Abstract
We analysed corrosion from a copper bowl dating from the Roman period (43–410 AD) found in a farm in Kent, UK. Despite its relatively good condition, the interior and exterior surface of the object had areas of deterioration containing green and brown-coloured corrosion which were sampled for characterization by a multi-analytical protocol. Basic copper chlorides atacamite and paratacamite were identified in the context of mineral phases along with chlorobenzenes in the green corrosion. Chlorobenzenes are common soil contaminants in rural areas from the use of pesticides, many of which were banned more than 50 years ago. Here we show that their presence is associated with accelerated corrosion, and this provides a threat to the preservation of archaeological metal objects in the ground.
Similar content being viewed by others
Introduction
In November 2016, a metal detectorist discovered a copper bowl (Fig. 1A) whilst scanning a grassy track in an orchard at a farm near Wingham in Kent, UK. After the discovery, the bowl was left protected in situ so that it could be excavated a month later by a team from Canterbury Archaeological Trust and Dover Archaeological Group. A 2-m area around the bowl was exposed but no cremation or burial deposits associated with the it were found. The archaeologists block-lifted the objects in its surrounding soil and transported it to the CSI: Sittingbourne laboratory1 for conservation (Figs. 1B,C) in preparation for its display at Sandwich Museum (Fig. 1D).
The recent history of the Roman Bowl–(A) the area where the bowl was found in relation to other Roman sites, exact findspot cannot be shown to protect the site (map created by Luciana da Costa Carvalho using https://webapps.kent.gov.uk/KCC.HeritageMaps.Web.Sites.Public/Default.aspx; (B) the interior and (C) exterior of the bowl during conservation and (D) the bowl on display at Sandwich Museum.
The bowl was found mid-way between the important Roman settlements at Richborough and Canterbury, close to two large Roman villas and Roman watermills2.
It was found buried at a depth of 40 cm in a clay soil with chalk inclusions. Features revealed during the excavation indicate that the bowl was deliberately placed in a ditch within a settlement site previously unknown to archaeology. Pottery sherds and coins found in the site indicate that it originated in the Late Iron Age, with continued occupation into the later Roman times1.
During conservation, samples of the brown and green corrosion from the interior and exterior surface of the bowl were collected for analysis by a protocol combining different analytical techniques developed to target organic residues trapped in copper corrosion3 comprising of:
-
X-ray powder Diffraction (XRD) for the identification of mineral phases;
-
Fourier Transform Infrared (FTIR) for the identification of the chemical fingerprint;
-
Gas Chromatography with quadrupole time-of-flight mass spectrometry (GC-QTOF-MS) with a thermal separation probe (TSP) for recovery and identification of organic molecules.
Results
Basic copper chlorides identified as mineral phases in the green corrosion
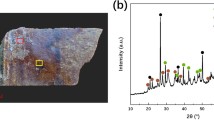
The mineral phases identified in the green corrosion from the interior of the bowl (Fig. 2A) were atacamite Cu2Cl(OH)3 and quartz. Atacamite was also identified as the main mineral phase in the exterior green corrosion (Fig. 2B), which also contained paratacamite Cu3(OH)6Cl2 and cuprite Cu2O. Atacamite and paratacamite are basic copper chlorides which interconvert4, and are typical copper corrosion products formed under acidic conditions and in an environment rich in chloride ions5.
The only mineral phase identified in the brown corrosion samples (Fig. 3) was quartz.
Spectroscopy analysis found evidence for chlorides and aromatic compounds in green corrosion
The FTIR spectra obtained for the green corrosion samples have poorly resolved bands and are practically identical (Fig. 4). The most intense bands appear at higher frequencies at around 3447, 3356 and 3317 cm−1 and are associated to O–H and/or N–H stretching vibrations. In the fingerprint region (i.e. below 1500 cm−1) bands are visible around 987, 582 and 514 cm−1. Some of these bands may result from the contribution of atacamite’s spectrum (Fig. 4 inset).
The broad band between 1600 and 1400 cm−1 may indicate the presence of an aromatic ring system, which normally appears as four bands. The absence of strong bands at 2900–2880 cm−1 indicates that waxes, resins or oils are either absent or present at quantities below the detection limit of the technique.
