Abstract
The mu opioid receptor (MOR) and the orphan GPR151 receptor are inhibitory G protein coupled receptors that are enriched in the habenula, a small brain region involved in aversion processing, addiction and mood disorders. While MOR expression in the brain is widespread, GPR151 expression is restricted to the habenula. In a previous report, we created conditional ChrnB4-Cre × Oprm1fl/fl (so-called B4MOR) mice, where MORs are deleted specifically in Chrnb4-positive neurons restricted to the habenula, and shown a role for these receptors in naloxone aversion. Here we characterized the implication of habenular MORs in social behaviors. B4MOR−/− mice and B4MOR+/+ mice were compared in several social behavior measures, including the chronic social stress defeat (CSDS) paradigm, the social preference (SP) test and social conditioned place preference (sCPP). In the CSDS, B4MOR−/− mice showed lower preference for the social target (unfamiliar mouse of a different strain) at baseline, providing a first indication of deficient social interactions in mice lacking habenular MORs. In the SP test, B4MOR−/− mice further showed reduced sociability for an unfamiliar conspecific mouse. In the sCPP, B4MOR−/− mice also showed impaired place preference for their previous familiar littermates after social isolation. We next created and tested Gpr151−/− mice in the SP test, and also found reduced social preference compared to Gpr151+/+ mice. Altogether our results support the underexplored notion that the habenula regulates social behaviors. Also, our data suggest that the inhibitory habenular MOR and GPR151 receptors normally promote social reward, possibly by dampening the aversive habenula activity.
Similar content being viewed by others
Introduction
Social interactions (maternal-, pair-, peer-, conspecifics-bonds) can either have benefits or costs1 and include various characteristics like social interest, social aptitude, social isolation/exclusion or social reciprocity. Social interactions are crucial in individual’s health: while positive social interactions (social reward) improve health and well-being throughout life, negative social interactions (social pain) can lead to pathological situations like health-damaging behaviors, stress, depression or suicide2,3. Reduced social reward valuation and/or increased reactivity to social rejection can be associated with different psychopathologies including depression4,5,6.
Mu opioid receptors (MORs) contribute to both positive and negative social behaviors. Social play behavior is either increased or decreased by morphine or naloxone, respectively7,8,9,10, and these effects extend to local injections in the nucleus accumbens—a central reward structure11. This was interpreted as an increased/decreased rewarding value of social play induced by MOR activation/blockade, respectively12. Using positron emission tomography in healthy human volunteers, social rejection was found to activate the MOR system in mood- and motivation-regulating brain sites (e.g. ventral striatum13), and this activation did not reach significance in patients suffering from major depressive disorder (MDD14). MOR activation in the nucleus accumbens also correlated with motivation for social interactions in healthy controls but not in MDD patients14. Thus, these data emphasize the MOR system as an important integrator of social information and MOR manipulation could be used to model social psychopathologies in animals. Indeed, mice lacking MORs display social attachment deficits (juveniles15,16) and an autistic-like behavior (adults17,18,19,20). MORs therefore influence different aspects of social behaviors, however circuit mechanisms and brain sites other than the nucleus accumbens have been little explored.
MORs are enriched in the medial subnucleus of the habenula (MHb21), an epithalamic structure implicated in mood-related dysfunctions22,23,24. Animal models of depression lead to an increase in metabolic activity in both nuclei of the habenula (medial and lateral25,26) and the MHb has been shown to be implicated in fear-27,28, aversion-29,30, or anxiety-28,31,32 responses. Emerging evidence also suggests a role for the MHb in social behaviors. Mice lacking the β4-subunit of the nicotine acetylcholine receptor, which is expressed almost exclusively in the MHb33, showed deficits in both nicotinic- and non-nicotinic reinforcing responses34,35 and, intriguingly, also showed increased heart rate in response to social isolation36. Further, mice lacking the Ca+ activated Cl-channel TMEM16A showed altered levels of anxiety/fear responses and social interactions31. Thus, concurrent with aversion processing, the MHb seems to modulate social behaviors. Whether the highly abundant MORs of the MHb are involved in social behaviors, has not been tested.
Recently we deleted MORs in ChrnB4-positive neurons (B4MOR−/− mice), leading to a 50% reduction of total habenular MORs. We found that mutant mice are less sensitive to naloxone aversion without evident reward processing deficiencies29, suggesting that MORs (inhibitory receptors) may act as a brake to reduce the well-established aversive activity of habenula. Here we investigated whether MOR activity at this brain site also influences social responses. To do so, we evaluated sociability behaviors in B4MOR mice and our results show reduced response in all the tests. Because the MOR is an inhibitory G protein coupled receptor (GPCR), we also tested whether another inhibitory GPCR would play a similar role. We found social deficits in mice lacking the orphan GPCR GPR151, which is almost exclusively expressed in the MHb37,38,39,40,41. Altogether our data demonstrate for the first time that habenular receptors MOR and GPR151 contribute to facilitate social behaviors and suggest that the tonic control of MHb activity by inhibitory GPCRs is essential to maintain healthy social interactions.
