Abstract
Adipocyte hypertrophy and expression of adipokines in subcutaneous adipose tissue (SAT) have been linked to steatosis, nonalcoholic steatohepatitis (NASH) and fibrosis in morbidly obese (BMI ≥ 40 kg/m2) subjects. It is unknown if this is also true for subjects with NAFLD with lesser degrees of obesity (BMI < 35 kg/m2). Thirty-two subjects with biopsy-proven NAFLD and 15 non-diabetic controls matched for BMI underwent fine-needle biopsies of SAT. Adipocyte volume was calculated. RNA-sequencing of SAT was performed in a subset of 20 NAFLD patients. Adipocyte volume and gene expression levels were correlated to the presence of NASH or significant fibrosis. Subjects with NAFLD had larger adipocyte volume compared with controls, (1939 pL, 95% CI 1130–1662 vs. 854 pL, 95% CI 781–926, p < 0.001). There was no association between adipocyte volume and the presence of NASH. Gene expression of adipokines previously described to correlate with NASH in morbid obesity, was not associated with NASH or fibrosis. Our results suggest that persons with NAFLD have larger SAT adipocytes compared with controls and that adipocytes are involved in the pathophysiology of hepatic steatosis in NAFLD. However, adipocyte volume was not associated with NASH or fibrosis in NAFLD subjects with varying degrees of obesity.
Similar content being viewed by others
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing globally and is today estimated to be approximately 25%1. This development parallels the increase of obesity and type 2 diabetes (T2D)2. NAFLD and T2D share insulin resistance (IR) as a common pathophysiological hallmark3,4. Recent data from the US, Europe, Australia, and New Zeeland shows that NAFLD is the most rapidly increasing indication for liver transplantation5,6,7. However, the majority of NAFLD patients will not develop advanced liver disease8. A major challenge is to identify the minority of NAFLD patients who carries a risk of disease progression9. Although the presence of liver fibrosis is the most important prognostic factor for subsequent morbidity and mortality in liver disease, nonalcoholic steatohepatitis (NASH) has been identified as an important predictor of disease progression10,11,12. The underlying pathogenic mechanisms as to why a subset of patients develop NASH is largely unknown. One hypothesis would be an interplay between the abdominal subcutaneous adipose tissue (SAT) and the liver through the release of adipokines13. Both morphological and physiological characteristics of SAT have been associated to NAFLD development and disease progression14,15. It has been demonstrated that adipocyte size correlates to the level of steatosis partly independent of other traits of the metabolic syndrome16. Further, in studies of morbidly obese patients undergoing bariatric surgery, adipocyte hypertrophy of SAT was associated with a higher prevalence of NAFLD, NASH and hepatic fibrosis17,18. However, it is unknown if this association applies also to patients with NAFLD with a lower body mass index (BMI).
Through the secretion of pro- or anti-inflammatory adipokines, the adipose tissue can exert direct effects on the liver19,20,21. Previous studies have found that the expression of specific adipokines correlate both to the presence of NASH and to hepatic fibrosis in NAFLD22,23,24,25. Despite several promising candidates, no single marker has been identified that alone can predict the presence of NASH or hepatic fibrosis with a high sensitivity. More recent studies have used gene array techniques to identify panels of adipokines that are associated with NASH and hepatic fibrosis26,27. These studies are based on selected cohorts of patients with morbid obesity (BMI ≥ 40) who are undergoing bariatric surgery. It is not known whether these patterns of adipokine expressions can predict presence of NASH or significant hepatic fibrosis in an unselected NAFLD population.
The aim of this study was to investigate the association between adipocyte volume or expression of adipokines in SAT, with features of NASH or hepatic fibrosis in patients with biopsy proven NAFLD.