There was little difference between the spectra of the brown corrosion samples (included in the Supplementary Information Figure S1). Both spectra contained poorly defined peaks: a broad band centred around 3400 cm-1 due to O–H/N–H stretching vibrations and a strong broad peak (better defined in the spectrum of the exterior sample) at around 1050 cm-1 for Si–O stretching in silicates and/or C–O stretching in polysaccharides6. As in the green corrosion samples, no peaks were visible in the 2900–2880 cm-1 region.
Chlorinated aromatic compounds identified in the green corrosion
Mass chromatograms obtained from the analysis of the green corrosion material by GC-QTOF-MS with TSP (Fig. 5) contain high intensity peaks identified as hexadecanoic acid [8] and chlorobenzenes [1, 2, 4 and 5]. Chlorobenzenes are synthetic compounds used in the production of organic chemicals (as solvents), in deodorants, fumigants, herbicides and as pesticides7. The mass chromatogram obtained from the analysis of the interior sample (Fig. 5A) also contains a major peak for octadecanoic acid [10], which appears at a lower intensity in the chromatogram of the exterior sample (Fig. 5B). Pentadecanoic acid [6] and methyl esters [7 and 9] were only identified in the interior sample, with unassigned peaks for long-chain alkanes, alkenes and alkanols, possibly of plant origin, present in both samples.
GC–MS mass chromatograms from the analysis of green corrosion material from the interior (A) and exterior (B) of the excavated bowl. Compounds identified by accurate mass and fragmentation pattern matching with NIST library labelled as: [1] 1,2,3-trichlorobenzene; [2] 1,2,4,5-tetrachlorobenzene; [3] 1,2,3,5-tetrachlorobenzene [4]; pentachlorobenzene; [5] hexachlorobenzene; [6] pentadecanoic acid; [7] hexadecanoic acid, methyl ester; [8] n-hexadecanoic acid; [9] methyl stearate; [10] n-octadecanoic acid; [*] non-specific compounds from plant material. Spectra associated with chlorobenzenes’ assignments included in Supplementary Figs. S2-6.
The mass chromatogram obtained from the analysis of the brown corrosion from the interior of the bowl (Fig. 6A) is dominated by two peaks: hexadecenoic acid [8] and diethyltoluamide (DEET) [4], its accurate mass and fragmentation pattern matching is included in Supplementary Figure S7. DEET was developed by the United States Army in 19468, and is the active ingredient in many insect-repellent products for topical use in humans and livestock9. There is also evidence of DEET in the exterior sample (peak 4 in Fig. 6B), where the second most prominent peak was identified as octadecanoic acid [9]. Amongst the unidentified compounds in both samples are possible compounds of plant origin and polycyclic substances, which could not be assigned to a specific compound.
GC–MS mass chromatograms from the analysis of brown corrosion material from the interior (A) and exterior (B) of the excavated bowl. Compounds identified by accurate mass and fragmentation pattern matching with NIST library labelled as: [1] Methenamine; [2] Ceteneo; [3] 2,4-Di-tert-butylphenol; [4] Diethyltoluamide (DEET); [5] N-ethyl-2-methyl-benzenesulfonamide,; [6] 2-Propanol, 1-chloro-,phosphate (3:1); [7] Hexadecanenitrile; [8] n-hexadecanoic acid; [9] octadecanoic acid; [*] plant material; [o] polycyclic compounds. Spectra associated with DEET assignment included in Supplementary Fig. S7.
Discussion
Factors affecting corrosion of the bowl
The most important parameters governing the corrosion of copper-alloy objects in soils are moisture, salt content, temperature, acidity and aeration10,11,12,13. The geology of the site where the bowl was found is generally described as boulder clay over chalk bedrock14,15, freely drained and slightly acidic16. According to short-term corrosion studies, copper and its alloys are expected to develop only sporadic localized pit corrosion in this type of soil17. However, it is expected that the chemical characteristics of the soil where the Roman bowl was found would have been affected by human agency over more than a thousand years during the time the object had been buried. A Swedish study13,18 found that recently-excavated bronzes were more extensively corroded compared to other bronze objects from the same site in museum collections. The researchers attributed the worst state of preservation of recently-excavated metal objects to an increase in soil acidity due to anthropogenic pollution in the last 50–100 years.