Materials and methods
Mice
Chrnβ4-Cre mice33 were originally crossed with Oprm1fl/fl mice42 to obtain ChrnB4-Cre × Oprm1fl/fl (abbreviated as B4MOR) mice lacking MORs in nicotinic acetylcholine receptor beta4 subunit-expressing neurons, primarily expressed in the medial habenula. The genetic mouse line was described earlier by our laboratory and maintained (at least ten generations) on a c57bl6:sv129 (50:50) background to produce experimental B4MOR−/− mice and their B4MOR+/+ littermates as in29,43. Homozygous Oprm1−/− mice (lacking MORs globally44) were created in our laboratory and are available at Jackson laboratories (B6.129S2-Oprm1tm1Kff/J Strain #:00755). Gpr151−/− mice (c57bl6 background) were created using a knock-in strategy (Institut Clinique de la Souris, PHENOMIN, http://www.phenomin.fr), so that the fluorescent reporter eGFP is produced instead of the GPR151 receptor in homozygous null mutants (Fig. 4A, and see Suppl Methods for details on the construction and validation).
Commercial c57bl6:sv129 mice (Jackson Laboratories) were used as interactors for B4MOR+/+ and B4MOR−/− mice in social preference and in the control procedure that paralleled the chronic social defeat stress procedure. CD-1 mice (Retired breeders, Charles River) were used as aggressors for the defeat. Commercial c57bl6:sv129 mice were used to judge the atypical behavior of B4MOR−/− versus B4MOR+/+ mice, as well as Oprm1−/− versus Oprm1+/+ mice in the reverse three chamber social test. Commercial c57bl/6J mice (Charles River) were used as interactors for Gpr151−/− and Gpr151+/+ mice in the social preference test.
All procedures in this report were conducted in accordance with ARRIVE guidelines, the guidelines set forth by the Canadian Council of Animal Care and by the Animal Care Committees of McGill University/Douglas Mental Health University Institute and were also approved by the Regional Committee of Ethic in Animals Experiment of Strasbourg (CREMEAS, APAFIS#31880-2021072316283769 v1).
Behavior
Descriptions of housing and behavioral procedures are in the Suppl Methods. This includes the description of chronic social defeat stress (CSDS) followed by a social interaction test (SIT), the social preference (SP) test, the social conditioned place preference test (sCPP), the food pellets self-administration paradigm, the reverse three chamber social test and the real time place preference testing.
Statistics
Data are presented as means ± SEM. Social behavior tests (SIT after CSDS, SP test, reverse SP test, sCPP) were represented across time (habituation/pre-test versus social) and were analyzed using RM ANOVAs. Significant main effects or interaction effects (P’s ≤ 0.05) were followed by Holm-sidak’s multiple comparisons tests. Refer to Suppl Table 1 for description of all statistical tests.
Results
Both control and defeated B4MOR−/− mice show reduced sociability to an unfamiliar CD1 mouse
Because the habenula is a notorious aversion center and MOR presumably inhibits the aversive habenula activity29,45, we first investigated the hypothesis that chronic social stress (social defeat) would trigger enhanced social avoidance in B4MOR−/− mice. We tested B4MOR−/− and B4MOR+/+ mice in a chronic social defeat stress paradigm46 for 10 consecutive days. Control mice were not exposed to physical aggressions. Twenty-four hours after the procedure, sociability for a novel CD1 mouse was tested in an open field (2.5-min habitation period followed by 2.5-min social interaction test; Fig. 1A).
B4MOR−/− mice show disinterest for a CD1-social target in the chronic social defeat paradigm. (A) Schematic representation of the chronic social defeat and control procedures followed by the social interaction test (the gray part represents the social interaction zone). The wire mesh enclosure was empty during the habituation of the test and contained an unfamiliar CD1 mouse during the social interaction test. (B) During habituation, mice spent generally more time in the corners than in the zone around the wire mesh enclosure (future social zone). (C) During the social interaction test, B4MOR+/+ control (undefeated) mice spent more time in the social zone and less time in the corners than B4MOR+/+ defeated mice. (D) Mice were clustered in four distinct categories based on their time spent in the social zone during the social interaction test. The less sociable cluster did not include B4MOR+/+ control mice, but all the three other groups were represented. *P’s < 0.05, B4MOR+/+ control mice versus B4MOR+/+ defeated mice. N’s = 7–10/group.
We found that the time spent in the CD1/social and corners/non-social zones depended upon the phase of the test (habituation versus social test), the previous social stress exposure, and the genotype (Fig. 1B,C). During habituation, where the wire mesh enclosure was empty, both B4MOR−/− and B4MOR+/+ mice spent significantly more time in the corners compared to the CD1 zone independently of the previous social stress experience (Stress x Zone interaction effect, P’s > 0.05; Fig. 1B), suggesting a general preference for corners in our experimental set up. However, during the social interaction test (SIT), where a novel CD1 mouse was inserted into the wire mesh enclosure, previous social stress exposure altered the time spent in the CD1 zone versus corners only in B4MOR+/+ mice (Stress x Zone interaction effect, F1,15 = 6.55, p = 0.02; Fig. 1C). Thus, as expected, defeated B4MOR+/+ spent more time in the corners and less in the social zone compared to control (undefeated) B4MOR+/+ mice (P’s < 0.05; Fig. 1C). On the contrary, both B4MOR−/− controls and defeated mice spent more time in corners, whether a CD1 mouse was present or not in the wire mesh enclosure, suggesting that basal sociability is reduced in the mutant mice.
