Abstract
Health-related quality of life (HRQoL) is lower in people with NAFLD compared to the general population. Sleep disturbance resulting in daytime sleepiness is common in patients with NAFLD, but the effect of daytime sleepiness on HRQoL in NAFLD is unclear. The prevalence and natural history of NAFLD vary in different ethnic groups, but there has been limited ethnic diversity in HrQoL studies to date. We aimed to assess whether daytime sleepiness is independently associated with reduced HRQoL in an ethnically diverse UK population. We conducted HRQoL assessments using SF-36 version 2 and Epworth Sleepiness Scale (ESS) questionnaires in 192 people with NAFLD. Multivariate linear regression was used to identify factors independently affecting HRQoL scales. People with NAFLD reported significantly reduced physical health-related SF-36 scores compared to the general UK population. South Asian NAFLD patients reported impairment in physical health, but not mental health, approximately a decade before White NAFLD patients. In multivariate linear regression, daytime sleepiness (ESS score > 10), was the most significant independent predictor of reduced physical health. Age, BMI and liver stiffness score were also significantly associated. HRQoL is impaired earlier in patients of South Asian ethnicity. ESS score > 10, indicative of excessive daytime sleepiness, is an independent predictor of reduced HRQoL in people with NAFLD regardless of ethnicity. Daytime sleepiness should be considered as a contributing factor to reduced HRQoL in clinical practice and when evaluating patient-related outcomes in clinical trials.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide affecting approximately 25% of the Western population and represents a spectrum of liver disease that ranges from benign steatosis to the inflammatory form, non-alcoholic steatohepatitis (NASH)1. Progression from benign steatosis to NASH can be associated with liver fibrosis, which can lead to cirrhosis, and significantly increases morbidity and mortality2.
Despite the growing awareness of the clinical outcomes associated with NAFLD, the wider impact of NAFLD from the patient’s perspective is not well understood. Health-related quality of life (HRQoL) assessments objectively examine the impact of a disease on an individual’s activities of daily living and allows for a common metric to compare to other diseases or to the general population. In addition to drug and disease related endpoints such as safety, tolerability and efficacy, changes in HRQoL have become important for regulatory bodies such as the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) when evaluating new therapeutic agents3. A number of disease-specific HRQoL tools have been developed although the most widely-used tool is the Short Form-36 (SF-36) questionnaire, which consists of 36 questions related to physical and emotional function and general wellbeing4. Relevant to the current study, this questionnaire that was developed in the United States, has been validated in different populations globally, including United Kingdom4, India5, Pakistan6 and Bangladesh7.
A number of studies have reported reduced HRQoL in people with NAFLD compared to the general population, particularly in physical health-related domains, believed to be related to obesity and type 2 diabetes mellitus (T2DM)8,9,10 albeit other co-morbidites have received little attention. Although the prevalence and natural history of NAFLD varies in different ethnic groups11, there has been limited ethnic diversity in HrQoL studies to date. Taken together, we do not know whether patient factors such as ethnicity or other co-morbidities contribute to impaired HRQoL in NAFLD.
Daytime sleepiness can be caused by sleep deprivation, sedating medication and neurological disorders although another common cause is obstructive sleep apnoea (OSA); a breathing disorder characterised by recurrent collapse of the upper airway during sleep, leading to chronic intermittent hypoxia and itself can impair HRQoL. Obesity, OSA and NAFLD share a number of patient-related risk factors and putative disease mechanisms12. The prevalence and severity of OSA vary in different ethnic groups13 but whether this variation carries through to impact on HRQoL is unknown. Symptoms that are associated with OSA such as daytime sleepiness, snoring, fragmented sleep, fatigue and cognitive impairment can be difficult to elicit in routine clinical care. Polysomnography, the gold standard for diagnosing OSA, is not appropriate as a screening tool as it is labour intensive, time consuming and expensive. Therefore, OSA can remain undiagnosed, and so its impact on HRQoL in NAFLD remains unknown. Epworth Sleepiness Scale (ESS) is a validated screening questionnaire designed to identify individuals who have symptoms which can be consistent with OSA14 but this tool is not routinely utilised in HRQoL assessments in NAFLD hence the limited data in this space.
