Abstract
We previously demonstrated that P. aeruginosa isolates that persisted in children with cystic fibrosis (CF) despite inhaled tobramycin treatment had increased anti-Psl antibody binding in vitro compared to those successfully eradicated. We aimed to validate these findings by directly visualizing P. aeruginosa in CF sputum. This was a prospective observational study of children with CF with new-onset P. aeruginosa infection who underwent inhaled tobramycin eradication treatment. Using microbial identification passive clarity technique (MiPACT), P. aeruginosa was visualized in sputum samples obtained before treatment and classified as persistent or eradicated based on outcomes. Pre-treatment isolates were also grown as biofilms in vitro. Of 11 patients enrolled, 4 developed persistent infection and 7 eradicated infection. P. aeruginosa biovolume and the number as well as size of P. aeruginosa aggregates were greater in the sputum of those with persistent compared with eradicated infections (p < 0.01). The amount of Psl antibody binding in sputum was also greater overall (p < 0.05) in samples with increased P. aeruginosa biovolume. When visualized in sputum, P. aeruginosa had a greater biovolume, with more expressed Psl, and formed more numerous, larger aggregates in CF children who failed eradication therapy compared to those who successfully cleared their infection.
Similar content being viewed by others
Introduction
Individuals with cystic fibrosis (CF) are susceptible to pulmonary infection with Pseudomonas aeruginosa due to defects in the cystic fibrosis transmembrane conductance regulator (CFTR) that result in impaired mucociliary clearance1. Without antimicrobial treatment, P. aeruginosa can establish chronic infection in the airways which is associated with more rapid lung function decline and premature death in patients with CF2,3. Early antibiotic eradication treatment (AET) of initial P. aeruginosa colonization is thus standard clinical care of children with CF and increasing the success rate of AET is key in improving long-term outcomes4,5.
There are few explanations as to why AET may fail in a patient with CF. We previously showed, using a biofilm model visualized by confocal microscopy, that initial P. aeruginosa isolates from children who failed AET had significantly increased anti-Psl antibody binding compared to isolates from those who cleared P. aeruginosa6. Increased anti-Psl antibody binding was associated with bacterial aggregation and tobramycin tolerance. Psl is a P. aeruginosa exopolysaccharide (EPS) that has been well described for its role in cell–cell and cell-substrate attachment adhesion and biofilm formation in vitro7,8,9,10,11,12. Although our findings were confirmed in separate collections of early P. aeruginosa clinical isolates from two distinct AET trials, these results were based on in vitro studies of P. aeruginosa in a glass slide chamber system.
Despite the use of clinical isolates and bacterial growth conditions mimicking CF airways, lab-based models examining the behaviour of CF pathogens and the effects of antibiotics may not accurately reflect conditions in patient airways13. Decisions on incubation times, growth media and antimicrobial concentrations, for example, can significantly alter experimental outcomes and it is very challenging to fully capture the complexities of the CF lung environment. With these challenges, it is perhaps not surprising that there is a disconnect between antimicrobial susceptibility testing results and clinical outcomes in CF14.
Recent advancements in the processing and imaging of respiratory tract specimens, however, have allowed the direct visualization and interaction of microbial species in patient samples. The microbial identification passive clarity technique (MiPACT), developed by DePas et al., fixes and visualizes bacteria using hybridization chain reaction to detect rRNA in CF sputum15. These methodologies obviate the need to make inferences in translating in vitro to in vivo results. The goal of this study was to validate our previous findings of increased anti-Psl antibody binding and bacterial aggregation in persistent P. aeruginosa isolates by directly visualizing P. aeruginosa biofilms in sputum in a prospective, observational trial of children with CF undergoing AET. We also aimed to determine whether we could, in fact, replicate in situ results from clinical specimens using our in vitro biofilm slide chamber model.
Results
Patient characteristics
The characteristics of enrolled patients are presented in Table 1. A total of 11 CF patients with new onset P. aeruginosa infection produced a sputum sample before treatment initiation and were included in the study; 7 successfully eradicated P. aeruginosa after 1 month of inhaled tobramycin (no P. aeruginosa positive sputum culture for at least 6 months following the initial positive culture) whereas 4 developed persistent infection despite therapy (P. aeruginosa positive sputum culture in the 6 months following the initial positive culture) (see Methods). Of the 4 who developed persistent infection, 3 had follow up P. aeruginosa isolates available for whole genome sequencing demonstrating persistence of the same strain (less than 10 single nucleotide polymorphism difference between the initial and follow up strain). There were no significant differences between the two groups in terms of demographics such as age, sex, baseline lung function,nutritional status or proportion of patients on CFTR modulator therapy. Of note, there was no significant difference in the proportion of patients with an initial mucoid P. aeruginosa isolate who eradicated compared to developed persistent infection.