We then clustered individual mice during the social interaction (time spent in the CD1 zone) based on their sociability index using the WARD method (Fig. S1). We found four sociability clusters, distinguishing resilient and susceptible subcategories of mice46,47 (Fig. 1D). There was a significant association between the four clusters and the stress/genotype groups (χ2(9) = 17.75, p = 0.04; Fig. 1D) which was driven by the undefeated B4MOR+/+ controls: removing this group from the analysis made the association non-significant (χ2(6) = 7.88, p = 0.25; Fig. 1D). This suggests that mice avoiding the social zone are equally distributed in defeated groups from both genotypes, but also in control (undefeated) B4MOR−/− mice—which was intriguing as this group was previously not exposed to chronic social stress. This finding is another indication that B4MOR−/− mice display reduced sociability at baseline.
B4MOR−/− mice show reduced social preference for a partner of the same strain
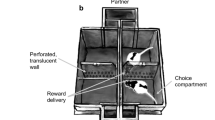
To further investigate a potential deficit of social interest in B4MOR−/− mice, we compared B4MOR−/− with B4MOR+/+ mice in the three-chamber social test, to assess social preference for an unfamiliar mouse. In this test, mice have free choice to spend time in the three chambers of the apparatus during a 10-min habituation period followed by a 5-min social test (Fig. 2A). During habituation, empty cups are placed in the two opposite chambers. Both B4MOR−/− and B4MOR+/+ mice similarly explored the two chambers during habituation (Fig. 2B,C). Then, an unfamiliar mouse was placed under one of the two cups and we compared the last 5-min bin of the habituation phase (5–10 min) with the 5-min social test (10–15 min). The time spent in the social versus object chamber depended upon the test phase only in B4MOR+/+ mice (Test x Chamber interaction effect, B4MOR+/+, F1,20 = 12.55, p = 0.002; Fig. 2B; B4MOR−/−, F1,23 = 2.46, p = 0.13; Fig. 2C). As expected, B4MOR+/+ mice spent more time in the social-paired chamber compared to the non-social one (p < 0.0001; Fig. 2B) leading them to be closer to the social cup versus the empty/object cup (p < 0.0001; Fig. 2D). However, B4MOR−/− mice spent a similar amount of time in the two chambers (Fig. 2C) leading them to be at equidistance between the two cups (Fig. 2E). Added to our previous results, these data suggest a social preference deficit in B4MOR−/− mice.
B4MOR−/− mice do not express social preference in the three-chamber social preference test. (A) Schematic representation of the social preference test. (B,C) Time spent in the social versus object compartment in B4MOR+/+ and B4MOR−/− mice, respectively. (D,E) Distance from the social versus object cup in B4MOR+/+ and B4MOR−/− mice, respectively. During the social test, B4MOR+/+ mice spent more time in the social compartment (B) and were closer to the social cup (D). B4MOR−/− mice spent a similar amount of time in both compartments (C) and were at equidistance from the two cups (E). *P’s < 0.05, Object compartment versus social compartment. #P’s < 0.05, habituation (Hab) versus social test. N’s = 21–24/group.
B4MOR−/− mice do not show conditioned place preference to social interactions
We next investigated the social reward-context association using a social Conditioned Place Preference (CPP) paradigm in B4MOR−/− and B4MOR+/+ mice (Fig. 3A). Reward-context association is an indicator of reward valuation of a conditioned stimulus48, in our case social stimuli. After a pre-test where mice could freely explore a CPP apparatus (50%–50% of time spent in the allocated social versus non-social compartment, Test Day 1, Fig. 3A,B), mice were socially isolated and were next conditioned to one compartment alone every morning and with their ex-littermates in the other compartment every afternoon for 8 successive days (Fig. 3A). While repeated social exposure to ex-littermates conditioned a place preference in B4MOR+/+ mice, this was not the case for B4MOR−/− mice (Genotype x Test Day interaction effect, F3,78 = 2.86, p = 0.04; versus Test Day 1, P’s < 0.05 at Test Days 14 and 18 in B4MOR+/+ mice; All other P’s > 0.05; Fig. 3B). Locomotor activity in the social compartment was also dependent upon previous social conditioning and genotype (Main effect of Test Day, F3,78 = 4.88, p = 0.004; Main effect of Genotype, F1,26 = 5.72, p = 0.02; Genotype x Test Day interaction effect, F3,78 = 2.92, p = 0.04; Fig. 3C) leading to decreased locomotor activity in the social-paired compartment on the last test day in B4MOR−/− mice versus B4MOR+/+ mice (p = 0.002, Fig. 3C). The CPP score was significantly higher in B4MOR+/+ mice on test day 18 (p = 0.04; Fig. 3D) but not on test days 10 (p = 0.88; Fig. 3D) and 14 (p = 0.08; Fig. 3D). Altogether, these results suggest that social reward-context association occurs only in B4MOR+/+ mice and that social reward is lower in B4MOR−/− mice compared to B4MOR+/+ mice.