We hypothesise that ESS-determined symptoms consistent with OSA are independently associated with HRQoL impairment in people with NAFLD. We conducted SF-36 and ESS questionnaires at the same hospital visit in an ethnically diverse population of patients with NAFLD in order to understand the effect of ethnicity on these patient-related outcome measures and to determine factors associated with impaired HRQoL.
Materials and methods
Patients over the age of 18 attending the hepatology outpatient clinics at Barts Health NHS Trust and for elective bariatric surgery at Homerton University Hospital Foundation Trust were invited to attend a further voluntary research visit, without financial incentive, for recruitment into non-commercial cross-sectional studies at Barts Liver Centre, Queen Mary University of London. Participants provided written informed consent and completed HRQoL assessments at enrolment. The studies were approved by the East London and City Regional Ethics Committee (reference numbers 18/LO/1759, 14/WA/1142) and performed in compliance with the Declaration of Helsinki. We included patients with evidence of liver steatosis, either by imaging (ultrasound, CT, MRI) or histology. Patients were excluded if they had any coexisting chronic liver disease diagnoses other than NAFLD, consumed alcohol greater than 14 units per week or had clinical features of decompensated cirrhosis. At enrolment, demographic data obtained included sex, age and ethnicity. Self-reported ethnicity was grouped into White, South Asian (Indian, Pakistani, Bangladeshi and Sri Lankan), Black or Other. Clinical and laboratory data obtained include body mass index (BMI), type 2 diabetes status, obstructive sleep apnoea diagnosis, glycated haemoglobin (HbA1c), drug history, platelet count, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Transient elastography was performed to measure liver stiffness for all recruited patients at enrolment according to standard clinical practice (reliable liver stiffness result based on successful reading rate > 60% and interquartile range of all readings < 30% of the median). Liver biopsy was performed in selected patients when clinically indicated or intra-operatively per-protocol at time of bariatric surgery. Each liver biopsy was reported by a single histopathologist (in routine clinical care) and were summarised according to the National Institutes of Health NASH clinical research network (Kleiner) criteria15.
Quality of life and daytime sleepiness assessments were conducted using Short Form-36 version 2 (SF-36v2) questionnaire and self-administered Epworth Sleepiness Scale (ESS) questionnaire. ESS scores were not performed in the bariatric cohort. SF-36 raw scores are combined to create scores for 8 sub-scales: physical functioning, role limitations due to physical health, emotional well-being, role limitations due to emotional problems, energy/fatigue, social functioning, pain, and general health. An overall physical health score (physical component scale [PCS]) and mental health score (mental component scale [MCS]) are derived from the sub-scale scores. PCS and MCS scores were compared against UK SF-36v2 normative data4. ESS questionnaire is a validated questionnaire used to assess daytime sleepiness, which consists of 8 questions scored from 0 to 3. ESS score greater than 10 is indicative of excessive daytime sleepiness, which may indicate OSA14.
Statistical analysis
We compared demographic and clinical parameters with individual sub-scales, PCS and MCS of the SF-36v2 and ESS score in all patients. A separate analysis was performed in biopsy-proven patients to correlate quality of life indices with histological parameters. Continuous variables were expressed as mean and standard deviation or percentage. Categorical variables were expressed as percentage or proportion. Statistical analysis included Student’s t-test for comparison of two means for parametric data, the Mann–Whitney U test for comparison of two means for non-parametric data. Univariate and multivariate linear regression were performed to identify variables significantly associated with SF-36v2 PCS. Age, BMI, liver stiffness score, serum ALT, serum AST were analysed as continuous variables for univariate and multivariate analyses. All variables with p < 0.05 in the univariate analysis were included in multivariate analysis. Post hoc power calculations were performed based on group mean differences found in SF-36v2 PCS using G*Power (version 3.1.9.2). All other data handling and analyses were performed using GraphPad Prism (version 9.1.1), SPSS (version 26.0) and R (version 3.5).