Results
Cohort characteristics
In total, 32 patients with a biopsy-proven diagnosis of NAFLD were included. Fifty-three percent were male, and the mean age was 55.2 years. In total, 20 (62.5%) subjects had NASH and 18 (56%) had significant or advanced fibrosis (F2-4). Fifteen subjects (47%) had T2D. The prevalence of T2D was higher among subjects with NASH, n = 13 (65%), compared to subjects without NASH, n = 2 (17%), (p = 0.008). HOMA-IR (8.2 vs. 4.7, p = 0.008) and BMI (31.8 vs. 27.9 kg/m2, p = 0.019) was higher in the NASH group compared to non-NASH. Characteristics of the study cohort at the time of SAT biopsy are shown in Table 1.
Adipocyte morphology and different traits of NAFLD
Subjects with NAFLD had significantly larger adipocytes compared to controls matched for BMI in both unadjusted analysis (p < 0.001) and when adjusting for sex, BMI and T2D (p < 0.001) (Table 2A.). In the sensitivity analysis, after excluding subjects with T2D from the NAFLD group, the difference remained significant (1855 pL, 95% CI 1559–2151 in non-diabetic NAFLD vs. 854 pL 95% CI 781–926 in controls; p < 0.001).
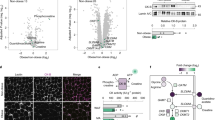
There was a trend towards a significant difference in adipocyte volume between subjects with NASH and non-NASH in the simple regression analysis, but not in the multiple regression adjusting for BMI, sex and T2D (Table 2B and Fig. 1A). No difference in adipocyte volume was detected between subjects with no or low-stage fibrosis compared with those with significant or advanced fibrosis (Table 2C and Fig. 1B). No association was observed between adipocyte volume and HOMA-IR within the NAFLD cohort.
Boxplot showing comparison of adipocyte volume between healthy controls matched for BMI and (A) NASH and non-NASH and (B) no- or mild fibrosis (F0-1) versus significant or advanced fibrosis (F2-4). P at solid brackets denotes significance for multiple regression analysis adjusted for T2D, sex and BMI. P at dashed bracket denotes significance for Kruskal–Wallis test.
Gene expression of adipokines in SAT and prediction of NASH and fibrosis
Applying the previously published 5-gene signature (CCL2, DMRT2, GADD45B, IL1RN, and IL8)26 to our cohort, we found that this model was not able to accurately predict NASH or fibrosis stage (Fig. 2). Next, we evaluated the five genes separately in a multiple logistic regression analysis adjusting for age, sex, BMI and T2D, but no association was found between the expression of either of the genes and presence of NASH or fibrosis stage (data not shown).
Violin plot showing the correlation between a previously published gene expression model and (A) NASH versus non-NASH, and (B) liver fibrosis stage. The y-axis (prediction) shows values calculated as the output of a published statistical model based on the expression of five genes in subcutaneous adipose tissue (CCL2, DMRT2, GADD45B, IL1RN, and IL8) 26.
We also explored our gene expression data from SAT for genes encoding adipokines and proinflammatory cytokines associated with NASH and fibrosis: Adiponectin, Leptin, TNF-α, IL-6, and IL-8. However, none of these adipokines were detected as differentially expressed genes between NASH and non-NASH or fibrosis F0-1 and F2-4 in the RNA-sequencing.
Genes differently expressed in subjects with NASH
Since we found no association between previously described adipokines and presence of NASH or fibrosis, we went on to do an exploratory investigation of the RNA-sequencing data. In total, 12,171 genes were analyzed and 86 showed significant association with NASH status from linear regression analysis. We further performed differential expression analysis on these 86 genes by comparing their expression in NASH subjects versus non-NASH subjects, leading to 31 genes with ANOVA p-value < 0.05. After Benjamini–Hochberg multiple hypothesis correction, 8 genes reached adjusted p-value < 0.05. For a more detailed description of all DEGs in the RNA-sequencing analysis, see Fig. 3 and Supplementary Table.