Pseudomonas aeruginosa visualization within sputum
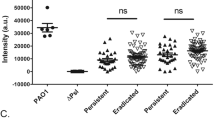
A sputum sample was obtained from each enrolled patient prior to initiation of inhaled tobramycin treatment, and P. aeruginosa was labelled using fluorescence in situ hybridization (FISH) and visualized using confocal microscopy. There was an increased P. aeruginosa biovolume (per sputum slice) as well as an increased number of larger P. aeruginosa aggregates in the sputum of CF children who subsequently failed eradication therapy compared to those who successfully cleared infection (Fig. 1). Representative images are shown in Fig. 2. The amount of P. aeruginosa in each sputum sample was also quantified using quantitative polymerase chain reaction (qPCR); P. aeruginosa concentrations as measured by qPCR did not correlate with P. aeruginosa biovolume visualized directly in fixed sputum specimens (Supplementary Fig. 1). Similarly, there was no correlation between sputum P. aeruginosa biovolume and semi-quantitative measures of P. aeruginosa growth on plating of the original sputum sample in the clinical microbiology laboratory (data not shown).
Patients with persistent infection had greater P. aeruginosa biovolume and greater number of larger aggregates compared to those with eradicated infection. P. aeruginosa visualization in initial sputum sample from negative controls (Negative PA, n = 3), positive controls (Chronic PA, n = 3), CF children who eradicated infection (Eradicated, n = 7) and CF children who developed persistent infection (Persistent, n = 4). Represented median (horizontal line) P. aeruginosa (A) average biovolume intensity (µm3)/sputum slice, (B) average number of aggregates/sputum slice, (C) average maximum aggregate size (µm)/sputum slice. **p < 0.01 using Mann–Whitney test.
Representative 3D confocal images of P. aeruginosa in the sputum of CF patients showing more numerous, larger aggregates in those with persistent compared to eradicated infection. P. aeruginosa structural differences are shown using a fluorescently-labelled molecular probe specific for P. aeruginosa (green), anti-Psl mAb (magenta), DAPI nucleic acid stain (blue) molecular probes and merged image, with ×100 oil objective lens.
Pseudomonas aeruginosa Psl was also visualized within sputum using fluorescently labelled anti-Psl antibody. Figure 3A shows that the amount of Psl antibody binding in sputum was greater in those who developed persistent infection compared to those who eradicated infection. However, once corrected for increased P. aeruginosa biovolume, Psl antibody binding per 100,000 µm3 P. aeruginosa biovolume was slightly higher in the eradicated compared to persistent group (p = 0.04, Fig. 3B). The proportion of anti-Psl antibody co-localized with P. aeruginosa (represented by the M2 coefficient) was similar in both the eradicated and persistent group (Fig. 3C). Figure 3D shows representative images of each category of sputum sample.
Greater overall anti-Psl antibody staining of P. aeruginosa in sputum from patients with persistent compared to eradicated infection. Initial sputum sample from negative controls (Negative PA, n = 3), positive controls (Chronic PA, n = 3), children with CF for whom infection was eradicated (Eradicated, n = 7) and children with CF who developed persistent infection (Persistent, n = 4). Horizontal line indicates the median. (A) average anti-Psl antibody binding intensity (µm3)/sputum slice, (B) average Psl antibody binding per 100,000 µm3 P. aeruginosa biovolume/sputum slice, (C) average proportion of anti-Psl antibody co-localized with P. aeruginosa represented by the M2 coefficient, (D) representative images from sputum from a patient with Persistent, Eradicated and Chronic infection, green: P. aeruginosa, magenta: anti-Psl antibody, white: Psl antibody co-localized with P. aeruginosa. *p < 0.05, ns: not significant using Mann–Whitney test.
Aggregation and antimicrobial resistance of P. aeruginosa isolates in glass slide chamber
To determine whether these P. aeruginosa characteristics observed in children with CF could be replicated in vitro, we then grew the initial P. aeruginosa isolates obtained from these patients for 48 h as biofilms in our glass slide chamber model. As with P. aeruginosa visualized within sputum, Psl antibody binding (per well) was greater in isolates from children who developed persistent infection compared to isolates from those who eradicated infection (Fig. 4A). However, once corrected for P. aeruginosa biovolume, Psl antibody binding per 100,000 µm3 P. aeruginosa biovolume was not different between persistent and eradicated isolates (Fig. 4B). Although the median P. aeruginosa biovolume in the slide chambers after 48 h of growth was greater for persistent compared to eradicated isolates, this difference was not statistically significant (Fig. 4C).