The site where the bowl was found has been used for agriculture since at least 1936 and, in the last ten-twenty years, planted as an orchard. Hexachlorobenzene (HCB), tri, tetra and penta-chlorobenzenes were used in agriculture as fungicides, herbicides, insecticides and pesticides19. In soils, highly substituted chlorobenzenes degrade via a range of reductive de-chlorination reactions20,21, essentially. Consisting of cleavage of the C–Cl bond following an electron transfer22,23, often by microbial action under anaerobic conditions24,25. Chlorobenzenes of lower substitution number are less toxic and more susceptible to microbial degradation26,27,28, with eventual mineralization into carbon dioxide, water and chloride ions29,30. The presence of metals31, including copper32,33,34,35, catalyse the degradation of HCB, with the opposite effect observed when co-existing chlorobenzenes are present under a neutral to alkaline pH36,37.
The identification of chlorobenzenes and basic copper chlorides in the bowl’s green corrosion suggests a relationship between these compounds because the soil where the object was found is characterized by a low concentration of chloride ions38. The heterogeneity of metallurgical features of the copper alloy39,40 used to produce the bowl and damage to the protective copper oxide layer would have supported the formation of anodic pits41. Anodic pits are positively-charged areas that are likely to have attracted chloride ions from the degradation of chlorobenzenes, leading to the formation of basic copper chlorides. This corrosion mechanism is supported by the fact that neither chlorobenzenes nor copper corrosion mineral phases were identified in the brown samples (composed by quartz mixed with non-chlorinated organic compounds).
Archaeological evidence for the effect of agrochemicals on the corrosion of buried copper alloy objects is limited to the effect of fertilizers42. In 2004, a group of scientists attempted to gather empirical evidence that agrochemicals accelerate the corrosion of metal objects buried in the soil43 but the difficulty in corroding copper in soil had a negative impact in their laboratory experiments44. Notwithstanding these difficulties, the researchers’ geochemical modelling predicts the following order of corrosiveness with respect to inorganic fertilisers: KCl (muriate of potash) > nitrogen > phosphorus45. The K-Cl bond disassociation energy is 427 kJ/mol while for C–Cl bond in thirteen chlorobenzenes range between 375 to 399 kJ/mol46, making chlorobenzenes more corrosive than KCl-based fertilizers.
Sources and fate of chlorobenzenes
Chlorobenzenes are synthetic compounds formed by the addition of up to 6 chlorine atoms to a benzene ring. Given the low solubility of chlorobenzenes in water (which is inversely proportional to the number of chlorine atoms), they are relatively resistant to chemical degradation. HCB is the most unreactive and well-studied chlorobenzene47,48. It was introduced as an agricultural pesticide in 194549, with emissions peaking in mid-1960s. Given its toxicity to humans50,51, and ability to accumulate in the environment and living organisms52, HCB is considered a persistent pollutant and its in agriculture is now restricted in most countries53.
Although HCB has been banned in the UK since 1975, analyses of 1968–1990 archived rural soils did not reveal a pronounced decline in HCB detected in soil samples collected since restrictions were introduced54. It is possible these results also include contributions from secondary sources such as industrial emissions, application of pesticides containing HCB51 such as dimethyl tetrachloroterephthalate (DCPA, traded as Dacthal), pentachloronitrobenzene (sold as PCNB Terraclor, Engage, and Defend), hexachlorocyclohexane (trade name Technical HCB or Mirex), pentachlorophenol and Picloram)55, sewage sludge56 and irrigation with contaminated water. The complexity of environmental contamination by persistent pollutants is exemplified by Diethyltoluamide (DEET)57 detected in the bowl’s brown corrosion. DEET is mainly associated with insect-repellent of personal use and an emerging pollutant in England’s groundwater systems, with the highest abundance found in natural settings58.