Discussion
In this cross-sectional study, we evaluated if adipocyte hypertrophy in subcutaneous adipose tissue was more common in subjects with NAFLD or associated with the presence of NASH. It is expected to find adipocyte hypertrophy in obese patients and consequently, in those with an obesity-related disease such as NAFLD28. However, the degree of obesity at which NAFLD occurs varies significantly between individuals29,30. In the present study, all subjects with NAFLD had hypertrophic adipocytes, independently of BMI and the presence of T2D compared with healthy controls. This result is in part congruent with the finding of a Finnish study in which subcutaneous adipose morphology from 119 non-diabetic subjects was correlated to the degree of liver steatosis measured with Magnetic Resonance Spectroscopy. They showed that 21% of the variation in liver fat content was explained by adipocyte size alone16. To our knowledge, our study is the first to confirm these findings in a cohort with biopsy proven NAFLD, which also includes subjects with T2D.
The results of the present study suggest that adipocyte hypertrophy is a common morphologic hallmark of SAT present in NAFLD and of importance for the pathophysiology of the disease. Previous studies have shown that with increasing levels of obesity and IR, hypertrophic adipocytes eventually reach a critical volume31,32. At this stage, lipolysis leads to an increased outflow of FFA to the circulation which can cause deposition of ectopic fat in the liver32,33,34. However, the BMI threshold at which adipocytes reach their maximum volume differs between individuals, just as NAFLD can occur at different degrees of overweight. From our results, we hypothesize that individuals who are inclined to develop adipocyte hypertrophy have a predisposition to develop NAFLD as a part of their lipid dysmetabolism.
We found no association between adipocyte volume in SAT and the presence of NASH or significant or advanced fibrosis, although there was a trend towards a significant difference in adipocyte volume associated with NASH. Our results comply with those of an Australian study on 216 obese subjects undergoing bariatric surgery, with a mean BMI of 46 kg/m2. They found no significant correlation between adipocyte diameter in SAT and histologically defined NASH or fibrosis35. However, in a similar study, adipocyte volume correlated positively to the presence of NASH, but only in females18. They collected histological samples from SAT and liver biopsies from 668 obese patients who underwent bariatric surgery. The population in that study was composed of morbidly obese subjects with a baseline BMI of 47.5 kg/m2 and a high proportion of females (78%). The lack of an association between SAT morphology and NASH or fibrosis suggests that the mechanisms behind liver inflammation and fibrosis is more complex than that of simple steatosis.
Next, we explored the expressions of specific adipokines in SAT and their association with NASH and significant fibrosis. First, we applied the model first described by du Plessis et al.26. We could not replicate any correlation between the previously published score and the presence of NASH or significant fibrosis. Like several other studies investigating the link between adipose tissue and NAFLD, the original trial evaluated subjects undergoing bariatric surgery. The median BMI was 40–42 kg/m2 and the proportion of females was approximately 75%19,27. It should be acknowledged that these studies were meticulously performed and based on large materials. Further, they used validation cohorts to strengthen their findings. However, the characteristics of the study cohorts might not be representative of the typical NAFLD population monitored in an outpatient hepatology clinic. In our opinion, the results from studies on patients with liver steatosis combined with morbid obesity, as in those undergoing bariatric surgery, can be difficult to implement in an ordinary clinical practice. The present results further strengthen that position.
Our study has several strengths. The outcome measurement of NAFLD, NASH and liver fibrosis was confirmed with liver biopsy, the gold standard for characterizing liver histopathology36. The biopsies were blinded and reviewed by an experienced liver pathologist. We included a group of healthy controls matched for age and BMI to compare adipocyte morphology with NAFLD patients. We performed highly accurate determination of gene expression in SAT by using RNA sequencing37,38.
Several weaknesses with our study should be acknowledged. The study cohort includes only 32 subjects, and the RNA-sequencing was performed in a subset of 20 subjects. Since this was an explorative cross-sectional study without an á priori hypothesis on the gene expression outcome, we were not able to do a calculation of statistical power. Therefore, a type II error regarding small differences in gene expressions between subgroups cannot be excluded. Likewise, our finding of a few genes associated with NASH should be interpreted with caution. The method for measuring adipocyte volume is user dependent. To address this, all samples were measured and reviewed by a single investigator (MH) and then checked for accuracy by an independent collaborator blinded to the clinical outcome. The measurements of absolute cell volume cannot be directly compared to similar analysis from other groups due to possible differences in the handling of biopsies and fixation of histopathology slides.