Greater overall anti-Psl antibody staining in glass slide chamber of P. aeruginosa isolates from patients with persistent compared to eradicated infection. Eradicated (n = 7) and persistent (n = 4) P. aeruginosa isolates grown for 48 h as biofilms in glass slide chamber and then labelled with fluorescent anti-Psl antibody for 3 h then visualized with confocal microscopy. Horizontal line, median. (A) average anti-Psl antibody binding intensity (µm3)/well, (B) average Psl antibody binding per 100,000 µm3 P. aeruginosa biovolume/well, (C) average P. aeruginosa biovolume intensity (µm3)/well. *p < 0.05, ns: not significant using Mann–Whitney test.
Given that persistent isolates failed to be cleared by tobramycin therapy in our patients, we aimed to determine whether this tobramycin tolerance could be measured in our in vitro model. Initial P. aeruginosa isolates from patients who subsequently developed persistent (persistent isolates) or eradicated infection (eradicated isolates) were grown for 24 h as biofilms in glass slide chambers and then exposed to high-dose (1000 µg/ml) tobramycin for 24 h. Tobramycin significantly reduced the biovolume of eradicated isolates but not persistent isolates (Fig. 5A). To replicate the CF lung environment, isolates were also grown in the presence of 5% pooled CF sputum supernatant and then treated with tobramycin; again, the biovolume of eradicated isolates was significantly reduced and there was a nonsignificant trend (p = 0.05) to significant reduction in the persistent isolates (Fig. 5A). The metabolic activity of isolates was also measured using an ATP assay and demonstrated significant reduction with tobramycin treatment for eradicated and persistent isolates in both growth conditions (Fig. 5B). The degree of bacterial aggregation (as measured by surface to biovolume ratio) was not different for eradicated and persistent isolates (Fig. 5C).
Differences in tobramycin response and aggregation between persistent and eradicated P. aeruginosa isolates in glass slide chamber. Eradicated (n = 7) and persistent (n = 4) P. aeruginosa isolates grown for 24 h as biofilms in glass slide chamber and then treated with 1000 µg/mL tobramycin for an additional 24 h then visualized with confocal microscopy. LB: Lysogeny Broth alone; + T: plus Tobramycin; SS: in presence of 5% pooled CF Sputum Supernatant. Horizontal line, median. (A) average P. aeruginosa biovolume intensity (µm3)/well, (B) average luminescence in relative light units (RLU) of ATP assay/well, (C) average degree of aggregation measured by surface to biovolume ratio/well. *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant using Mann–Whitney test.
Discussion
To our knowledge, this study is the first to demonstrate that children with CF who fail to eradicate new-onset P. aeruginosa infection with tobramycin therapy are infected with more numerous, larger aggregates of P. aeruginosa expressing increased Psl due to the increased biovolume of P. aeruginosa. In vitro, increased anti-Psl antibody binding was also seen in persistent compared to eradicated isolates but once corrected for biovolume, this difference was not significant; tobramycin resistance and greater bacterial aggregation was not consistently observed in persistent isolates in our glass slide chamber model, highlighting the importance of direct in vivo observation of P. aeruginosa pulmonary infections in CF patients.
Prior studies, including ours, have attempted to identify P. aeruginosa characteristics associated with failure of AET in CF. Phenotypes associated with chronic infection, such as mucoidy status and wrinkly phenotypes typical of biofilm producers, have previously been shown to have a weak association with failure of AET16,17. Surprisingly, an increased amount of P. aeruginosa, as measured by sputum bacterial density counts, for instance, has not been identified as a risk factor for inhaled tobramycin eradication treatment failure. Sputum bacterial density measurements are not performed routinely by the clinical microbiology laboratory for unexpected incident infections and more importantly, are based on planktonic growth of homogenized, serially diluted and plated sputum samples. The increased P. aeruginosa biovolume identified in our patients who went on to develop persistent infection was only noted on direct visualization of their embedded and fixed sputum samples. Sputum P. aeruginosa biovolume did not correlate with either semi-quantitative measures of growth on plates or P. aeruginosa concentrations measured in sputum by PCR.