Potential policy implications
In the UK, the arrival of the twentieth century marked the development of archaeology as a professional discipline59. Bombing of cities during the Second World War exposed many archaeological sites, with the creation of many Planning Acts to control the redevelopment of these sites and funds allocated to support excavations. English Heritage was created in 1983 to advise the government on heritage matters, with similar institutions created in Wales, Scotland and Northern Ireland. Initially English Heritage had its own field excavation unit, which was eventually closed in 1990. This closure coincided with the adoption of Planning Policy Guidance Note 16 (PPG16), which made archaeology a material factor to be considered before determining planning applications for the redevelopment of sites. PPG16 states that when construction works would disturb the archaeological remains present in a site, the developer may be obliged to excavate, record and publish those assets. The adoption of PPG16 and subsequent legislation secured access to sites and funding for archaeological excavations, creating an unprecedented demand for commercial archaeological services60. By 2007, 93% of all archaeological excavations in the UK were developer-led61.
Under this legal framework, it is implied that archaeological remains buried underground are largely protected from damage, particularly in agricultural land if buried below the topsoil (and thus protected from mechanical disturbances). In the case of metal objects, it is presumed that corrosion rates would have slowed down since initial deposition, with chemical reactions reaching an equilibrium with the burial environment. Excavation exposes these objects to different environmental conditions from those in the deposition environment such as an increase in oxygen levels and humidity which can promote further corrosion processes.
A small number of studies have been published linking corrosion of archaeological metal objects to agricultural activities42,62 but these have been limited to the identification of “unusual” mineral phases. However, our study indicates that archaeological copper alloy objects provide a sink for chlorobenzenes, producing more familiar diagnostic chemistry (i.e. atacamite and paratacamite), therefore presenting an opportunity to systematically evaluate corrosion products from freshly-excavated objects. Such evaluation would provide a clearer picture of the impact of soil pollutants on archaeological objects and the means to monitor their distribution.
In the UK metal detectorists are responsible for most of the metal finds coming out of rural lands, which are reported under the Portable Antiquities Scheme (PAS)63. Corrosion samples could be submitted for characterization through the PAS platform enabling correlation of results with historical records of land use and soil environmental surveys. Such an initiative would provide metal detectorists, who are often marginalized by heritage professionals64, an opportunity to contribute to cultural and environmental heritage management policies. Publishing material to raise awareness about the effect of pollutants on copper alloy objects amongst this community and mechanism for reporting it would be the first step to facilitate engagement.
Conclusion
We found evidence of chlorobenzenes amidst corrosion from an archaeological copper alloy bowl excavated from a rural site. Chlorobenzenes are synthetic compounds released in the environment through agricultural and industrial activities. Given their toxicity and persistence in the environment, many countries have taken steps to control their release. Our study provides the first evidence that chlorobenzenes are associated with accelerated corrosion mechanisms linked to archaeological material and demonstrates that they are a threat to the preservation of archaeological metals in the ground.
Methods
Fourier-transform infrared (FTIR)
Samples were analysed in transmittance mode using the KBr pellet method in an Excalibur Series Varian UMA600. Measurements were taken between the 4000-400 cm−1 range, 64 scans and included background subtraction. Data was processed using Digilab Resolutions Pro 4.0 software and figures created with Spectragryph v.1.2.12.
Gas chromatography quadrupole time-of-flight mass spectrometry using a thermal separation probe (GC-QTOF-MS with TSP)
Around 4 mg of sample ground to a powder in an agate mortar was transferred to a glass microvial and placed inside the TSP attached to an Agilent 7890B gas chromatograph equipped with a Restek Rxi-5 ms column (30 m × 320 μm × 0.25 μm) attached to an Agilent 7250 GC/QTOF mass spectrometer equipped with a low-energy-capable electron ionization source operating at 70 eV. The TSP was set at 300 °C. The oven temperature was set at 40 °C for one minute, increasing by 20 °C/minute until 320 °C, where it was held for five minutes. Helium was used as a carrier gas, at 1.43 mL/min flow rate and 8.70 psi pressure. The equilibration time was set at 0.5 min and the sample injection was splitless. The mass range was 50 to 650 m/z. All samples were run in triplicate.
Data analysis was performed with Agilent Mass Hunter Qualitative Analysis 10.0 with compound assignments using NIST Library 17. Only compounds with a match factor (MF) and reverse match factor (RMF) > 700 and accuracy mass error below 50 ppm were shortlisted.