Conclusion
Our findings establish an association between subcutaneous adipocyte hypertrophy and NAFLD, but not to NASH. It supports the theory that adipocyte hypertrophy is an important factor in the pathophysiology of NAFLD. Prediction models of SAT gene expression seen in morbidly obese subjects undergoing bariatric surgery cannot be translated into a typical cohort of NAFLD patients. Our finding demands for further research focusing on the interplay between NAFLD and gene expressions of subcutaneous adipose tissue in large cohorts of patients recruited from clinical settings.
Material and methods
Study population
Between May 2016 and April 2018 patients with biopsy proven NAFLD who were followed at the outpatient clinic at the Department of Upper GI, Karolinska University Hospital, were screened for study participation. Exclusion criteria were: (1) alcohol consumption ≤ 140 g/week for women or ≤ 210 g/week for men, (2) treatment with either insulin, Glucagon-like peptide 1-analogue or glitazones, (3) chronic liver disease other than NAFLD, (4) pregnancy or lactation, (5) coagulopathy or treatment with anti-coagulants, and (6) reduction of BMI ≥ 1,5 kg/m2 since time of liver biopsy. All participants signed an informed consent, and the study was approved by the Regional Ethics Committee in Stockholm, Sweden (Dnr 2011/13–31/1). All interventions were made in accordance with local regulations at Karolinska University hospital regarding clinical research and with the declaration of Helsinki. At the visit for the SAT biopsy, data on age, sex, body mass index (BMI), presence of T2D, homeostatic model assessment for insulin resistance (HOMA-IR) and alanine aminotransferase (ALT) were collected. For comparison of SAT adipocyte size, a group of 15 healthy controls were selected from a cohort of non-diabetic subjects that has been described in a previous study39 and were matched on BMI (to the nearest value within +/− 1.0 kg/m2) in a 1:1 ratio to 15 of the NAFLD subjects. In the original study, the controls were not screened for NAFLD, but were non-diabetic (mean fP-insulin = 6.0 ± 3.3 mU/l), non-obese and with normal measures of body composition (mean waist-to-hip ratio = 0.88 ± 0.05 and mean body fat = 22 ± 8 kg). These characteristics of metabolically healthy individuals indicate a very low likelihood of them having NAFLD. Baseline characteristics of the 15 controls included in the present study are reported in Table 1.
Liver biopsies and histological assessment
All participants had previously been diagnosed with NAFLD through percutaneous liver biopsy as part of a clinical work-up within 12 months from inclusion. All biopsies were reassessed by an expert liver pathologist (OD) blinded to clinical data. The NAFLD activity score (NAS) was calculated as the unweighted sum of the degree of steatosis (0–3), lobular inflammation (0–3) and hepatocellular ballooning (0–2) according to Kleiner et al.40. NASH was diagnosed according to the fatty liver inhibition of progression (FLIP) algorithm, as the presence of both fat, lobular inflammation, and ballooning41. Fibrosis stage was assessed according to the classification by Kleiner40.
