Abstract
We explored the association between COVID-19 severity and vitamin D status using information from Danish nation-wide health registers, the COVID-19 surveillance database and stored blood samples from the national biobank. 25-hydroxyvitamin D (25(OH)D) was measured using tandem mass spectroscopy. The association between 25(OH)D levels and COVID-19 severity, classified hierarchical as non-hospitalized, hospitalized but not admitted to an intensive care unit (ICU), admitted to ICU, and death, was evaluated by proportional odds ratios (POR) assuming proportionality between the four degrees of severity. Among 447 adults tested SARS-CoV-2 positive in the spring of 2020, low levels of 25(OH)D were associated with a higher risk of severe COVID-19. Thus, odds of experiencing more severe COVID-19 among individuals with insufficient (25 to < 50 nmol/L) and sufficient (≥ 50 nmol/L) 25(OH)D levels were approximately 50% of that among individuals with deficient levels (< 25 nmol/L) (POR = 0.49 (95% CI 0.25–0.94), POR = 0.51 (95% CI 0.27–0.96), respectively). Dividing sufficient vitamin D levels into 50 to < 75 nmol/L and ≥ 75 nmol/L revealed no additional beneficial effect of higher 25(OH)D levels. In this observational study, low levels of 25(OH)D were associated with a higher risk of severe COVID-19. A possible therapeutic role of vitamin D should be evaluated in well-designed interventional studies.
Similar content being viewed by others
Introduction
The majority of those infected with SARS-CoV-2 will have mild symptoms, but some will experience severe clinical symptoms necessitating hospitalization, and in worst cases, death1,2. It is still uncertain why some are more prone to develop a more severe outcome of COVID-19, but risk factors such as older age, male sex, obesity, non-white race and co-morbidities seem to be of importance2. Findings of a potential beneficial effect of vitamin D on the incidence and the severity of acute respiratory tract infections suggests that vitamin D deficiency also could be linked to the outcome of COVID-19 illness3,4.
Several studies have reported lower levels of vitamin D among individuals who test positive for SARS-CoV-2 as compared with those who test negative5,6,7. Other studies were not supportive of an association between vitamin D status and risk of SARS-CoV-2 infection8,9,10 but, in at least one case, the null result could be explained by the use of vitamin D levels measured several years in the past10. Clinical severity among hospitalized COVID-19 patients, evaluated according to the possible existence of unconsciousness, respiratory symptoms, organ dysfunction, need of invasive mechanical ventilation, or death have also been linked to lower vitamin D levels in some studies11,12,13,14,15, but not all16,17,18,19.
Despite the discrepancy in previous studies, findings from many of the systematic reviews and meta-analyses support the hypothesis that vitamin D deficiency is related to a higher SARS-CoV-2 infection risk and worse disease outcome20,21,22,23. However, clinical and methodological heterogeneity hamper comparison of the previous studies resulting in less reliable pooled risk estimates22. Especially selection bias, lack of adjustment for relevant confounders, timing of vitamin D measurements, and varying definitions of vitamin D deficiency are issues of great concern5,6,12,14,15,16,18,22,24,25,26.
Vitamin D is known to modulate the immune system, and a possible beneficial effect of vitamin D on the outcome of COVID-19 could be through the prevention of the overreaction of the immune system e.g. the cytokine storm characteristic of severe COVID-1927,28. Vitamin D may also play a role in the production of antimicrobial peptides in the respiratory epithelium, the maintenance of the integrity of the cellular junctions and the control of cellular proliferation and angiogenesis27,28. Furthermore, genetic variations in the vitamin D binding protein gene, specifically SNP in rs7041 locus, have been found to correlate with the prevalence of COVID-19 and mortality rates, suggesting that genetic factors may also have a role29,30.
COVID-19 hospitalizations continue, despite the availability of COVID-19 vaccines, and it is therefore urgent to determine whether vitamin D deficiency has a role in the severity of COVID-19 infection. In the present study, we assess the association between vitamin D status and COVID-19 severity among Danish COVID-19 patients using the Danish National Patient Registry, the Cancer Registry, the COVID-19 surveillance database, which contains information on all SARS-CoV-2 PCR tests carried out in Denmark, and blood samples stored in the National Biobank. Using these resources enable us to select the most suitable blood sample for every COVID-19 patient and adjust for relevant confounders.