Through direct visualization of P. aeruginosa within sputum, we were also able to observe more numerous, larger aggregates in those who failed AET. Bacterial aggregation is a well-recognized mechanism of antibiotic tolerance and immune evasion18. There are many factors that contribute to P. aeruginosa aggregation within CF airways. Intrinsic bacterial characteristics include the expression of Psl. Our collaborators have previously shown that P. aeruginosa aggregates in CF sputum express Psl and that such aggregation likely occurs via an EPS-mediated polymer-bridging mechanism19. We noted increased anti-Psl antibody binding both in vivo (in patient sputum) as well as in vitro (isolates cultured in the glass slide chamber model) for patients with CF with persistent compared to eradicated infection. However, in contrast to our previous publication, once adjusted per 100,000 µm3 of P. aeruginosa biovolume, this difference was no longer significant, suggesting that persistent isolates do not have increased expression of Psl per cell, rather there were more bacterial cells, each expressing the same amount of Psl, in persistent compared to eradicated isolates6. In addition to specific P. aeruginosa characteristics, there are extrinsic factors, conditions in the CF airway, that promote bacterial aggregation. For example, Singh et al. demonstrated that secreted neutrophil elastase in sputum supernatant can cleave P. aeruginosa flagella, decreasing motility and triggering aggregation20. Of note, this aggregation can occur despite mutations in biofilm related genes, underscoring the difference between aggregation and biofilm formation. The composition of sputum itself can also affect aggregation as mucin has been shown to enhance aggregation as has the presence of host polymers, via a depletion mechanism21,22. Despite growing our persistent isolates in vitro in pooled CF sputum supernatant (comprising of excreted cellular products but without host or other bacterial cells), we could not replicate the increased aggregation observed in the sputum of patients who developed persistent infection. Similarly, exposure of both eradicated and persistent isolates to tobramycin led to a decrease in metabolic activity, which may represent a bacterial protective response23. Direct analysis of the composition of the sputum from our enrolled patients was beyond the scope of this study, however, future investigation in this area is warranted.
This study used MiPACT to directly visualize bacterial communities in airways24. Other investigators have used these methods to describe the composition of airway infections in patients with CF, with chronic obstructive lung disease (COPD) and with ventilator associated pneumonia (VAP)15,25. The current study, however, is the first to use MiPACT to identify differences in bacterial characteristics that correlate with response to antibiotic therapy. This is of particular importance to CF patients as there has been no test developed to date that can predict clinical outcomes of antibiotic treatment14. The complexities of the CF lung environment, with its unique growth conditions, polymicrobial interactions and drug delivery challenges, are very difficult, if not impossible, to replicate in the laboratory13. Even in our glass slide chamber model based on biofilm growth and incorporating host factors in the form of CF sputum supernatant, we were unable to consistently demonstrate tobramycin tolerance and bacterial aggregation by persistent isolates in vitro. Our prior in vitro studies demonstrating tobramycin tolerance and greater aggregation in persistent P. aeruginosa isolates only did so with the addition of Staphylococcus aureus filtrate, SpA or anti-Psl antibody, the clinical relevance of which is unclear6,26. The use of the MiPACT methodology to visualize other bacterial species and host immune cells as well as their geospatial interaction has the potential to further our understanding of more complicated CF infections such as those associated with pulmonary exacerbations15,27.
There were several limitations to this study. The patient sample size was small and included only older patients who could produce sputum, limiting the generalizability of the findings to CF children with new-onset P. aeruginosa infection detected from throat swabs. Furthermore, obtaining sputum samples has become more difficult with the introduction of CFTR modulator therapy, reducing the ability of CF patients to expectorate28. Despite the small number of patients enrolled, however, statistically significant differences in P. aeruginosa aggregate formation were demonstrated in sputum between the patient groups. In addition, due to the smaller volume of sputum produced by children, there was not enough sample to perform serial dilutions to calculate sputum bacterial density [in colony forming units (CFU)/ml]. However, semi-quantitative measures of P. aeruginosa load identified by plating of the original sputum sample in the clinical microbiology laboratory were available for comparison. Finally, this study focused on characteristics of only P. aeruginosa itself associated with failure of AET but there are likely other factors, such as interactions with other organisms and host responses, that play a role26. Other investigators have demonstrated that polymorphonuclear leukocytes respond aggressively toward a biofilm matrix consisting of both Psl and alginate exopolysaccharides, responses not considered in our current study29.
In conclusion, this study demonstrated that children with CF who failed to eradicate P. aeruginosa respiratory infection with inhaled tobramycin were infected with Psl-expressing P. aeruginosa that form more numerous and larger aggregates than in those who successfully clear their infection. These groups of patients could not reliably be distinguished using standard in vitro methods, highlighting the need for direct visualizations of infections within airways to predict antibiotic response in CF.
Methods
Study design and patient population
This was a prospective, observational study conducted at SickKids (Toronto, Canada) from March 2020–2022. Children with CF were enrolled if they had a new onset P. aeruginosa infection defined as a sputum culture positive for P. aeruginosa with at least 3 preceding negative cultures in the prior 12 months16,30. After enrollment, a sputum culture was obtained prior to starting inhaled tobramycin for 28 days. Per the SickKids AET protocol, if the sputum culture obtained 1 week following the end of inhaled tobramycin treatment was still positive for P. aeruginosa, an additional 28 days of inhaled tobramycin was prescribed; if the sputum culture was still positive for P. aeruginosa following the second round of inhaled tobramycin, the patient was admitted to hospital and intravenous tobramycin and ceftazidime was prescribed for 2 weeks followed by an additional 28 days of inhaled tobramycin31.