X-ray powder diffraction (XRD)
The sample was pulverized and set on a silicon plate and analysed using a PANalytical X'Pert PRO Cu alpha instrument set to operate in continuous mode at 40 kV/40 mA with a scanned area set between 1 and 70° 2θ, 0.02 step size and 3° per minute.
Data was processed using QualX© software with phase identification using the Crystallography Open Database (COD).
Data availability
The raw datasets are available from the corresponding author on reasonable request.
References
Goodburn-Brown, D. & Price, V. CSI: Sittingbourne: Conservation science investigations in a town center shopping mall. In: Science and Technology for the Conservation of Cultural Heritage (eds Rogerio-Candelera M.Á., Lazzari, M. & Cano Cerdán, E.) 393–6 (CRC Press, 2013).
Parfitt K & Richardson A. Report on excavations off Preston Hill, Wingham. (Canterbury, CAT, 2016).
Carvalho, LdC. Beyond Copper Soaps : Characterization of Copper Corrosion Containing Organics. Springerbriefs in Applied Sciences and Technology series. (SpringerNature, 2022)
Pollard, A. M. et al. Synthesis and stabilities of the basic copper(II) chlorides atacamite, paratacamite and botallackite. Mineral. Mag. 53, 557–563 (1989).
Scott, D. A. A review of copper chlorides and related salts in bronze corrosion and as painting pigment. Stud. Conserv. 45, 39–53 (2000).
Smidt, E. & Meissl, K. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 27, 268–276 (2007).
US Environmental Protection Agency. Health Assessment for Chlorinated Benzenes EPA/600/8–84–015F. (USEPA, 1985).
US Environmental Protection Agency. Reregistration eligibility decision (RED). DEET. EPA738-R-98-010. (USEPA, 1998).
Cheng, Y. et al. Chemical characteristics and origins of nitrogen-containing organic compounds in PM2.5 aerosols in the lower fraser valley. Environ. Sci. Technol. 40, 5846–5852 (2006).
Tylecote, R. F. The effect of soil conditions on the long-term corrosion of buried tin-bronzes and copper. J. Archaeol. Sci. 6, 345–368 (1979).
Mattsson, E. et al. Deterioration of archaeological material in soil: results on bronze artefacts (Swedish National Heritage Board, Stockholm, 1997).
Selwyn, L. Metals and corrosion: A handbook for the conservation professional (Canadian Conservation Institute, 2004).
Scott, D. A. Copper and bronze in art: Corrosion, colorants, conservation. (Getty Conservation Institute, Los Angeles, 2002).
British Geological Survey (BGS) ID: 12083418 : BGS Reference: TR25NW60 British National Grid (27700) : 624000,158000 http://mapapps2.bgs.ac.uk/geoindex/home.html?layer=BGSBoreholes
British Geological Survey (BGS) ID: 18139205 : BGS Reference: TR25NW77 British National Grid (27700) : 624700,158000 http://mapapps2.bgs.ac.uk/geoindex/home.html?layer=BGSBoreholes
Cranfield Soil and Agrifood Institute. SoilScapes. http://www.landis.org.uk/soilscapes/
Nord, A. G. et al. Factors influencing the long-term corrosion of bronze artefacts in soil. Prot. Met. 41, 309–316 (2005).
Ullén, I. et al. The degradation of archaeological bronzes underground: Evidence from museum collections. Antiquity 78, 380–390 (2004).
Courtney, K. D. Hexachlorobenzene (HCB): A review. Environ. Res. 20, 225–266 (1979).
Hardman, D. J. Biotransformation of halogenated compounds. Crit. Rev. Biotechnol. 11, 1–40 (1991).
Bollag, J.-M. Microbial Transformation of Pesticides. In Advances in Applied Microbiology 18 (ed. Perlman, D.) 75–130 (Academic Press, 1974).
Dolfing, J. & Harrison, B. K. Redox and reduction potentials as parameters to predict the degradation pathway of chlorinated benzenes in anaerobic environments. FEMS Microbiol. Ecol. 13, 23–29 (1993).
Yin, H. et al. Insights into electroreductive dehalogenation mechanisms of chlorinated environmental pollutants. ChemElectroChem 7, 1825–1837 (2020).