B4MOR−/− mice do not show conditioned place preference to social interactions. (A) shows a schematic representation of the social conditioned place preference paradigm. (B) The time spent in the social-paired compartment started at 50% in both genotypes on test day 1 and increased after social conditioning only in B4MOR+/+ mice. (C) Locomotor activity (beam breaks and release) in the social-paired compartment was higher in B4MOR+/+ mice versus B4MOR−/− mice after social conditioning. (D) CPP score was higher in B4MOR+/+ mice versus B4MOR−/− mice on test day 18. #P’s < 0.05, versus Test Day 1. *P’s < 0.05, B4MOR+/+ versus B4MOR−/− mice. N’s = 13–15/group.
In this test, B4MOR−/− mice deficits to value social interactions as rewarding could be the consequence of learning and/or memory deficits. We then tested whether low reward valuation also applied to palatable food. B4MOR−/− and B4MOR+/+ mice self-administered similar amounts of chocolate pellets under FR1, FR5 and PR (Fig. S2) suggesting that food reinforcement was not altered in B4MOR−/− mice. When tested under reversal conditions (active and inactive ports were switched), the flexibility to learn the new task was similar for B4MOR−/− and B4MOR+/+ mice. Of note, our previous work showed that B4MOR−/− mice also show intact Morphine CPP29. Altogether, these data suggest that no generalized learning and/or memory deficit seems to impair the social reward-context association in B4MOR−/− mice.
Gpr151−/− mice also show reduced social preference for a partner of the same strain
To extend our study to another inhibitory habenular receptor, we used a genetic mouse line where the GPR151 receptor was removed. The construction of this mouse line was designed as to show eGFP expression upon deletion of the Gpr151 gene coding sequence (Fig. 4A). Analysis of the Gpr151 transcript in coronal brain slices of Gpr151−/− mice versus Gpr151+/+ mice by sFISH (see Suppl Methods) confirmed the absence of Gpr151 mRNA in the habenular complex of Gpr151−/− mice (Fig. 4B). Also, sectioning coronal brain slices of Gpr151−/− mice with a 50°–60° angle allowed us to visualize eGFP-positive cells in which the GPR151 receptors were removed. As expected, the eGFP fluorescence was detected throughout the habenulo-interpeduncular pathway in Gpr151−/− mice but not Gpr151+/+ mice (Fig. 4C). There was almost no eGFP expression in the dorsal part of the medial habenula (Fig. 4C), which agrees with results reporting no detection of GPR151 in Substance P-expressing cells38.
Generation and characterization of Gpr151−/− knock-in mice. (A) Construction of the Gpr151−/− knock-in mouse line. (B) Gpr151 mRNA in Gpr151+/+ and Gpr151−/− brain slices using FISH. (C) eGFP signal detected by immunohistochemistry in a 50°–60° coronal brain section of a Gpr151−/− mouse throughout the habenular pathway: medial habenula (MHb), lateral habenula (LHb), fasciculus retroflexus (fr) and interpeduncular nucleus (IPN).
Because MOR and GPR151 are both inhibitory habenular receptors, we hypothesized that the social reward deficiencies measured in B4MOR−/− mice could also be measured in Gpr151−/− mice. We assessed social preference for an unfamiliar mouse of the same strain in Gpr151−/− mice and Gpr151+/+ mice using the three-chamber social test (Fig. 5A). For both genotypes, there was no discrimination between the two opposite chambers during habituation (Fig. 5B,C). During the subsequent social test, the preference for the social-paired versus non-social paired chamber occurred in Gpr151+/+ (p = 0.028, Fig. 5B) but not in Gpr151−/− mice (Fig. 5C, also see Fig. S3 for data in female mice). Gpr151+/+ mice were then closer to the social cup (p = 0.048, Fig. 5D) while Gpr151−/− stayed at equidistance from the two cups (Fig. 5E). These data suggest that the social reward deficit observed in B4MOR−/− mice could extend to other models of mice lacking inhibitory habenular receptors.
Gpr151−/− mice do not express social preference in the three-chamber social preference test. (A) Schematic representation of the social preference test. (B,C) Time spent in the social versus object compartment in Gpr151+/+ and Gpr151−/− mice, respectively. (D,E) Distance from the social versus object cup in Gpr151+/+ and Gpr151−/− mice, respectively. During the social test, Gpr151+/+ mice spent more time in the social compartment (B) and were closer to the social cup (D). Gpr151−/− mice spent a similar amount of time in both compartments (C) and were at equidistance from the two cups (E). *P’s < 0.05, object compartment versus social compartment. N’s = 19–20/group.
Discussion
In this report, we first show that mice lacking MORs in the MHb present reduced sociability in the chronic social defeat paradigm at baseline (no stress), suggesting a social interaction deficit. We next show that these mutant mice show reduced sociability in the three-chamber social preference test, as well as reduced place preference to a social context, confirming the alteration of social behaviors. We finally show reduced sociability in the social preference test in mice lacking GPR151, an orphan GPCR enriched in the MHb. Altogether, these data support a role for Gi/o coupled GPCRs of the MHb in promoting social behaviors, likely through the tonic inhibition of MHb activity.