AET failure was defined as a P. aeruginosa positive sputum culture in the 6 months following the initial positive culture; all enrolled patients had a minimum of 6 months of follow up time in the study. If a patient failed AET, the initial P. aeruginosa isolate (obtained prior to commencing inhaled tobramycin) was defined as persistent; otherwise, the initial isolate was defined as eradicated.
Negative sputum controls that were negative for P. aeruginosa by qPCR were included from CF children who had never grown P. aeruginosa in their respiratory tract. Positive sputum controls that were positive for P. aeruginosa by qPCR were included from CF children with chronic P. aeruginosa infection32. DNA extraction and qPCR was performed as described previously30.
Sputum processing and imaging
Sputum processing was performed using an adapted MiPACT (tissue clearing) technique, previously described15,24. In brief, expectorated sputum specimens were collected from enrolled patients in a sterile cup and immediately stored at 4 °C. Within 24 h of collection, sputum plugs (approximately 0.2 g) were fixed in 4% paraformaldehyde (PFA), washed with 1× phosphate-buffered saline (PBS) then sectioned into small slices (approximately 5 mm diameter). Individual sputum slices were added to separate wells of a Nunc™ Lab-Tek™ eight-chamber cover glass slide (Thermo Fisher Scientific, Mississauga, ON), consisting of 250 µL hydrogel mixture. The sputum-hydrogel mixture (within the chamber wells) was sealed inside a BD GasPak™ EZ container (VWR, Mississauga, ON) with an anaerobic pack, then allowed to polymerize for 3 h, at 37 °C. Following polymerization, the solidified sample was cleared in 8% sodium dodecyl sulfate (SDS) solution (adjusted to pH 8) for 3–5 days, at 37 °C. After clearance, the sputum hydrogels were washed with 1× PBS and stored in 0.01% (wt/vol) sodium azide solution, at 4 °C until imaging.
To visualize P. aeruginosa and the exopolysaccharide, Psl, within the sputum hydrogels, a fluorescent in situ hybridization (FISH) technique was performed, as previously described24 with minor adjustments. In brief, sputum hydrogels were sectioned into three thin slices (approximately 1 mm thickness ea.), then added to 500 µL of hybridization buffer with 4 µL of 100 µM PsearA-Alexa488 (green) probe. The PsearA probe, targeting the 16S rRNA gene fragment of P. aeruginosa, was allowed to incubate with the sputum hydrogels for 18–24 h, at 46 °C. The sputum hydrogels were rinsed in a wash buffer, then incubated in 1 mL of fresh wash buffer for 6 h, at 48 °C. Following DNA-probe hybridization, sputum hydrogels were washed with 1× PBS and incubated in 2% bovine serum albumin (BSA) solution for 18–24 h, in dark, at room temperature. The next day, sputum hydrogels were removed and added to 500 µL of fresh 2% BSA solution with 4 µL of 2 mg/mL Psl0096-Alexa594 (red) anti-Psl antibody, then incubated for 6 h, in dark, at room temperature. The sputum hydrogels were washed with 1× PBS, then incubated in 250 µL of refractive index matching solution (RIMS) with 4 µL of 10 µg/mL DAPI, in dark, at room temperature with gentle rotation. The sputum hydrogel slices were mounted on a microscope slide and sealed with a 1.7 mm CoverWell™ Perfusion Chamber (Sigma-Aldrich, Oakville, ON).
Images used for analysis were acquired using a Quorum Wave FX Borealis Spinning Disk Confocal Microscope, based on a Yokohama CSU-10 scan head. Fluorescently labeled (red) Psl0096 antibody, PsearA P. aeruginosa (green) probe and DAPI (blue) nucleic acid stain were excited using 561, 491 and 405 nm excitation lines, respectively. The sputum hydrogels were visualized with a 20×/0.75 Zeiss lens, coupled to a 1.6 × magnification coupler. Image acquisition was performed using the Quorum Volocity 6.3 software. A total of 6 z-stack images were acquired per slide in increments of 0.3 µm. All image processing of hydrogel z-stack images was performed using Volocity. P. aeruginosa colonization within sputum was quantified using the biovolume intensity, maximum object size (minimum measurement considered as an aggregate set as 2 µm3) and population number functions. Psl antibody binding within P. aeruginosa biofilms was calculated using the total voxel ratio of the red to green channel. Last, the Manders (M2) coefficient was selected to calculate the degree of Psl to PA co-localization. All images are included in the Supplemental materials as Supplementary Fig. 2.