Ethics approval
The studies were approved by the local research ethics committees (reference numbers 18/LO/1759, 14/WA/1142) and performed in compliance with the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent at study enrolment.
Consent to publication
All participants provided written informed consent at study enrolment.
Results
A total of 266 patients were enrolled from January 2015 to January 2020, and after excluding 64 due to incomplete datasets, our cohort comprised 192 patients (Supplementary Table 1). All 192 patients completed an SF-36v2 questionnaire and 181 (94%) completed an ESS questionnaire. The mean age was 51.7 years, 42.2% were female (Table 1). Mean body mass index was 32.8 kg/m2, 47.4% had T2DM, mean liver stiffness measurement (LSM) was 10.2 kPa and 13.5% had known diagnosis of OSA. Mean ESS score (non-bariatric surgery cohort only) was 7.4 and 24.9% of patients had ESS score > 10. The mean Physical Component Summary (PCS) and Mental Component Summary (MCS) were statistically significantly lower than published scores from the general UK population4, with a greater effect seen in PCS (35.33 vs 50.02, p < 0.001) than in MCS (48.47 vs 50.05, p = 0.031) (Supplementary table 2).
Compared to patients with ESS ≤ 10, those with ESS score > 10 had a higher BMI (36.7 kg/m2 vs 31.3 kg/m2, p < 0.0001), higher prevalence of T2DM (58.9% vs 42.7%, p = 0.044), with lower PCS (26.9 vs 38.2, p < 0.0001) and MCS (39.3 vs 51.5, p < 0.0001) (Table 2). No differences in age, gender or ethnicity were observed. Similar proportions of patients reported use of medications with potential somnolent profiles (beta blockers, opioids, antihistamines, antidepressants) in both groups (14.3% vs 17.8%, p = 0.672). The chronicity of medication use was not recorded. Liver histology was available for 40 patients (Supplementary table 1) and no significant correlations between ESS score > 10 and histological parameters of NAFLD were identified (Supplementary table 3) in this group. 26.7% of patients with ESS score > 10 had non-obese BMI (adjusted for South Asian ethnicity16).
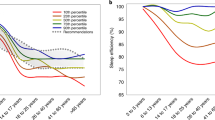
The two largest ethnic groups were White (n = 83, 43.2%) and South Asian (n = 80, 41.7%). Compared to White patients, South Asian patients were younger (49.3y vs 55.0y, p = 0.004), with lower mean BMI (29.8 kg/m2 vs 35.5 kg/m2, p < 0.001), had a higher prevalence of T2DM (55.5% vs 37.4%, p = 0.022), albeit with comparable LSM scores (9.4 kPa vs 10.9 kPa, p = 0.221), ESS scores (7.0 vs 7.1, p = 0.915), serum levels of ALT (53.4 IU/ml vs 52.7 IU/ml, p = 0.917) and AST (44.1 IU/ml vs 37.9 IU/ml, p = 0.179) (Table 1). PCS, but not MCS, reduced with advancing age (Fig. 1A,D). We found a reduction in PCS, but not MCS, at an earlier age (by approximately 9 years) in patients of South Asian ethnicity compared to White ethnicity (Fig. 1B,C,E,F, Supplementary Table 4). PCS was lower in the 45–60 age group compared to under 45 age group in South Asian patients, but in patients of White ethnicity, a significant decline in PCS only occurred in patients aged over 60. There were no differences in PCS and MCS when White and South Asian groups of all ages were compared (Fig. 2A,B). Across all ethnicities, the presence of T2DM was associated with lower scores in role limitations due to physical health, general health and PCS compared to people with NAFLD who did not have diabetes (49.4 vs 67.5, p = 0.007; 42.5 vs 53.2, p = 0.002; 32.5 vs 37.8, p = 0.016 respectively) (Supplementary Table 2).
Physical and Mental Component Scores stratified by age. PCS physical component summary, MCS mental component summary. (A) All NAFLD PCS stratified by age; (B) South Asian PCS stratified by age; (C) White PCS stratified by age; (D) All NAFLD MCS stratified by age; (E) South Asian MCS stratified by age; (F) White MCS stratified by age.