Bosma, T. N. P. et al. Reductive dechlorination of all trichloro- and dichlorobenzene isomers. FEMS Microbiol. Ecol. 4, 223–229 (1988).
Adrian, L. & Görisch, H. Microbial transformation of chlorinated benzenes under anaerobic conditions. Res. Microbiol. 153, 131–137 (2002).
Reineke, W. & Knackmuss, H. J. Microbial metabolism of haloaromatics: Isolation and properties of a chlorobenzene-degrading bacterium. Appl. Environ. Microbiol. 47, 395–402 (1984).
Schraa, G. et al. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. Strain A175. Appl. Environ. Microbiol. 52, 1374–1381 (1986).
Spain, J. C. & Nishino, S. F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl. Environ. Microbiol. 53, 1010–1019 (1987).
Guerin, T. F. Ex-situ bioremediation of chlorobenzenes in soil. J. Hazard. Mater. 154, 9–20 (2008).
Brahushi, F. et al. Fate processes of chlorobenzenes in soil and potential remediation strategies: A review. Pedosphere 27, 407–420 (2017).
Alonso, F. et al. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 102, 4009–4092 (2002).
Raut, S. S. et al. Efficacy of zero-valent copper (Cu0) nanoparticles and reducing agents for dechlorination of mono chloroaromatics. Chemosphere 159, 359–366 (2016).
Weidlich, T. The influence of copper on halogenation/dehalogenation reactions of aromatic compounds and its role in the destruction of polyhalogenated aromatic contaminants. Catalysts 11, 378 (2021).
Hagenmaier, H. et al. Copper-catalyzed dechlorination/hydrogenation of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and other chlorinated aromatic compounds. Environ. Sci. Technol. 21, 1085–1088 (1987).
Alderman, S. L. et al. An infrared and X-ray spectroscopic study of the reactions of 2-chlorophenol, 1,2-Dichlorobenzene, and chlorobenzene with model CuO/silica fly ash surfaces. Environ. Sci. Technol. 39, 7396–7401 (2005).
Jiang, L. et al. Influence of degradation behavior of coexisting chlorobenzene congeners pentachlorobenzene, 1,2,4,5-tetrachlorobenzene, and 1,2,4-trichlorobenzene on the anaerobic reductive dechlorination of hexachlorobenzene in dye plant contaminated soil. Water Air. Soil Pollut. 226, 1–9 (2015).
Ramanand, K. et al. Reductive dehalogenation of chlorinated benzenes and toluenes under methanogenic conditions. Appl. Environ. Microbiol. 59, 3266–3272 (1993).
Rawlins, B. G. et al. Chlorine (Cl). In The Advanced Soil Geochemical Atlas of England and Wales. 58–61 (BGS, 2012).
Scott, D. A. Metallography and microstructure of ancient and historic metals (Getty Conservation Institute & Archetype Books, 1991).
Casaletto, M. P. et al. Production of reference “ancient” Cu-based alloys and their accelerated degradation methods. Appl. Phys. A: Mater. Sci. Process. 83, 617–622 (2006).
McNeil, M. & Selwyn, L. S. Electrochemical processes in metallic corrosion. In Handbook of Archaeological Sciences (eds. Brothwell, D. R & Pollard, A. M.) 605–14 (Wiley, Chichester, 2001).
Rémazeilles, C. & Conforto, E. A buried roman bronze inkwell–Chemical interactions with agricultural fertilizers. Stud. Conserv. 53, 110–117 (2008).
Pollard, A. M. et al. Assessing the influence of agrochemicals on the rate of copper corrosion in the vadose zone of arable land Part 1: Field experiments. Conserv. Manag. Archaeol. Sites. 6, 363–376 (2004).
Pollard, A. M. et al. Assessing the influence of agrochemicals on the rate of copper corrosion in the vadose zone of arable land Part 2: Laboratory simulations. Conserv. Manag. Archaeol. Sites. 7, 225–239 (2006).
Wilson, L. et al. Assessing the influence of agrochemicals on the nature of copper corrosion in the vadose zone of arable land Part 3: Geochemical modelling. Conserv. Manag. Archaeol. Sites 7, 241–260 (2006).