Methods
The study cohort
Residual blood samples from clinical biochemistry departments at two major hospitals in the Capital Region of Copenhagen have routinely been sent to and stored by the Danish National Biobank (DNB) at Statens Serum Institut (SSI). Biological samples in the DNB are by the unique personal ID number assigned to every Danish citizen by the Civil Registration System (CRS)31 linked to the National Patient Registry (NPR)32,33 which enabled us to match stored blood samples in DNB drawn from individuals registered with a hospital contact due to COVID-19 up to the beginning of May 2020.
Selection of blood samples for vitamin D measurements
A blood sample collected 1–30 days before registration of a COVID-19 diagnosis was preferred. If this was not possible, a blood sample drawn on the date of registration or 1–2 days after was selected, and if this could not be fulfilled, we selected the most recent blood sample collected up to 24 months before the COVID-19 registration. If none of the blood samples were drawn in this period, the person was excluded from the study.
Measurements of vitamin D
For each participants’ sample, 25-hydroxyvitamin D (25(OH)D) was measured using 30 µl of blood plasma. We measured 25-hydroxyvitamin D3, (25(OH)D3), which is the main circulating form of vitamin D, and the related ergosterol derived-form 25-hydroxyvitamin D2 (25(OH)D2). The assay was done at SSI and is highly sensitive using minimal sample cleanup to reduce sample loss during extraction, chemical derivatization to enhance 25(OH)D2 and 25(OH)D3 ionization, and liquid chromatography tandem mass spectroscopy coupled with multiple reaction monitoring34. Vitamin D level was calculated as the sum of 25(OH)D3 and 25(OH)D2.
Information on SARS-CoV-2 test results
To confirm the diagnosis of COVID-19, the study cohort was linked to the SSI COVID-19 surveillance database, which includes results for every SARS-CoV-2 PCR test carried out in Denmark since February 2020. The database is based on information from the Departments of Clinical Microbiology and Test Center Denmark and is updated daily. In addition to test results, it also contains information on hospitalizations, admissions to ICU, and COVID-19 related death35.
Severity of COVID-19
We defined onset of COVID-19 as date of a positive SARS-CoV-2 test and not as date of a hospital contact as we consider date of test to be closest to onset of first symptoms. A hospital contact related to COVID-19 was defined as a registration in the NPR with a diagnosis of COVID-19 (ICD-10 code; DB342A or DB972A) as primary or secondary diagnosis within 14 days after a positive test. However, if a hospitalized person with the above diagnostic codes tested positive within 2 days after admission, this hospitalization was also considered related to COVID-19. We defined four categories of increasing severity: (1) no hospital admission (incl. hospital contacts < 12 h), (2) hospitalized (≥ 12 h), but not admitted to ICU, (3) admitted to ICU, regardless of duration of hospital contact and (4) death within 30 days after a positive SARS-CoV-2 test.
Potential confounders
As older age, male sex, chronic diseases, obesity, and non-white race have been associated with vitamin D deficiency36,37, as well as with an increased risk of severe COVID-192, we consider those covariates as potential confounders. For each cohort member we therefore obtained detailed information on pre-existing diseases registered 5 years to 1 month prior to the positive SARS-CoV-2 test using information from the NPR32,33 and Cancer Registry32,38. The categories of pre-existing diseases were defined according to a modified version of the Charlson comorbidity index39 and we used the updated weights40 (Supplementary Table 1). Obesity was defined as any registration of clinical obesity in the NPR (ICD-10 codes: E65 or E66). We have no specific information on race/ethnicity, accordingly we used country of origin as proxy of race. Country of origin was defined using data from CPR on family relations and place of birth according to an algorithm developed by Statistics Denmark41.
Statistical methods
25(OH)D levels were modelled in predefined categories, (< 25 nmol/L (deficient), 25 to < 50 nmol/L (insufficient), and ≥ 50 nmol/L (sufficient)) and as a continuous variable. We a posteriori divided the category of sufficient 25(OH)D levels into two levels; 50 to < 75 nmol/L and ≥ 75 nmol/L.
Assuming proportionality between the 4 categories of severity we used ordinal logistic regression to calculate proportional odds ratios (POR) to evaluate the possible association between severity of COVID-19 and vitamin D levels. We calculated profile likelihood based 95% confidence intervals to account for the non-normal distribution of the POR. We furthermore stratified the analyses according to age at SARS-CoV-2 test (< 65 and ≥ 65 years) and sex.