Three dimensional representative images at 100 × oil immersion were acquired using a Leica SP8 Lightning Confocal STED microscope. The PsearA P. aeruginosa (green) probe, Psl0096 antibody (magenta) and DAPI (blue) nucleic acid stain were excited using 592, 488 and 405 nm excitation lines, respectively. The sputum hydrogels were visualized with a 100×/1.4 (STED) oil lens. Image acquisition and processing was performed using the Leica LAS, Lightning Module, Navigator software. Representative z-stack images of eradicated and persistent P. aeruginosa isolates were captured in increments of 0.25 µm.
Pseudomonas aeruginosa isolate recovery from sputum
A portion of the sputum collected from the study patients was used for P. aeruginosa isolate recovery. Sputum specimens were spread plated on MacConkey agar (with 8% crystal violet), then incubated for 48 h at 42 °C. Individual morphotypes were then grown on blood agar for an additional 24 h at 37 °C. Identification of P. aeruginosa was confirmed by Matrix-assisted desorption/ionization-time of flight (MALDI-ToF) and the isolates were frozen at − 80 °C until subsequent examination via glass slide chamber model and ATP assay. If the isolate could not be recovered from the sputum sample obtained for MiPACT, the initial P. aeruginosa isolate identified by the clinical microbiology laboratory in the initial sputum sample from the patient was recovered for the following experiments.
Pseudomonas aeruginosa biofilm growth in glass slide chamber and imaging
Pseudomonas aeruginosa isolates recovered from the sputum specimens were grown as biofilms using a Nunc Lab-Tek II, 8-chambered cover glass slide (Thermo Fisher Scientific, Mississauga, ON), previously described33. In brief, P. aeruginosa was grown in LB (optimal for growing biofilms in the glass slide chamber) for 24 h at 37 °C with shaking (225 rpm). The culture was diluted 1:100, then grown to an early log phase of 0.1 OD at 600 nm. Of this culture, 200 µL was added to the wells of a glass slide chamber, then incubated for 24 h at 37 °C. The next day, media was removed and either 200 µL of LB alone or LB with tobramycin (final concentration 1000 µg/mL) was added to designated wells, then incubated for 24 h at 37 °C. Following incubation, media was removed and 200 µL of Syto-9™ live-cell fluorescent stain in LB was added to the wells, then incubated for 45 min. The wells were washed 2× with LB and LB was added back into the wells prior to visualization via confocal laser scanning microscopy (CLSM). This procedure was also replicated in the presence of 5% pooled CF sputum supernatant, based on a modified prior protocol34,35; sputum supernatant comprised of excreted cellular products but not host or bacterial cells, given the non-specific binding of Syto-9™ live-cell fluorescent stain. This procedure was repeated to attain 3 biological replicates for each P. aeruginosa isolate.
To measure Psl antibody binding, P. aeruginosa was grown in the same manner to an early log phase of 0.1 OD at 600 nm, of which 220 µL was added to the wells of an 8-chambered cover glass slide and incubated for 24 h at 37 °C. Following incubation, media was removed and fresh LB was added back into the wells, then allowed to incubate for an additional 24 h. The next day, media was removed and either 200 µL of LB alone or LB with Psl0096-Alexa594 (red) anti-Psl antibody36 (final concentration 56 µg/mL) was added to designated wells, then incubated for 90 min at RT. Following incubation, media was removed and 200 µL of Syto-9™ in LB was added to the wells, then incubated for an additional 45 min. The wells were washed 2× with LB before CLSM in LB.
Quantification of biovolume intensity and Psl antibody binding within P. aeruginosa biofilms was calculated, as previously described herein, using Volocity. To measure P. aeruginosa aggregation, surface-to-biovolume ratio was quantified using Comstat2 software as a plugin to ImageJ, as previously described33.
ATP assays of P. aeruginosa biofilms
Cell metabolic activity of P. aeruginosa biofilms was assessed using an adapted ATP assay, previous described6,35. In brief, P. aeruginosa was grown overnight in cation-adjusted Muller-Hinton broth (CAMHB) (standardized media for antimicrobial susceptibility testing) at 37 °C with shaking (225 rpm). The overnight culture was diluted 1:100 in CAMHB, then grown to an early log phase of 0.1 OD at 600 nm. Of this culture, 220 µL was added to the wells of a white Greiner Medium Binding 96-well plate (Sigma-Aldrich, Oakville, ON) in absence or presence of 5% sputum supernatant, then incubated for 24 h at 37 °C without shaking. Following incubation, media was removed and 200 µL of CAMHB in absence or presence of sputum supernatant (final sputum dilution, 5%) and tobramycin (final concentration 1000 µg/mL) was added to designated wells, then incubated for 24 h at 37 °C. The next day, media was removed and the wells were washed 2X with CAMHB and 100 µL of CAMHB was added back into the wells. To measure ATP in the cell-attached fraction of the wells, the base of each well was disrupted by scraping and 100 µL of Bac Titer-Glo™ reagent (Promega, Madison, WI) was added. The plates were gently mixed on an orbital shaker for 10 min in the dark before luminescence reading as per the manufacturer's protocol. This procedure was repeated to attain three biological replicates for each P. aeruginosa isolate.