Zhang, R. et al. Theoretical study of the C–Cl bond dissociation enthalpy and electronic structure of substituted chlorobenzene compounds. CJCP 22, 235–240 (2009).
Canada, H. et al. Canadian environmental protection act: Priority substances list assessment report: Hexachlorobenzene (Canada Communication Group, 1993).
Starek-Świechowicz, B. et al. Hexachlorobenzene as a persistent organic pollutant: Toxicity and molecular mechanism of action. Pharmacol. Rep. 69, 1232–1239 (2017).
Berntssen, M. H.G. et al. Chapter 20–Chemical contamination of finfish with organic pollutants and metals. In Chemical Contaminants and Residues in Food 2nd Edition (eds Schrenk, D. & Cartus, A.) 517–51 (Elsevier Science & Technology, Cambridge, 2017).
Pontillo, C. A. et al. Action of hexachlorobenzene on tumor growth and metastasis in different experimental models. Toxicol. Appl. Pharmacol. 268, 331–342 (2013).
Dhaibar, H. A. et al. Hexachlorobenzene, a pollutant in hypothyroidism and reproductive aberrations: A perceptive transgenerational study. Environ. Sci. Pollut. Res. 28, 11077–11089 (2021).
Iyer, P. & Makris, S. Chapter 12 - developmental and reproductive toxicology of pesticides. In Hayes' Handbook of Pesticide Toxicology 3rd Edition (ed. Krieger, R.) 381–440 (Elsevier Science & Technology, Saint Louis, 2010).
Barber, J. L. et al. Hexachlorobenzene in the global environment: Emissions, levels, distribution, trends and processes. Sci. Total Environ. 349, 1–44 (2005).
Meijer, S. N. et al. Organochlorine pesticide residues in archived UK Soil. Environ. Sci. Technol. 35, 1989–1995 (2001).
Bailey, R. E. Global hexachlorobenzene emissions. Chemosphere 43, 167–182 (2001).
Wang, M.-J. et al. Chlorobenzenes in field soil with a history of multiple sewage sludge applications. Environ. Sci. Technol. 29, 356–362 (1995).
Santos Dos, M. M. et al. DEET occurrence in wastewaters: Seasonal, spatial and diurnal variability–mismatches between consumption data and environmental detection. Environ. Int. 132, 105038 (2019).
Manamsa, K. et al. A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci. Total Environ. 568, 712–726 (2016).
Schofield, J. et al. Archaeological practice in Great Britain: A heritage handbook (Springer, 2011).
Aitchison, K. No Going Back: Remembering when British archaeology changed forever. In Training and Practice for Modern Day Archaeologists (eds Jameson, J. H. & Eogan, J.) 53–67 (Springer, New York, 2013).
Aitchison, K. After the “gold rush”: Gobal archaeology in 2009. World Archaeol. 41, 659–671 (2009).
Ingo, G. M. et al. Uncommon corrosion phenomena of archaeological bronze alloys. Appl. Phys. A: Mater. Sci. Process. 83, 581–588 (2006).
Portable Antiquities Scheme. https://finds.org.uk/
Ferguson, N. An assessment of the positive contribution and negative impact of hobbyist metal detecting to sites of conflict in the UK (ProQuest Dissertations Publishing, 2013).
Acknowledgements
We thank Walter Ahmet (Finds Liaison Officer for Kent County Council) for facilitating the archaeological investigation of the area where the bowl was found, Dr Amber Thompson (Oxford Chemical Crystallography Service Manager) for training and granting access to XRD equipment, Dr Colin Johnston (Manager of Oxford Materials Characterization Service) for supervision and granting access to FTIR equipment and Dr James Wickens (former manager of Oxford Mass Spectrometry Research Facility) for providing training on GC-QTOF-MS with TSP and compound assignments.
Author information
Authors and Affiliations
Contributions
L.C., D.G. and M.P. designed the research. L.C. carried out the analyses, processed data and prepared the manuscript and figures. J.M. and M.P. supervised the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carvalho, L.d., Goodburn-Brown, D., McCullagh, J.S.O. et al. The influence of pesticides on the corrosion of a Roman bowl excavated in Kent, UK. Sci Rep 12, 14521 (2022). https://doi.org/10.1038/s41598-022-17902-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17902-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.