverse effects of selinexor have been shown to be dose-dependent, combining selinexor with venetoclax potentially allows for a more tolerable and therapeutic option for patients, such as the ones described, that are older, are heavily pre-treated, or not candidates for further intensive therapy. This may also serve as an option for patients who may not have access to clinical trials for relapsed and refractory disease or for certain reasons request a strictly oral regimen. It could also act as a bridge to therapies that require manufacturing time, such as CAR-T therapy.
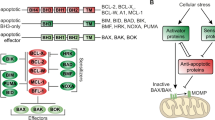
The synergistic mechanism of the combination and selective preference in t(11;14) multiple myeloma is an area of ongoing investigation. However, our preliminary studies suggest a role for Cyclin D1 itself as a target of selinexor and synergistic effect of the combination with venetoclax. Cyclin D1 is a cargo protein of XPO1, the direct target of selinexor, but the activity of venetoclax against Cyclin D1 expression is not well defined. Previous studies have correlated venetoclax sensitivity to the expression of BCL2, especially in cell lines or patients who possess t(11;14)21. Positive correlation between Cyclin D1 and BCL2 and the role in oncogenesis has been described in other tumor types22,23. Our results did not show a difference in BCL2 levels after the combination therapy but other studies suggest the balance of BCL2 and other anti-apoptotic proteins, such as BCL-XL and MCL-1 against the pro-apoptotic proteins is more important than BCL2 expression levels alone24,25,26,27. Accordingly, we saw increases in MCL-1 levels with venetoclax monotherapy that was abrogated by the addition of selinexor. This effect was seen in both t(11;14) and non-t(11;14) myeloma cell lines but was more significantly decreased in the t(11;14). While MCL-1 has not been reported to be a direct cargo of XPO1, others have reported decreased MCL-1 levels by selinexor therapy potentially by disrupting protein and downregulated expression of mRNA28,29. Expression of MCL-1 is also regulated by NFκB signaling, and we observed decreases in NFκB p65 after combination therapy.
In conclusion, though limited to four patients, we observed responses in four out of four patients the administration of selinexor and venetoclax combination was safe in t(11;14) translocated relapsed and refractory MM patients. Preclinical studies in a xenograft mouse model of t(11;14) multiple myeloma also showed combination efficacy and tolerability. Based on these results, we are planning a prospective clinical trial to test the effectiveness of venetoclax and selinexor combination in t(11;14) multiple myeloma patients.
Methods
Patients
Patients received treatment under regular clinical care but retrospectively provided written informed consent to be included in this study. The Sylvester Comprehensive Cancer Center (SCCC) institutional review board approved the study. Responses were assessed by IMWG criteria30.
Animals
Three-month-old female NSG-SGM3 mice were used for xenograft studies approved by the University of Miami Institutional Animal Care and Use Committee (IACUC) guidelines (Protocol #20-079).
Cell lines
RPMI-8226 and U266-B1 cell lines were purchased from ATCC (Manassas, VA). OPM2 and KMS12BM cell lines were a kind gift from Dr. Leif Bergsagel from Mayo Clinic. Cell lines were tested for mycoplasma with the MycoAlert mycoplasma detection kit (Catalog # LT07-218, Lonza, Morristown, NJ). RPMI-8226 and OPM2 were cultured in RPMI-1640 with 10% FBS. U266-B1 and KMS12BM were cultured in RPMI-1640 with 15% FBS and 20% FBS, respectively. Cell lines were maintained at 37 °C in a CO2 incubator and passaged every 2–3 days. Selinexor (Catalog # S7252, KPT-330) and venetoclax (Catalog # S8048, ABT-199) were obtained from Selleck Chemicals (Houston, TX).