Genomic analysis
Whole genome sequencing raw data was trimmed using TRIMMOMATIC with parameters LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:80. We used SKA to compare samples within patients, before and after treatment. To identify the multi-locus sequence typing (MLST) profiles we used ska type function and for single nucleotide polymorphism (SNP) detection and distance estimation between samples, we used ska fastq, compare, merge and distance functions. Default parameters were used in every case37,38.
Statistical analysis and institutional approvals
All statistical analyses were done using GraphPad 5.0. Continuous variables were compared using non-parametric Mann–Whitney test; p-value of < 0.05 was considered significant. This study was approved by the SickKids Research Ethics Board (REB#1000079038); written informed consent was obtained from all participants and research conducted in accordance with all relevant guidelines and regulations.
Data availability
All images that support the findings of this study are available in the Supplemental materials as Supplementary Fig. 2. All genomic data are available through BioProject ID: PRJNA867399 (http://www.ncbi.nlm.nih.gov/bioproject/867399).
References
Shteinberg, M., Haq, I. J., Polineni, D. & Davies, J. C. Cystic fibrosis. Lancet 397, 2195–2211. https://doi.org/10.1016/S0140-6736(20)32542-3 (2021).
Pamukcu, A., Bush, A. & Buchdahl, R. Effects of Pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr. Pulmonol. 19, 10–15 (1995).
McColley, S. A. et al. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr. Pulmonol. 52, 909–915. https://doi.org/10.1002/ppul.23715 (2017).
Mogayzel, P. J. Jr. et al. Cystic Fibrosis Foundation pulmonary guideline. Pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann. Am. Thorac. Soc. 11, 1640–1650. https://doi.org/10.1513/AnnalsATS.201404-166OC (2014).
Gascon Casaredi, I. S. M., Waters, V., Seeto, R., Blanchard, A., Ratjen, F. Impact of antibiotic eradication therapy of Pseudomonas aeruginosa on long term lung function in cystic fibrosis. J. Cystic Fibros. (2022) (in press).
Morris, A. J. et al. The role of Psl in the failure to eradicate Pseudomonas aeruginosa biofilms in children with cystic fibrosis. NPJ Biofilms Microbiomes 7, 63. https://doi.org/10.1038/s41522-021-00234-3 (2021).
Jackson, K. D., Starkey, M., Kremer, S., Parsek, M. R. & Wozniak, D. J. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186, 4466–4475. https://doi.org/10.1128/JB.186.14.4466-4475.2004 (2004).
Ma, L., Lu, H., Sprinkle, A., Parsek, M. R. & Wozniak, D. J. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. J. Bacteriol. 189, 8353–8356. https://doi.org/10.1128/JB.00620-07 (2007).
Byrd, M. S. et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 73, 622–638. https://doi.org/10.1111/j.1365-2958.2009.06795.x (2009).
Yang, L. et al. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 13, 1705–1717. https://doi.org/10.1111/j.1462-2920.2011.02503.x (2011).
Chew, S. C. et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. MBio 5, e01536-e11514. https://doi.org/10.1128/mBio.01536-14 (2014).
Ma, L. et al. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5, e1000354. https://doi.org/10.1371/journal.ppat.1000354 (2009).
Waters, V. J. et al. Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Clin. Infect. Dis. 69, 1812–1816. https://doi.org/10.1093/cid/ciz364 (2019).
Somayaji, R. et al. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: A systematic review. J. Cystic Fibros. https://doi.org/10.1016/j.jcf.2019.01.008 (2019).
DePas, W. H. et al. Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. MBio https://doi.org/10.1128/mBio.00796-16 (2016).
Vidya, P. et al. Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 35, 67–74. https://doi.org/10.1007/s10096-015-2509-4 (2016).
Mayer-Hamblett, N. et al. Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin. Infect. Dis. 59, 624–631. https://doi.org/10.1093/cid/ciu385 (2014).
Bjarnsholt, T. et al. The in vivo biofilm. Trends Microbiol. 21, 466–474. https://doi.org/10.1016/j.tim.2013.06.002 (2013).