The MHb regulates social behaviors
Only few studies have proposed a role of the MHb in social behaviors31,36. Indeed, habenula function is mainly studied in the context of mood disorders. Habenular activity was reported to be increased in MDD patients49. Consistent with this finding, animal models of depression show increased metabolic activity in both the medial (MHb) and the lateral (LHb) nuclei of the habenula while neural activity is generally lowered in other brain areas25,26. The function of the LHb in depressive-like states has been well characterized50 and reduced LHb activity parallels reduced depressive-like symptoms51,52. Neural activity in the MHb and the interpeduncular nucleus (IPN)—its main projection site—is also increased in animal models of depression25. MORs have their strongest expression in the MHb21 and the MOR system is already known to regulate depressive-like states53,54. We therefore here evaluated the role of habenular MORs29 using a model of depression, the chronic social defeat paradigm55,56, with the assumption that MOR activity may alleviate the aversive consequences of stress, and limit social avoidance in this test. Unexpectedly, while no genotype effect was observed in the stress response, mice lacking habenular MORs showed a social deficit at baseline. This was a first indication for a role of the MHb in the regulation of social behaviors, which we further confirmed through several social testing paradigms and the genetic deletion of another inhibitory GPCR specific to the MHb.
MORs promote social behaviors at the level of the MHb
The implication of MORs in reward- and pain-related processes is well characterized57,58, and was also shown to extend to the social aspects of behavior3. In the literature, the alteration of MOR function was measured in different mental health disorders with social deficiencies, including depression14,53,59,60,61. Thus, constitutive MOR knockout mice (Oprm1−/− mice) present a large range of social deficiencies, including a decrease in social attachment, reciprocal direct social interactions, social preference, social conditioned place preference, ultrasound vocalizations (USVs) emission in response to social cues and social exploratory activation to USVs15,16,17,18,19,20. The pharmacology also indicates that MOR activation/blockade increases/decreases social responses, respectively7,8,9,10,11,12. MOR activity at the level of the mesolimbic pathway partly contributes to these effects11 but nothing is known at the level of the habenula, where MOR density is extremely high21.
In this study, mice lacking MORs in the MHb show reduced sociability (or propensity to spend time with another mouse rather than an empty chamber, see62) in two different tasks. In the first task (chronic social defeat), undefeated B4MOR−/− mice behaved like defeated B4MOR+/+ mice with a reduced social exploration (Fig. 1), suggesting that in the absence of social stress, at baseline levels, habenular MORs facilitate sociability to congeners with different phenotypic traits. In this procedure however, mice were isolated for 10 days, which could induce a social distress by itself possibly recruiting the opioid system7,63 and be sufficient to impair future sociability in mutant mice. In the second sociability task (three-chamber social preference), mice were group housed and social preference for an unfamiliar peer from the same strain was tested. Under these different conditions, B4MOR−/− mice did not dissociate between the social and the empty compartment (Fig. 2), characterizing them as not sociable or indifferent to the social target. Together, the two testing procedures concur to demonstrate that MORs operate at the level of the MHb to promote sociability. Whether this mechanism acts in concert with the mesolimbic circuitry, or perhaps the dorsal raphe strongly involved in social behaviors64,65, remains to be clarified.
MORs in the MHb encode the hedonic value of social stimuli
We previously highlighted a role of habenular MORs in aversive states29,45 and using Mor-Cre mice66, we recently showed that the selective activation of Hb-IPN MORs positive cells was aversive45. Thus, MORs function could balance between reward and aversion processes at the level at the habenula. Decreased sociability in B4MOR−/− mice could be the consequence of both processes, either an increase in social aversion or a decrease in social reward. Our results tend to the latter hypothesis as social stimuli-context association did not occur in B4MOR−/− mice—without the expression of an avoidance to the social-context (Fig. 3). This suggests that the hedonic value of social interactions with siblings is reduced in mice lacking habenular MORs.
It has recently been shown that mice lacking MORs constitutively demonstrate an “atypical” social behavior leading judge mice to decrease their interaction time with them18. We did not find this effect in mice lacking MORs in the MHb (Fig. S4), suggesting that an “atypical” behavior does not explain decreased sociability observed in B4MOR−/− mice. Added to this result, social reward-context association occurred in both B4MOR+/+ mice conditioned only with B4MOR+/+ and with mixed genotypes (data not shown). Thus, abnormal/atypical social behavior does not seem to contribute to deficits in social interactions behavior in B4MOR−/− as it is likely the case for constitutive Oprm1−/− mice17,18,61.