Subcutaneous adipose tissue biopsies and fat cell measures
Participants were examined after an overnight fast. Approximately 2 g of SAT was aspirated under sterile conditions using a 14 G puncture needle and a 10 ml Hepafix® aspiration syringe (Braun, Kornberg, Germany). SAT was placed on a nylon filter net (Sefar™, Heiden, Switzerland) and washed with 0.9% sterile saline solution. About 500 mg of SAT was snap frozen in liquid nitrogen and stored in − 80 °C freezer for later RNA-sequencing. The remaining SAT was fixed in formalin and embedded in paraffin. Paraffin-embedded SAT was cut in Sects. 7–10 µm thick, mounted on microscopy slides, and stained with hematoxylin & eosin using standard protocols. The CellInsight™CX5 system (Thermo Fisher Scientific, Waltham, US) was used to generate images of histological sections. Mean adipocyte diameter was measured using ImageJ software with the Watershed adipocyte segmentation plugin. Adipocyte volume was calculated by the formula described by Goldrick42. The rationale for this formula has been described previously31,43. In brief, the diameter of a sphere is calculated by the formula \(\pi \times \frac{{d^{3} }}{6}\). The mean diameter of adipocytes in a histological section (d) is a normally distributed variable but its cube (d3) is skewed, and the arithmetic mean of d3 can therefore not be used to calculate mean fat cell volume. The average fat cell volume is instead better approximated by using the formula \(\frac{\pi }{6}\left( {3\sigma^{2} \times d^{2} } \right)d\) where σ is the variance of the diameter.
RNA-sequencing – measurement of adipokines in SAT
Raw reads from RNA-seq data were aligned to human genome (hg38) using the STAR aligner44. Uniquely aligned reads mapping to the RefSeq gene annotations were used for gene expression estimation at reads per kilobase transcript and million mapped reads (RPKMs) using the rpkmforgenes software45. Low quality samples were excluded from downstream analysis when they failed to meet the following criteria for retaining cells: (1) ≥ 50,000 sequence reads; (2) ≥ 30% of reads uniquely aligned to the genome; (3) ≥ 40% of these unique reads mapping to RefSeq annotated exons; (4) ≥ 1000 genes with RPKM ≥ 2. From the results of the RNA-sequencing we first evaluated the expression of a 5-gene signature that previously has been developed to predict the prevalence of NASH and fibrosis in morbidly obese subjects26. These five genes were CCL2, DMRT2, GADD45B, IL1RN, and IL8. Next, we explored other NASH-associated genes by ordinary least squares regression model using NASH status, age, sex, BMI, and DM2 as factors in combination with differential expression analysis.
Statistics
Adipocyte volume is presented as median and interquartile range due to a skewed distribution. Continuous data are presented as mean ± SD and categorical data as percentages. For continuous parameters, differences between groups were calculated using a t-test for normally distributed data. Differences in categorical parameters were calculated using the Chi2-test. The Kruskal Wallis-test was used for differences between multiple groups.
For comparison of adipocyte volume, subjects with NAFLD were categorized into groups based on the presence of NASH (NASH vs. non-NASH) and fibrosis stage (non- or low stage fibrosis (F0-1) vs. significant or severe fibrosis (F2-4)). Difference in adipocyte volume was compared using a Mann–Whitney U-test, due to a skewed distribution of data, and multiple regression was used to adjust for age, sex, BMI and T2D. Also, as a sensitivity analysis, subjects with T2D were excluded from the NAFLD group.
We performed ordinary least squares linear regression analysis on gene expressions. Genes associated with NASH status at a significance level of p < 0.05 were subjected to differential expression analysis. Finally, by comparing NASH subjects and non-NASH subjects using ANOVA followed by Benjamini–Hochberg multiple hypothesis corrections, genes were identified as NASH-associated genes based on adjusted p-value < 0.05 and at least one condition with median log2-RPKM of 1.
Data availability
The data supporting the findings of this trial was generated at Karolinska Institutet, Stockholm, Sweden. The raw RNA-seq data has been deposited to The European Genome-phenome Archive (EGA) (https://ega-archive.org/) and is awaiting approval. The accession number is pending but will be available upon request when the data has been published. Anonymized clinical data are available from the corresponding author on reasonable request. This excludes sharing of data that conflicts with the statement of confidentiality in the informed consent.
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md.) 64, 73–84. https://doi.org/10.1002/hep.28431 (2016).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801. https://doi.org/10.1016/j.jhep.2019.06.021 (2019).
Bugianesi, E. et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48, 634–642. https://doi.org/10.1007/s00125-005-1682-x (2005).