Jennings, L. K. et al. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 34, 108782. https://doi.org/10.1016/j.celrep.2021.108782 (2021).
Staudinger, B. J. et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 189, 812–824. https://doi.org/10.1164/rccm.201312-2142OC (2014).
Secor, P. R., Michaels, L. A., Ratjen, A., Jennings, L. K. & Singh, P. K. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 115, 10780–10785. https://doi.org/10.1073/pnas.1806005115 (2018).
Landry, R. M., An, D., Hupp, J. T., Singh, P. K. & Parsek, M. R. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol. Microbiol. 59, 142–151. https://doi.org/10.1111/j.1365-2958.2005.04941.x (2006).
Lopatkin, A. J. et al. Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat. Microbiol. 4(12), 2109–2117. https://doi.org/10.1038/s41564-019-0536-0 (2019).
Jackson, L. et al. Visualization of Pseudomonas aeruginosa within the Sputum of Cystic Fibrosis Patients. J. Vis. Exp. JoVE https://doi.org/10.3791/61631 (2020).
Kolpen, M. et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax https://doi.org/10.1136/thoraxjnl-2021-217576 (2022).
Beaudoin, T. et al. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes 3, 25. https://doi.org/10.1038/s41522-017-0035-0 (2017).
O’Toole, G. A. et al. Model systems to study the chronic, polymicrobial infections in cystic fibrosis: Current approaches and exploring future directions. MBio 12, e0176321. https://doi.org/10.1128/mBio.01763-21 (2021).
Waters, V. Beneath the surface: Assessing cystic fibrosis pulmonary infections in the era of highly effective modulator therapy. J. Cyst. Fibros. 20, 723–724. https://doi.org/10.1016/j.jcf.2021.06.008 (2021).
Rybtke, M., Jensen, P. O., Nielsen, C. H. & Tolker-Nielsen, T. The Extracellular Polysaccharide Matrix of Pseudomonas aeruginosa Biofilms Is a Determinant of Polymorphonuclear Leukocyte Responses. Infect. Immun. 89(1), e00631-20. https://doi.org/10.1128/IAI.00631-20 (2020).
Blanchard, A. C. et al. Early detection using qPCR of Pseudomonas aeruginosa infection in children with cystic fibrosis undergoing eradication treatment. J. Cyst. Fibros. https://doi.org/10.1016/j.jcf.2018.02.008 (2018).
Blanchard, A. C. et al. Effectiveness of a stepwise Pseudomonas aeruginosa eradication protocol in children with cystic fibrosis. J. Cyst. Fibros. 16, 395–400. https://doi.org/10.1016/j.jcf.2017.01.007 (2017).
Lee, T. W., Brownlee, K. G., Conway, S. P., Denton, M. & Littlewood, J. M. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Cyst. Fibros. 2, 29–34 (2003).
Morris, A. J., Li, A., Jackson, L., Yau, Y. C. W. & Waters, V. Quantifying the effects of antimicrobials on in vitro biofilm architecture using COMSTAT software. JoVE. https://doi.org/10.3791/61759 (2020).
Kennedy, S. et al. Activity of tobramycin against cystic fibrosis isolates of Burkholderia cepacia complex grown as biofilms. Antimicrob. Agents Chemother. 60, 348–355. https://doi.org/10.1128/AAC.02068-15 (2016).
Beaudoin, T. et al. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci. Rep. 8, 14728. https://doi.org/10.1038/s41598-018-33016-7 (2018).
DiGiandomenico, A. et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 209, 1273–1287. https://doi.org/10.1084/jem.20120033 (2012).
Harris, S. SKA: Split Kmer analysis toolkit for bacterial genomic epidemiology. bioRxiv https://doi.org/10.1101/453142 (2018).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Acknowledgements
This study was funded by the Cystic Fibrosis Foundation (CFF WATERS19AO-1) and Glyconet (ID-03). MRP is funded by the Cystic Fibrosis Foundation (CFF PARSEK17IO).
Author information
Authors and Affiliations
Contributions
A.J.M., S.P., S.E., A.R., L.G. and P.W. performed the experiments. N.M. obtained the sputum samples. A.J.M., S.P., S.E., A.R., L.G., P.W., Y.C.W.Y., P.L.H., M.R.P., L.R.H., D.N., D.S.G., B.C., D.J.W. and V.W. contributed to the study design, data interpretation and analysis, and writing of the manuscript. A.D. contributed experimental materials and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morris, A.J., Yau, Y.C.W., Park, S. et al. Pseudomonas aeruginosa aggregation and Psl expression in sputum is associated with antibiotic eradication failure in children with cystic fibrosis. Sci Rep 12, 21444 (2022). https://doi.org/10.1038/s41598-022-25889-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25889-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.