To adjust for variation in 25(OH)D due to seasonality during blood sample collection, we regressed the 25(OH)D levels on the periodic function βsine sin(2ΠMi/12) + βcosinus cos(2ΠMi /12), where Mi is month of sample collection. The residuals from this model were added to the specific 25-hydroxyvitamin D means derived from the model to create an adjusted 25(OH)D as described by Munger et al.42. Besides adjusting for seasonality in vitamin D, we a priori included age, sex, Charlson comorbidity index, obesity and country of origin in the model.
Supplementary analyses
We evaluated the validity of the proportional odds assumption, by estimating odds ratios of the pairwise comparisons of COVID-19 hospitalization, ICU and fatal COVID-19 vs no hospitalization, respectively.
Sensitivity analyses
To further account for possible variations in vitamin D levels over calendar periods in 2018, 2019 and 2020 we restricted cohort members to those who had their blood samples drawn from the 1st of February 2020 to the 1st of May 2020. In a second sensitivity analysis we restricted cohort members to those who had a blood sample drawn 1–30 days before the positive SARS-CoV-2 test. We did this in order to minimize the risk of reverse causality i.e. that the COVID-19 illness would affect the person’s vitamin D level, and in order to eliminate the possibility of changes in vitamin D related behaviors after the blood sample was drawn e.g. beginning vitamin D supplementation.
A posteriori analysis
Vitamin D levels appeared surprisingly high among the deceased COVID-19 cases. We speculate that this could reflect prescribed vitamin D supplementation to frail older persons. We therefore omitted death from the four categories of COVID-19 severity and estimated the odds of experiencing a more severe outcome of COVID-19 among individuals who survived the COVID-19.
Ethics
This study was approved by the Danish National Committee on Health Research Ethics (H-20028195) and the Statens Serum Institut’s compliance department. We used already stored blood samples originally collected for other purposes. These blood samples were routinely sent to and stored by the National Biobank in Denmark. As the study was carried out (1) without active participation, (2) with no contact with any of the study participants, (3) without any health risks or other burdens for the persons whose data were used, and (4) including a large number of participants (originally approx. 2000), the Danish National Committee on Health Research Ethics granted us a dispensation from for the rule of obtaining consent in accordance with the Committee Act, section 16, subsection 3. All the analyses and the reporting of results have been done completely anonymously. The project group ensured that none of the blood sample owners had actively refused that their biological material could be used for research—this information is available in the Danish national “Tissue Use Register”.
Results
Overall, 587 individuals with a least one blood sample in the DNB were registered with COVID-19 in the Danish Biobank Register. 120 persons did not have a blood sample drawn within the accepted time interval and 9 did not have a physical blood sample in the DNB, reducing the number of COVID-19 cases to 458. After linkage with the COVID-19 surveillance database 8 out the 458 did not have a positive SARS-CoV-2 test, and finally 3 persons were excluded as they had no address in Denmark at the time of the test (Fig. 1). The distribution of sex, mean age and vitamin D level of the 8 patients who did not have a positive SARS-CoV-2 test despite a COVID-19 registration did not differ from that among the 447 patients included in the study (data not shown).
Table 1 shows the characteristics of the 447 cohort members according to predefined categories of vitamin D levels. Overall, 11%, 30% and 59% of the cohort members had vitamin D deficiency, insufficiency or sufficiency, respectively (Table 1). Vitamin D level was statistically significantly associated with sex, age, country of origin and comorbidity, but not with obesity or time interval between date of blood sample drawn and SARS-CoV-2 test. Against expectations, we observed that 68% (145/212) of the cohort members ≥ 70 years had sufficient vitamin D levels versus 50% among individuals < 70 years (118/235), and that vitamin D sufficiency was more common among individuals with comorbidities than among individuals without comorbidities (68% (115/168) versus 53% (148/279), respectively).
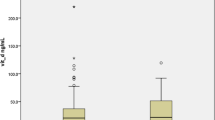
Among the 447 cohort members, 126 (28%) were not hospitalized, 205 (46%) were hospitalized for COVID-19, but not admitted to an ICU, 34 (8%) were admitted to an ICU and 82 (18%) died from COVID-19. Figure 2 shows individual vitamin D levels in a boxplot according to COVID-19 severity, age (< 65 year and ≥ 65 years) and sex. Lower levels of vitamin D were associated with higher COVID-19 severity among males younger than 65 years of age but not among women of same age. Among the older men and women, the relationship between vitamin D levels and severity of COVID-19 resembled a U-formed association i.e. highest vitamin D levels were observed among those who were not hospitalized and among those who did not survive COVID-19 (Fig. 2).