Björkström, K. et al. A personalized treatment program in persons with type 2 diabetes is associated with a reduction in liver steatosis. Eur. J. Gastroenterol. Hepatol. https://doi.org/10.1097/meg.0000000000001882 (2020).
Younossi, Z. M. et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. https://doi.org/10.1016/j.cgh.2020.05.064 (2020).
Holmer, M. et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. Off. J. Int. Assoc. Study Liver 38, 2082–2090. https://doi.org/10.1111/liv.13751 (2018).
Calzadilla-Bertot, L. et al. Increasing incidence of nonalcoholic steatohepatitis as an indication for liver transplantation in Australia and New Zealand. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 25, 25–34. https://doi.org/10.1002/lt.25361 (2019).
Dam-Larsen, S. et al. Final results of a long-term, clinical follow-up in fatty liver patients. Scand. J. Gastroenterol. 44, 1236–1243. https://doi.org/10.1080/00365520903171284 (2009).
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402, https://doi.org/10.1016/j.jhep.2015.11.004 (2016).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397. https://doi.org/10.1053/j.gastro.2015.04.043 (2015).
Hagstrom, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273. https://doi.org/10.1016/j.jhep.2017.07.027 (2017).
Ratziu, V. et al. Liver fibrosis in overweight patients. Gastroenterology 118, 1117–1123. https://doi.org/10.1016/s0016-5085(00)70364-7 (2000).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Adipokines in nonalcoholic fatty liver disease. Metabolism 65(8), 1062–1079. https://doi.org/10.1016/j.metabol.2015.11.006 (2016).
Skurk, T., Alberti-Huber, C., Herder, C. & Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92, 1023–1033. https://doi.org/10.1210/jc.2006-1055 (2007).
Mendez-Sanchez, N. et al. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 19, 2034 (2018).
Petaja, E. M. et al. Adipocyte size is associated with NAFLD independent of obesity, fat distribution, and PNPLA3 genotype. Obesity (Silver Spring, Md.) 21, 1174–1179. https://doi.org/10.1002/oby.20114 (2013).
Tordjman, J. et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J. Hepatol. 56, 1152–1158. https://doi.org/10.1016/j.jhep.2011.12.015 (2012).
Bedossa, P. et al. Systematic review of bariatric surgery liver biopsies clarifies the natural history of liver disease in patients with severe obesity. Gut 66, 1688–1696. https://doi.org/10.1136/gutjnl-2016-312238 (2017).
D’Incao, R. B. et al. Adipokine levels versus hepatic histopathology in bariatric surgery patients. Obes. Surg. 27, 2151–2158. https://doi.org/10.1007/s11695-017-2627-4 (2017).
Estep, J. M. et al. Expression of cytokine signaling genes in morbidly obese patients with non-alcoholic steatohepatitis and hepatic fibrosis. Obes. Surg. 19, 617–624. https://doi.org/10.1007/s11695-009-9814-x (2009).
Adolph, T. E., Grander, C., Grabherr, F. & Tilg, H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int. J. Mol. Sci. 18, 1649 (2017).
Polyzos, S. A. et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59, 30–43. https://doi.org/10.1007/s00125-015-3769-3 (2016).
Polyzos, S. A., Toulis, K. A., Goulis, D. G., Zavos, C. & Kountouras, J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism 60(3), 313–326. https://doi.org/10.1016/j.metabol.2010.09.003 (2011).
Rotter, V., Nagaev, I. & Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278, 45777–45784. https://doi.org/10.1074/jbc.M301977200 (2003).
Cao, Q., Mak, K. M., Ren, C. & Lieber, C. S. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J. Biol. Chem. 279, 4292–4304. https://doi.org/10.1074/jbc.M308351200 (2004).
du Plessis, J. et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 149, 635–648. https://doi.org/10.1053/j.gastro.2015.05.044 (2015).
Wolfs, M. G. et al. Determining the association between adipokine expression in multiple tissues and phenotypic features of non-alcoholic fatty liver disease in obesity. Nutr. Diabetes 5, e146. https://doi.org/10.1038/nutd.2014.43 (2015).