GPR151, as MORs in the MHb, facilitates sociability
Our results on the implication of MHb MORs in social behavior led us to question the role of other inhibitory habenular receptors in social interactions. GPR151 is an orphan GPCR densely and almost exclusively expressed in the habenular pathway37,38,39,40,41, and this highly restricted expression pattern in the brain makes this particular GPCR a highly valuable potential target for brain disease. Little is known about the function of GPR151, but an implication of GPR151 in nicotine intake was shown, supposing a role for this receptor in drug addiction and reward processes40. We hypothesized that, as for MHb MORs, GPR151 may act as a brake on the habenulo-interpeduncular pathway. The mechanism by which these receptors would be endogenously active is still to be determined, but if this is the case, we would expect Gpr151−/− mice to show impaired sociability. Indeed, while Gpr151+/+ mice showed sociability for a peer (three-chamber social preference), Gpr151−/− mice were indifferent to a social partner (Fig. 5). This result extends our data from B4MOR mice and supports the more general notion that deleting inhibitory GPCRs from the MHb reduces social behaviors, likely via enhanced activity of the habenular pathway. Of note, mice are nocturnal animals but were tested during the light phase which could have impacted sociability. However, social interaction behavior can be evaluated during both the dark and the light phase of the circadian cycle and there is no clear influence of the testing time67.
As habenular function is related to anxiety responses28,31,32, we can hypothesize that the observed decrease in sociability could result from changes in anxiety levels. However, we found that B4MOR−/− and B4MOR+/+ mice similarly explored the center of an open field apparatus (Fig. S5) and Antolin-Fontes et al.40, reported that Gpr151−/− mice spend similar amount of time in the open arms of an elevated plus maze apparatus compared to Gpr151+/+ mice. Further, the latency to first entry in the social compartment was not impaired in B4MOR and GPR151 null mutants (Fig. S6), suggesting altogether that mutant mice are not less sociable because of higher anxiety.
Conclusion
Altogether, our results suggest that habenular Gi/o receptors participate to the attribution of the hedonic value of social interactions. Whether this function is related to hedonic or motivational aspects of social interactions is unknown and could be determined using operant social self-administration models68. It is interesting to note that double in situ hybridization allowed us to observe habenular cells expressing MOR only, GPR151 only or both receptors (Fig. S7). Thus, it will be interesting in the future to quantify the proportion of habenular cells expressing both receptors and eventually address the question of potential interactions between the two receptors in the MHb. Also it will be important to precisely characterize cells of the MHb responsible for the regulation of social behaviors as MOR- and GPR151-positive neurons overlap cholinergic neurons known to populate the MHb21,38, as well as Substance P neurons (MOR, see21). Finally, a next step could be the identification of circuit mechanisms underlying the roles of habenular MOR and GPR151 in the regulation of social behaviors. We recently showed that optostimulation of habenular MOR-positive neurons projecting to the IPN is aversive45, an activity that could partly contribute to the impaired sociability observed in mice lacking habenular MORs. However similar optostimulation parameters applied to habenular GPR151-positive neurons projecting to the IPN did not seem to be aversive (Fig. S8). Although this finding requires further confirmation, it is possible that distinct cellular and/or circuit mechanisms underlie the apparent similar role of MORs and GPR151 receptors in regulating social behaviors, and these remain to be determined.
In summary, our findings reveal the importance of MHb activity, and receptors modulating this activity, in the control of social behaviors. This study opens new therapeutic perspectives, because social interactions deficits are associated with several psychopathologies including addiction69 and depression3, therefore targeting habenula function may benefit to this particular dimension of psychiatric conditions. In particular, the development of GPR151 agonists may deliver highly specific drugs with minimal adverse effects, in high demand in the area of mental health.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Feldman, R. The neurobiology of human attachments. Trends Cogn. Sci. 21(2), 80–99 (2017).
Cacioppo, J. T., Cacioppo, S., Capitanio, J. P. & Cole, S. W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767 (2015).
Lutz, P. E., Courtet, P. & Calati, R. The opioid system and the social brain: implications for depression and suicide. J. Neurosci. Res. 98(4), 588–600 (2020).
Catalano, L. T., Heerey, E. A. & Gold, J. M. The valuation of social rewards in schizophrenia. J. Abnorm. Psychol. 127(6), 602–611 (2018).
Kumar, P. et al. Increased neural response to social rejection in major depression. Depress Anxiety. 34(11), 1049–1056 (2017).
Sharma, A. et al. Divergent relationship of depression severity to social reward responses among patients with bipolar versus unipolar depression. Psychiatry Res. Neuroimaging. 254, 18–25 (2016).
Panksepp, J., Herman, B. H., Vilberg, T., Bishop, P. & DeEskinazi, F. G. Endogenous opioids and social behavior. Neurosci Biobehav. Rev. 4(4), 473–487 (1980).
Achterberg, E. J. M., van Swieten, M. M. H., Houwing, D. J., Trezza, V. & Vanderschuren, L. Opioid modulation of social play reward in juvenile rats. Neuropharmacology 159, 107332 (2019).
Guard, H. J., Newman, J. D. & Roberts, R. L. Morphine administration selectively facilitates social play in common marmosets. Dev. Psychobiol. 41(1), 37–49 (2002).
Siegel, M. A., Jensen, R. A. & Panksepp, J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav. Neural Biol. 44(3), 509–514 (1985).
Trezza, V., Damsteegt, R., Achterberg, E. J. & Vanderschuren, L. J. Nucleus accumbens mu-opioid receptors mediate social reward. J. Neurosci. 31(17), 6362–6370 (2011).