In the main analysis, the odds of experiencing a more severe outcome of COVID-19 (progressing to the next severity category), among individuals with insufficient and sufficient levels of 25(OH)D were approximately 50% of that among individuals with deficient levels (POR = 0.49 (95% CI 0.25–0.94) and POR = 0.51 (95% CI 0.27–0.96), respectively) adjusted for age, sex, comorbidity, seasonality, obesity and country of origin (hereinafter referred to as aPOR). Treating vitamin D levels as a continuous variable, the odds of experiencing a more severe outcome of COVID-19 was reduced by 3% per 5 nmol/L increase in vitamin D level (aPOR = 0.97 (95% CI 0.93–1.01)). However, this estimate did not reach statistically significance. No beneficial effect of increasing levels of vitamin D was observed in the analysis dividing the category of vitamin sufficiency into 50 to < 75 nmol/L and ≥ 75 nmol/L (Table 2).
Analyses stratified according to age (< 65 years, ≥ 65 years) and sex revealed estimates compatible with a higher severity of COVID-19 among vitamin D deficient males in all age groups and among women less than 65 years of age, whereas no such association was seen among women 65 years of age or older (Table 2). Numbers were few in the stratified analyses, as reflected by the rather wide confidence intervals surrounding the aPOR estimates, of which many did not reach statistically significance.
Supplementary analyses
To evaluate the validity of the proportional odds assumption, we estimated the odds of progression in COVID-19 disease severity from non-hospitalized to hospitalized, non-hospitalized to ICU treatment and finally from non-hospitalized to death, according to vitamin D level. Overall, these results supported the validity of the main analysis based on proportional odds regression in that sufficient and insufficient levels protected against severe COVID-19 vs no hospitalization in pairwise comparisons (Supplementary Table 2).
Sensitivity and a posteriori analyses
In the sensitivity analyses restricting cohort members to those with blood samples drawn in the spring of 2020 (n = 276) or to those with blood samples drawn within 1–30 days before positive test (n = 76), estimates remained supportive of a higher severity of COVID-19 among vitamin D deficient persons (Supplementary Table 3). Similar findings were observed in the a posteriori analyses in which we reduced the four severity levels to three levels by excluding cohort members who died within 30 days of a positive test (Supplementary Table 4).
Discussion
We studied the association between vitamin D level and severity of COVID-19 among 447 individuals who tested positive within the first 3 months of the SARS-CoV-2 epidemic in Denmark. Overall, we found that individuals with vitamin D deficiency were at a higher risk of progressing to a more severe clinical outcome of COVID-19.
Most of the previous hospital-based studies observed an association between vitamin D deficiency and a more critical outcome of COVID-19. Vitamin D deficiency has been associated with elevated risk of oxygen support43, ICU admission24, invasive mechanical ventilation11, severe sepsis/septic shock24, lung damage15, hypoxia13, death11,14,15, higher level of the inflammatory marker CRP13 and severe/critical COVID-19 illness defined according to severity scales developed by WHO or CDC13,14. However, other studies observed no association between vitamin D deficiency and clinical outcome of COVID-19, such as ICU treatment19, requirement for mechanical ventilation16,19, mortality16,19,25, length of hospitalization17,19 and severe/critical COVID-1917 (WHO definitions).
Although some previous studious found no effect, a recent meta-analysis carried out by Dissanayake et al. including nearly 2 million adults and 76 studies concluded that vitamin D deficiency/insufficiency probably increases susceptibility to COVID-19 and severe COVID-19, although with a high risk of bias and heterogeneity, whereas association with mortality is less robust22. Future rigorous prospective studies, using a standardized definition of vitamin D deficiency, insufficiency and sufficiency will be essential.