Choe, S. S., Huh, J. Y., Hwang, I. J., Kim, J. I. & Kim, J. B. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 7, 30. https://doi.org/10.3389/fendo.2016.00030 (2016).
Loomis, A. K. et al. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. J. Clin. Endocrinol. Metab. 101, 945–952. https://doi.org/10.1210/jc.2015-3444 (2016).
Mantovani, A., Byrne, C. D., Bonora, E. & Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 41, 372–382. https://doi.org/10.2337/dc17-1902 (2018).
Eriksson-Hogling, D. et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int. J. Obes. 2005(39), 893–898. https://doi.org/10.1038/ijo.2015.18 (2015).
Lundgren, M. et al. Fat cell enlargement is an independent marker of insulin resistance and “hyperleptinaemia”. Diabetologia 50, 625–633. https://doi.org/10.1007/s00125-006-0572-1 (2007).
Jaworski, K., Sarkadi-Nagy, E., Duncan, R. E., Ahmadian, M. & Sul, H. S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1–G4. https://doi.org/10.1152/ajpgi.00554.2006 (2007).
Armstrong, M. J. et al. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes. Metab. 16, 651–660. https://doi.org/10.1111/dom.12272 (2014).
Ooi, G. J. et al. Effect of body mass index, metabolic health and adipose tissue inflammation on the severity of non-alcoholic fatty liver disease in bariatric surgical patients: a prospective study. Obes. Surg. 29, 99–108. https://doi.org/10.1007/s11695-018-3479-2 (2019).
Bedossa, P. Histological assessment of NAFLD. Dig. Dis. Sci. 61, 1348–1355. https://doi.org/10.1007/s10620-016-4062-0 (2016).
Song, C. et al. RNA-Seq analysis identifies differentially expressed genes Insubcutaneous adipose Tissuein Qaidamford cattle, Cattle-Yak, and Angus cattle. Animals 9, 1077. https://doi.org/10.3390/ani9121077 (2019).
Kukurba, K. R. & Montgomery, S. B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 951–969, 2015. https://doi.org/10.1101/pdb.top084970 (2015).
Rydén, M. et al. The adipose transcriptional response to insulin is determined by obesity, not insulin sensitivity. Cell Rep. 16, 2317–2326. https://doi.org/10.1016/j.celrep.2016.07.070 (2016).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321. https://doi.org/10.1002/hep.20701 (2005).
Bedossa, P., & FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 60(2), 565–575 (2014).
Goldrick, R. B. Morphological changes in the adipocyte during fat deposition and mobilization. Am. J. Physiol. 212, 777–782. https://doi.org/10.1152/ajplegacy.1967.212.4.777 (1967).
Hirsch, J. & Gallian, E. Methods for the determination of adipose cell size in man and animals. J. Lipid Res. 9, 110–119 (1968).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. https://doi.org/10.1093/bioinformatics/bts635 (2013).
Ramsköld, D., Wang, E. T., Burge, C. B. & Sandberg, R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput. Biol. 5, e1000598. https://doi.org/10.1371/journal.pcbi.1000598 (2009).
Funding
Open access funding provided by Karolinska Institute. The study was financed by Region Stockholm (ALF20181157, ALF20200576, and Clinical Research Appointment K2017-4579), CIMED (20180889) and the Swedish Cancer Society (170690).
Author information
Authors and Affiliations
Contributions
Study conception and design: H.H., P.S. Acquisition of data: M.H., H.H., O.D., P.C. Statistical analysis: M.H., P.C. Analysis and interpretation of data: M.H., P.C., P.S. Drafting of manuscript: M.H. Critical revision: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holmer, M., Hagström, H., Chen, P. et al. Associations between subcutaneous adipocyte hypertrophy and nonalcoholic fatty liver disease. Sci Rep 12, 20519 (2022). https://doi.org/10.1038/s41598-022-24482-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24482-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.