Cell viability assays
Cell lines were seeded at 1 × 104 cells/well in a 6 × 6 matrix of white, clear-bottom 96-well plates. Each cell line was exposed to increasing concentrations of each drug and a vehicle-only control (DMSO). After 72 h of incubation at 37 °C in a 5% CO2 incubator, the effects of cell viability were measured using CellTiter-Glo viability assay according to the manufacturer’s instructions (Catalog # G7573, Promega, Madison, WI). Synergy was analyzed via the Bliss independence model using Synergy Finder software (synergy.fimm.fi) as well as via the Chou–Talalay method by using CompuSyn software (combosyn.com). Synergy Finder model synergy score values above 10 indicates synergistic effects and values below −10 indicates antagonistic drug effects. CompuSyn combination index (CI) values CI < 1 are synergistic, CI = 1 is additive, and CI > 1 are antagonistic.
Immunoblotting
Cells were plated at 1 × 106 cells/well in a six-well plate. Whole-cell lysates were prepared with IP lysis buffer supplemented with protease and phosphatase inhibitors (Thermo Scientific, Waltham, MA). Protein concentration was determined using BCA Protein Assay kit (Thermo Scientific, Waltham, MA). Ten micrograms of total protein was separated by electrophoresis on a 4–12% bis-tris protein gel and transferred onto PVDF membranes and probed with antibodies against Cyclin D1 (Catalog # 554181, BD Biosciences, San Jose, CA); XPO1 and p53 (Catalog # sc-5595 and sc-263 Santa Cruz Biotechnology, Dallas, TX); and MCL-1 and p65 (Catalog # 4572 and 4764 Cell Signaling Technologies, Danvers, MA), β-actin (Catalog # A1978 Sigma Aldrich) all were used at 1:1000 dilution in 1% BSA. Membranes were visualized by Clarity Western ECL substrate (BioRad, Hercules, CA) following the manufacturer’s protocol. The following treatments were used: DMSO-only control, selinexor (200 nM), venetoclax (1 µM), and combination. β-actin was used as a loading control. Treatment of U266-B1 and RPMI-8226 was 24 h and treatment of KMS12BM and OPM2 was 16 h. Western blot quantification was performed using ImageJ and normalized against β-actin. All blots were derived from the same experiment and processed in parallel. Uncropped blot images are provided in Source Data File.
Xenograft studies and in vivo treatments
The NSG-SGM3 mice were weighed, followed by anesthetized by exposure to 1–5% vaporized isoflurane in 100% oxygen. One million KMS12BM cells were injected into the mice subcutaneously. The following drugs were administered with a minimum of nine mice per experimental group: a vehicle on the same schedule as the treated groups, selinexor (5 mg/kg; oral gavage three times a week), venetoclax (100 mg/kg; oral gavage daily), and the combination on the same schedule. Mice were monitored daily, and tumor burden was measured using high-frequency ultrasound (Vevo3100, Visualsonics). Mice were sacrificed when tumor size reached >2 mm3 or if weight loss was >20% for two consecutive days.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data will be made available by contacting the corresponding authors.
References
Bergsagel, P. L. et al. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc. Natl Acad. Sci. USA 93, 13931–13936 (1996).
Fonseca, R. et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood 99, 3735–3741 (2002).
Lakshman, A. et al. Natural history of t(11;14) multiple myeloma. Leukemia 32, 131–138 (2018).
Specht, K. et al. Different mechanisms of cyclin D1 overexpression in multiple myeloma revealed by fluorescence in situ hybridization and quantitative analysis of mRNA levels. Blood 104, 1120–1126 (2004).
Touzeau, C. et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 28, 210–212 (2014).
Touzeau, C., Maciag, P., Amiot, M. & Moreau, P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia 32, 1899–1907 (2018).
Prado, G., Kaestner, C. L., Licht, J. D. & Bennett, R. L. Targeting epigenetic mechanisms to overcome venetoclax resistance. Biochim. Biophys. Acta Mol. Cell Res. 1868, 119047 (2021).
Kumar, S. K. et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 21, 1630–1642 (2020).
Gandhi, U. H. et al. Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma. Clin. Lymphoma Myeloma Leuk. 18, 335–345 (2018).
Alt, J. R., Cleveland, J. L., Hannink, M. & Diehl, J. A. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14, 3102–3114 (2000).
Benzeno, S. & Diehl, J. A. C-terminal sequences direct cyclin D1-CRM1 binding. J. Biol. Chem. 279, 56061–56066 (2004).