Heterogeneity could be due to different definitions of vitamin D deficiency and of COVID-19 severity. We followed the definition given by the Danish National Board of Health claiming that vitamin D levels less than 25 nmol/L are considered deficient and levels ≥ 50 nmol/L as sufficient44, but in other studies vitamin D deficiency was defined as < 30 nmol/L12 or < 50 nmol/L14,15 and sufficient vitamin D levels as ≥ 75 nmol/L17. We did, however, a posteriori divide the highest level of vitamin D into 50 nmol/L to < 75 nmol/L and ≥ 75 nmol/L and found no evidence of an increasing beneficial effect of higher vitamin D levels on COVID-19 severity.
We defined severity of COVID-19 according to data routinely recorded in Danish Nationwide Health and administrative registers which facilitates a uniform and easy replicable rating of severity. In contrast to many of the other studies on COVID-19 we included non-hospitalized COVID-19 patients, which could have caused bias. However, to get a SARS-CoV-2 test in Denmark in the spring of 2020, a person had to be referred by a physician who had to assess whether a clinical evaluation at a hospital was required. If this was not the case the person was referred to a regional test center45. We therefore consider the majority of non-hospitalized in our study as representative of those referred to a hospital for clinical evaluation, but due to milder symptoms the assessing hospital physician did not considered inpatient admission necessary45. Certain discrepancies in the procedures of COVID-19 testing and registrations in the Danish health care system may however have existed at the beginning of the epidemic.
We are aware of the fact that by defining COVID-19 mortality as deceased within 30 days after a positive SARS-CoV-2 test, we may have included individuals who did not die due to COVID-19, but due to unrelated conditions. However, the proportion of deceased individuals infected with SARS-CoV-2 who died due to other causes than COVID-19 within 30 days after the test is considered to be low in Denmark until December 2021, the appearance of the omicron variant46.
A rather high proportion of the deceased COVID-19 patients in the present study had sufficient vitamin D levels i.e. ≥ 50 nmol/L. We speculate that this could be due to vitamin supplementation among the more vulnerable and elderly population. To evaluate the influence of confounding by indication we a posteriori repeated our analysis omitting study cohort members who did not survive COVID-19 and found compatible estimates.
Another important source to heterogeneity is timing of vitamin D measurements in relation to the test. Previous studies have tested vitamin D levels at time of admission, some during admission and few within the preceding 10 years22. Due to risk of reverse causality and possible vitamin D modifying conditions after the blood samples were drawn, e.g. use of vitamin D supplementation, we would have preferred to measure vitamin D in blood samples drawn 1–30 days before start of symptoms. This criterion could only be met for 18% of the COVID-19 patients in the present study. Although estimates lost statistical significance and confidence intervals were broad, we consider the results based on this smaller group of participants compatible with vitamin D deficiency being associated with an elevated risk of a more severe outcome of COVID-19.
We addressed the association between vitamin D status and COVID-19 severity among individuals infected in the spring of 2020 i.e. at the beginning of the epidemic. Several different variants of SARS-CoV-2 have appeared since then, with different patterns of severity and contagiousness, thus our findings may not be applicable to all SARS-CoV-2 infected individuals. Furthermore, COVID-19 vaccinations was initiated in Denmark in December 2020. The association between vitamin D status and severity of COVID-19 in the present study is therefore observed in a group of non-vaccinated persons.
We defined a COVID-19 related hospital contact as a registration in the NPR with a diagnosis of COVID-19 (ICD-10 code; DB342A or DB972A) as primary or secondary diagnosis within 14 days after a positive test or 2 days before a positive test. In situations where COVID-19 is recorded as a secondary diagnosis, we may have included some individuals hospitalized due to other causes than COVID-19, who was incidentally found SARS-CoV-2 positive. However, 87% of the COVID-19 hospitalizations in the present study were hospital contacts with a primary diagnosis of DB342A or DB972A. Another limitation could be false positive PCR test results. The specificity of the PCR test in Denmark has, however, been estimated as 99.85%, and accordingly we do not consider false positive PCR tests to have any influence on our results47.
Chronic health conditions have been shown to affect vitamin D levels and COVID-19 severity. We therefore adjusted for pre-existing diseases and obesity, however we cannot rule out the existence of residual confounding due to other health conditions not included in the Charlson comorbidity index.
Conclusion
In this Danish observational study of 447 COVID-19 patients, we observed that deficient vitamin D levels were associated with an elevated risk of progressing to a more severe COVID-19 outcome.