Vanderschuren, L. J., Niesink, R. J., Spruijt, B. M. & Van Ree, J. M. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur. J. Pharmacol. 276(3), 257–266 (1995).
Hsu, D. T. et al. Response of the mu-opioid system to social rejection and acceptance. Mol. Psychiatry. 18(11), 1211–1217 (2013).
Hsu, D. T. et al. It still hurts: Altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry. 20(2), 193–200 (2015).
Moles, A., Kieffer, B. L. & D’Amato, F. R. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science 304(5679), 1983–1986 (2004).
Cinque, C. et al. Modeling socially anhedonic syndromes: Genetic and pharmacological manipulation of opioid neurotransmission in mice. Transl. Psychiatry. 2, e155 (2012).
Becker, J. A. et al. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology 39(9), 2049–2060 (2014).
Toddes, C., Lefevre, E. M., Brandner, D. D., Zugschwert, L. & Rothwell, P. E. Mu opioid receptor (Oprm1) copy number influences nucleus accumbens microcircuitry and reciprocal social behaviors. J. Neurosci. 41, 7965 (2021).
Wohr, M., Moles, A., Schwarting, R. K. & D’Amato, F. R. Lack of social exploratory activation in male mu-opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Soc. Neurosci. 6(1), 76–87 (2011).
Gigliucci, V. et al. Region specific up-regulation of oxytocin receptors in the opioid oprm1 (−/−) mouse model of autism. Front. Pediatr. 2, 91 (2014).
Gardon, O. et al. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience 277, 595–609 (2014).
Aizawa, H., Amo, R. & Okamoto, H. Phylogeny and ontogeny of the habenular structure. Front. Neurosci. 5, 138 (2011).
Hikosaka, O. The habenula: From stress evasion to value-based decision-making. Nat. Rev. Neurosci. 11(7), 503–513 (2010).
Namboodiri, V. M., Rodriguez-Romaguera, J. & Stuber, G. D. The habenula. Curr. Biol. 26(19), R873–R877 (2016).
Shumake, J., Edwards, E. & Gonzalez-Lima, F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 963(1–2), 274–281 (2003).
Caldecott-Hazard, S., Mazziotta, J. & Phelps, M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J. Neurosci. 8(6), 1951–1961 (1988).
Agetsuma, M. et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 13(11), 1354–1356 (2010).
Yamaguchi, T., Danjo, T., Pastan, I., Hikida, T. & Nakanishi, S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron 78(3), 537–544 (2013).
Boulos, L. J. et al. Mu opioid receptors in the medial habenula contribute to naloxone aversion. Neuropsychopharmacology 45(2), 247–255 (2020).
Soria-Gomez, E. et al. Habenular CB1 receptors control the expression of aversive memories. Neuron 88(2), 306–313 (2015).
Cho, C. H. et al. TMEM16A expression in cholinergic neurons of the medial habenula mediates anxiety-related behaviors. EMBO Rep. 21(2), e48097 (2020).
Kobayashi, Y. et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front. Behav. Neurosci. 7, 17 (2013).
Proulx, C. D., Hikosaka, O. & Malinow, R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 17(9), 1146–1152 (2014).
Harrington, L. et al. Role of beta4* nicotinic acetylcholine receptors in the habenulo-interpeduncular pathway in nicotine reinforcement in mice. Neuropsychopharmacology 41(7), 1790–1802 (2016).
Husson, M. et al. Beta4-nicotinic receptors are critically involved in reward-related behaviors and self-regulation of nicotine reinforcement. J. Neurosci. 40(17), 3465–3477 (2020).
Salas, R., Pieri, F., Fung, B., Dani, J. A. & De Biasi, M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J. Neurosci. 23(15), 6255–6263 (2003).
Aizawa, H., Kobayashi, M., Tanaka, S., Fukai, T. & Okamoto, H. Molecular characterization of the subnuclei in rat habenula. J. Comp. Neurol. 520(18), 4051–4066 (2012).
Broms, J., Antolin-Fontes, B., Tingstrom, A. & Ibanez-Tallon, I. Conserved expression of the GPR151 receptor in habenular axonal projections of vertebrates. J. Comp. Neurol. 523(3), 359–380 (2015).
Broms, J. et al. Monosynaptic retrograde tracing of neurons expressing the G-protein coupled receptor Gpr151 in the mouse brain. J. Comp. Neurol. 525(15), 3227–3250 (2017).
Antolin-Fontes, B. et al. The habenular G-protein-coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc. Natl. Acad. Sci. USA 117(10), 5502–5509 (2020).
Ehrlich, A. T. et al. Expression map of 78 brain-expressed mouse orphan GPCRs provides a translational resource for neuropsychiatric research. Commun. Biol. 1, 102 (2018).
Weibel, R. et al. Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: Insight from conditional knockout mice. PLoS ONE 8(9), e74706 (2013).
Han, J. et al. Mu opioid receptors on hippocampal GABAergic interneurons are critical for the antidepressant effects of tianeptine. Neuropsychopharmacology 47(7), 1387–1397 (2022).
Matthes, H. W. et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383(6603), 819–823 (1996).