Alao, J. P. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol. Cancer 6, 24 (2007).
Musgrove, E. A., Caldon, C. E., Barraclough, J., Stone, A. & Sutherland, R. L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 11, 558–572 (2011).
Qie, S. & Diehl, J. A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 94, 1313–1326 (2016).
Chari, A. et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381, 727–738 (2019).
Grosicki, S. et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet 396, 1563–1573 (2020).
Kumar, S. et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130, 2401–2409 (2017).
Taylor, J. et al. Altered nuclear export signal recognition as a driver of oncogenesis. Cancer Discov. 9, 1452–1467 (2019).
Fischer, M. A. et al. Venetoclax response is enhanced by selective inhibitor of nuclear export compounds in hematologic malignancies. Blood Adv. 4, 586–598 (2020).
Shang, E. et al. Dual Inhibition of Bcl-2/Bcl-xL and XPO1 is synthetically lethal in glioblastoma model systems. Sci. Rep. 8, 15383 (2018).
Punnoose, E. A. et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol. Cancer Ther. 15, 1132–1144 (2016).
Beltran, E. et al. A cyclin-D1 interaction with BAX underlies its oncogenic role and potential as a therapeutic target in mantle cell lymphoma. Proc. Natl Acad. Sci. USA 108, 12461–12466 (2011).
Lin, H. M., Lee, Y. J., Li, G., Pestell, R. G. & Kim, H. R. Bcl-2 induces cyclin D1 promoter activity in human breast epithelial cells independent of cell anchorage. Cell Death Differ. 8, 44–50 (2001).
Bose, P., Gandhi, V. & Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 58, 1–17 (2017).
Gupta, V. A. et al. Venetoclax sensitivity in multiple myeloma is associated with B-cell gene expression. Blood 137, 3604–3615 (2021).
Touzeau, C. et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 30, 761–764 (2016).
Tai, Y. T. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014).
Luedtke, D. A. et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via Mcl-1. J. Cell Mol. Med. 22, 6099–6111 (2018).
Zhu, Z. C., Liu, J. W., Yang, C., Zhao, M. & Xiong, Z. Q. XPO1 inhibitor KPT-330 synergizes with Bcl-xL inhibitor to induce cancer cell apoptosis by perturbing rRNA processing and Mcl-1 protein synthesis. Cell Death Dis. 10, 395 (2019).
Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17, e328–e346 (2016).
Acknowledgements
We would like to thank the patients and their families. The work was supported by funding from the American Society of Hematology and the National Institutes of Health (1K08CA230319).
Author information
Authors and Affiliations
Contributions
Conceptualization: N.N., J.H., and J.T. Investigation: S.C., T.T., R.D., E.R., S.M., G.P., A.P., J.A., M.A., J.J., T.B, F.M, D.K, D.B., and J.C. Writing—original draft preparation: N.N., S.C., and J.T. Writing—reviewing and editing: N.N., S.C., F.M., O.L., J.H., and J.T.
Corresponding authors
Ethics declarations
Competing interests
T.B.: AbbVie: membership on an entity’s Board of Directors or advisory committees, speakers bureau; Novartis: consultancy, membership on an entity’s Board of Directors or advisory committees. F.M.: OncLive: honoraria; Medscape: consultancy, honoraria. D.K.: Arcellx: honoraria, membership on an entity’s Board of Directors or advisory committees; BMS: honoraria, membership on an entity’s Board of Directors or advisory committees. O.L.: adaptive: honoraria; binding site: honoraria; BMS: honoraria; Cellectis: honoraria; Amgen: honoraria; Janssen: honoraria; Celgene: research funding; Janssen: other: IDMC; Janssen: research funding; Takeda: other: IDMC; Amgen: research funding; GSK: honoraria. J.T.: Karyopharm: honoraria.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, N., Chaudhry, S., Totiger, T.M. et al. Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t(11;14). npj Precis. Onc. 6, 73 (2022). https://doi.org/10.1038/s41698-022-00315-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-022-00315-2