The possible role of vitamin D supplementation in the treatment of COVID-19 has already been assessed in randomized controlled trials, but as pointed out in recent meta-analyses, large variations in vitamin D supplementation schemes in the hitherto published studies means that no definitive conclusion can be drawn on the therapeutic effect of vitamin D, until larger well-designed interventional studies addressing issues such as the appropriate dose, duration and mode of vitamin D administration have been completed48,49,50.
Data availability
The data in the present study are considered person-sensitive, and cannot be shared due to data protection regulations.
Change history
31 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-28993-3
References
Gandhi, R. T., Lynch, J. B. & Del Rio, C. Mild or moderate Covid-19. N. Engl. J. Med. 383(18), 1757–1766 (2020).
St Sauver, J. L. et al. Factors associated with severe COVID-19 infection among persons of different ages living in a defined midwestern US population. Mayo Clin. Proc. 96(10), 2528–2539 (2021).
Martineau, A. R. et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 356, i6583 (2017).
Sabetta, J. R. et al. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 5(6), e11088 (2010).
Meltzer, D. O. et al. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open 3(9), e2019722 (2020).
D’Avolio, A. et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 12(5), 1359 (2020).
Merzon, E. et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 287(17), 3693–3702 (2020).
Li, Y. et al. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open 4(5), e2111634 (2021).
Raisi-Estabragh, Z. et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health (Oxf) 42(3), 451–460 (2020).
Hastie, C. E. et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 14(4), 561–565 (2020).
Angelidi, A. M. et al. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: A cohort of COVID-19 hospitalized patients. Mayo Clin. Proc. 96(4), 875–886 (2021).
Luo, X. et al. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people [corrected]. J. Nutr. 151(1), 98–103 (2021).
Maghbooli, Z. et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One 15(9), e0239799 (2020).
Dror, A. A. et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS One 17(2), e0263069 (2022).
De Smet, D. et al. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am. J. Clin. Pathol. 155(3), 381–388 (2021).
Hernandez, J. L. et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 106(3), e1343–e1353 (2021).
Davoudi, A. et al. Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection. BMC Infect. Dis. 21(1), 450 (2021).
Hastie, C. E., Pell, J. P. & Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 60, 545–548 (2021).
AlKhafaji, D. et al. The impact of vitamin D level on the severity and outcome of hospitalized patients with COVID-19 disease. Int. J. Gen. Med. 15, 343–352 (2022).
Liu, N. et al. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 104, 58–64 (2021).
Pereira, M. et al. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 62, 1308–1316 (2022).
Dissanayake, H. A. et al. Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 107, 1484–1502 (2022).
Petrelli, F. et al. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol. 211, 105883 (2021).
Charoenngam, N. et al. Association of vitamin D status with hospital morbidity and mortality in adult hospitalized patients with COVID-19. Endocr. Pract. 27(4), 271–278 (2021).
Jude, E. B. et al. Vitamin D deficiency is associated with higher hospitalisation risk from COVID-19: A retrospective case-control study. J. Clin. Endocrinol. Metab. 106, e4708–e4715 (2021).
Carpagnano, G. E. et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 44(4), 765–771 (2021).
Alshahawey, M. COVID-19 and vitamin D deficiency; the two pandemics. Are they correlated?. Int. J. Vitam. Nutr. Res. 91(5–6), 383–384 (2021).
Benskin, L. L. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front. Public Health 8, 513 (2020).
Alshahawey, M. A genetic insight into vitamin D binding protein and COVID-19. Med. Hypotheses 149, 110531 (2021).
Karcioglu Batur, L. & Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 93(3), 1409–1413 (2021).
Pedersen, C. B. et al. The Danish Civil Registration System. A cohort of eight million persons. Dan. Med. Bull. 53(4), 441–449 (2006).
Sundhedsdatastyrelsen. De nationale sundhedsregistre. https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre (Accessed 10 April 2022).
Schmidt, M. et al. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 7, 449–490 (2015).
Eyles, D. et al. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin. Chim. Acta 403(1–2), 145–151 (2009).
https://miba.ssi.dk/forskningsbetjening/tilgaengelig-data (Accessed 10 April 2022).
Hansen, L. et al. Vitamin D status and seasonal variation among Danish children and adults: A descriptive study. Nutrients 10(11), 1801 (2018).
Amrein, K. et al. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 74(11), 1498–1513 (2020).
Gjerstorff, M. L. The Danish Cancer Registry. Scand. J. Public Health 39(7 Suppl), 42–45 (2011).