Bailly, J., Allain, F., Tirel, C., Petit, F., Darcq, E., & Kieffer, B. Neurons expressing mu opioid receptors of the habenula promote negative affect in a projection-specific manner. bioRxiv:2021:2021.09.13.460041 (2021).
Golden, S. A., Covington, H. E. 3rd., Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6(8), 1183–1191 (2011).
Torres-Berrio, A. et al. DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218. Biol. Psychiatry. 81(4), 306–315 (2017).
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict. Biol. 11(1), 2–38 (2006).
Morris, J. S., Smith, K. A., Cowen, P. J., Friston, K. J. & Dolan, R. J. Covariation of activity in habenula and dorsal raphé nuclei following tryptophan depletion. Neuroimage 10(2), 163–172 (1999).
Lecca, S., Meye, F. J. & Mameli, M. The lateral habenula in addiction and depression: An anatomical, synaptic and behavioral overview. Eur. J. Neurosci. 39(7), 1170–1178 (2014).
Li, B. et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470(7335), 535 (2011).
Winter, C., Vollmayr, B., Djodari-Irani, A., Klein, J. & Sartorius, A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav. Brain Res. 216(1), 463–465 (2011).
Lutz, P. E. & Kieffer, B. L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 36(3), 195–206 (2013).
Tejedor-Real, P., Mico, J. A., Maldonado, R., Roques, B. P. & Gibert-Rahola, J. Implication of endogenous opioid system in the learned helplessness model of depression. Pharmacol. Biochem. Behav. 52(1), 145–152 (1995).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762), 864–868 (2006).
Kudryavtseva, N. N., Bakshtanovskaya, I. V. & Koryakina, L. A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 38(2), 315–320 (1991).
Bodnar, R. J. Endogenous opiates and behavior: 2018. Peptides 132, 170348 (2020).
Le Merrer, J., Becker, J. A., Befort, K. & Kieffer, B. L. Reward processing by the opioid system in the brain. Physiol. Rev. 89(4), 1379–1412 (2009).
Ashok, A. H., Myers, J., Reis Marques, T., Rabiner, E. A. & Howes, O. D. Reduced mu opioid receptor availability in schizophrenia revealed with [(11)C]-carfentanil positron emission tomographic imaging. Nat. Commun. 10(1), 4493 (2019).
Nummenmaa, L. et al. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology 45(11), 1953–1959 (2020).
Oddi, D., Crusio, W. E., D’Amato, F. R. & Pietropaolo, S. Monogenic mouse models of social dysfunction: implications for autism. Behav. Brain Res. 251, 75–84 (2013).
Moy, S. S. et al. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 3(5), 287–302 (2004).
Stein, D. J., van Honk, J., Ipser, J., Solms, M. & Panksepp, J. Opioids: From physical pain to the pain of social isolation. CNS Spectr. 12(9), 669–670 (2007).
Matthews, G. A. et al. Dorsal Raphe dopamine neurons represent the experience of social isolation. Cell 164(4), 617–631 (2016).
Walsh, J. J. et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560(7720), 589–594 (2018).
Bailly, J. et al. Targeting morphine-responsive neurons: Generation of a knock-in mouse line expressing cre recombinase from the mu-opioid receptor gene locus. eNeuro. 7(3), 433 (2020).
Yang, M., Weber, M. & Crawley, J. Light phase testing of social behaviors: Not a problem. Front. Neurosci. 2, 186–191 (2008).
Venniro, M. & Shaham, Y. An operant social self-administration and choice model in rats. Nat. Protoc. 15(4), 1542–1559 (2020).
Heilig, M., Epstein, D. H., Nader, M. A. & Shaham, Y. Time to connect: Bringing social context into addiction neuroscience. Nat. Rev. Neurosci. 17(9), 592–599 (2016).
Acknowledgements
We thank the Institut Clinique de la Souris—PHENOMIN, as well as Domain Therapeutics and Boehringer Ingelheim for their support to create the GPR151 knockout mouse line. Image acquisition and image analysis were performed on the Imaging Platform of the CRBS, PIC-STRA UMS 38, Inserm, Unistra. We thank Pascal Kessler for his help in the Imaging Platform. We thank Dr. Cecilia Flores for generously providing the equipment needed to conduct the chronic social defeat stress paradigm, Dr. Philip Vassilev for sharing his expertise to perform the technique and Tara Thomson for her help to conduct the experiment. We thank Dr. Sami Ben Hamida for scientific discussions around the project and technical advice on the different behavioral procedures. Finally, we thank DaWoon Park, Marine Garat, Florence Petit and Grace Adegunju.
Funding
This work was supported by the National Institute of Health (# DA005010 and # DA048796 to BLK), the Canada Fund for Innovation and the Canada Research Chairs to ED and BLK.
Author information
Authors and Affiliations
Contributions
Study design and funding: F.A., E.D. and B.L.K. Investigations and data analysis: F.A., E.D., S.D. and M.C. Manuscript writing: A.F., E.D. and B.L.K. All authors approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allain, F., Carter, M., Dumas, S. et al. The mu opioid receptor and the orphan receptor GPR151 contribute to social reward in the habenula. Sci Rep 12, 20234 (2022). https://doi.org/10.1038/s41598-022-24395-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24395-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.