Quan, H. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 43(11), 1130–1139 (2005).
Ternavasio-de la Vega, H. G. et al. The updated Charlson comorbidity index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia. Epidemiol. Infect. 146(16), 2122–2130 (2018).
Statistics Denmark. https://www.dst.dk/en/Statistik/dokumentation/documentationofstatistics/immigrants-and-descendants-discontinued-/statistical-presentation (Accessed 18 November 2021).
Munger, K. L. et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296(23), 2832–2838 (2006).
Gavioli, E. M. et al. An evaluation of serum 25-hydroxy vitamin D levels in patients with COVID-19 in New York City. J. Am. Nutr. Assoc. 41, 201–206 (2022).
The National Board of Health, Denmark. Forebyggelse, diagnostik og behandling af D-vitaminmangel. https://www.sst.dk/-/media/Udgivelser/2020/D-vitamin/D-vitamin-baggrundsnotat-27052010-med-opdatering-af-UL-2016-og-D-vitamintilskud-2020.ashx?la=da&hash=20549ACABF9846A21DC0EA6AE2F3FB0AB08FB5AA (2010).
The National Board of Health, Denmark. Retningslinjer for håndtering af covid-19 i sundhedsvæsenet. https://www.sst.dk/da/Udgivelser/2022/Retningslinjer-for-haandtering-af-covid-19 (2020).
Statens Serum Institut. Overvågning af dødeligheden i Danmark under covid-19 epidemien. https://www.ssi.dk/-/media/arkiv/subsites/covid19/overvaagningsdata/ugentlige-opgoerelser-med-overvaagningsdata/overvaagning-af-doedeligheden-i-danmark-17022022.pdf?la=da (2022).
Staerk-Ostergaard, J. et al. Evaluation of diagnostic test procedures for SARS-CoV-2 using latent class models. J. Med. Virol. 94(10), 4754–4761 (2022).
Pal, R. et al. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 45(1), 53–68 (2022).
Bania, A. P. K. et al. Therapeutic Vitamin D supplementation following COVID-19 diagnosis: Where do we stand?—A systematic review. J. Pers. Med. 12, 419 (2022).
Stroehlein, J. K. et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 5, CD015043 (2021).
Acknowledgements
The Danish Departments of Clinical Microbiology (KMA) and Statens Serum Institut carried out laboratory analyses, registration, and release of the national SARS-CoV-2 surveillance data for the present study.
Funding
COVID-19 research at the Danish National Biobank has been supported by a grant from Novo Nordisk Foundation NNF20SA0062871.
Author information
Authors and Affiliations
Contributions
A.A. contributed to the conception and idea of the work. N.M.N., A.S.C., L.B. and A.A. contributed to the design of the study. N.M.N., A.S.C., L.B., S.G.B. contributed to the acquisition of data. N.M.N., A.H., L.B., S.G.B. and T.G.J. contributed to the analyses of the data. All authors contributed to the interpretation of the data and the results in the manuscript. N.M.N. drafted the manuscript, made Fig. 1, T.G.J. made Fig. 2. All the authors revised the manuscript critically and has approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: Sanne Grundvald Boelt was omitted from the author list in the original version of this Article. The Author Contributions section now reads: “A.A. contributed to the conception and idea of the work. N.M.N., A.S.C., L.B. and A.A. contributed to the design of the study. N.M.N., A.S.C., L.B., S.G.B. contributed to the acquisition of data. N.M.N., A.H., L.B., S.G.B. and T.G.J. contributed to the analyses of the data. All authors contributed to the interpretation of the data and the results in the manuscript. N.M.N. drafted the manuscript, made Fig. 1, T.G.J. made Fig. 2. All the authors revised the manuscript critically and has approved the final version.”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nielsen, N.M., Junker, T.G., Boelt, S.G. et al. Vitamin D status and severity of COVID-19. Sci Rep 12, 19823 (2022). https://doi.org/10.1038/s41598-022-21513-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21513-9
This article is cited by
-
High-dose vitamin D3 supplementation shows no beneficial effects on white blood cell counts, acute phase reactants, or frequency of respiratory infections
Respiratory Research (2024)
-
Association between vitamin D deficiency and post-acute outcomes of SARS-CoV-2 infection
European Journal of Nutrition (2024)
-
Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy
Inflammopharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.