In univariate linear regression, age, BMI, diabetes diagnosis, liver stiffness score, ESS score > 10 and OSA diagnosis were negatively associated with PCS (Table 3). OSA and diabetes diagnoses no longer had significant associations with PCS on multivariate linear regression, but the other variables remained statistically significant. ESS score > 10 was the most significant independent predictor of PCS. Sensitivity analyses showed that after firstly excluding those with known diagnosis of OSA (n = 26) (Supplementary Table 5); secondly excluding those with use of medications with potential somnolent profiles and known diagnosis of OSA (n = 48), ESS score > 10 remained the strongest independent predictor of PCS (Supplementary Table 6). Post hoc power analysis determined the total study size of 192 was adequately powered (1 − β = 0.997) to detect statistically significant differences PCS between ESS ≤ 10 v and ESS > 10 groups (Supplementary Table 7).
Discussion
This study, conducted in an ethnically diverse urban population showed that high Epworth Sleepiness Score, indicative of excessive daytime sleepiness, is a significant independent predictor of impaired HRQoL in patients with NAFLD. Overall, people with NAFLD had lower quality of life (particularly physical health scores) compared to the general population, in keeping with previous reports8,9,10. We found that ethnicity is associated with the age of onset of decline in physical health scores in people with NAFLD which was significantly earlier in patients of South Asian compared to White ethnicity. However, no significant differences in OSA prevalence or ESS scores were observed.
Other than NAFLD, previous studies have demonstrated variability in the prevalence of daytime sleepiness and OSA in different ethnicities, associated with a range of conditions including obesity, type 2 diabetes, cardiovascular disease and depression. In an atherosclerosis study of 2230 participants, higher prevalence of daytime sleepiness and undiagnosed OSA were reported in those of Black, Hispanic and Chinese ethnicities compared to White ethnicity17. The National Health and Wellness Survey from 7239 diabetic patients found the strongest predictors of sleep disturbance and daytime sleepiness were obesity, White ethnicity, female gender, low income, and smoking18. Longitudinal analysis of 41,094 participants of the UK Biobank found obesity, ethnicity (Black, Asian and mixed), depression, and social deprivation were the main factors associated with poor sleep and associated symptoms19. In Asian populations, daytime sleepiness and OSA were associated with subclinical atherosclerosis and diabetes irrespective of BMI20,21. The variability in these observations are not fully understood, but are likely to be multifactorial, associated with genetic, biological, social and environmental factors. Recent genetic studies have identified methylation sites in multiple genes specifically associated with daytime sleepiness in African Americans22. Differences in craniofacial structures have been linked to elevated risk of OSA and associated symptoms despite lower rates of obesity in Asian populations compared to White and Black ethnicities23,24.
In our study, 13.5% of participants had a diagnosis of OSA at enrolment, but a significantly higher proportion (24.9%) recorded ESS scores > 10. To our knowledge, this is the first report that shows ESS score > 10 is a significant independent predictor of physical HRQoL, in addition to factors known to be associated with impaired physical health scores in NAFLD; older age, female gender, BMI, presence of T2DM, degree of fibrosis including cirrhosis8,9,10,25,26,27. In our study, ESS score > 10 was associated with metabolic co-morbidites (obesity, T2DM), and liver stiffness score by transient elastography. Our study was not designed to detect associations of HRQoL or sleepiness with histology and the lack of statistical associations may be related to the low proportion of patients in our study who had liver biopsy as part of routine clinical care (21%). Nevertheless, our findings were congruent with findings by Newton et al.28, which reported perceived fatigue experienced by patients with NAFLD was associated with daytime sleepiness, but not liver disease severity. However, a Swiss study of sleep disturbance in patients with biopsy-proven NAFLD, insulin resistance and elevated serum transaminase levels29 showed an association between sleepiness and NASH on biopsy albeit the Swiss cohort differed in that patients had lower mean BMI, ESS and fibrosis scores, and higher mean ALT. Continuous positive pressure ventilation (CPAP) is the primary treatment for OSA, associated with improvements in OSA symptoms, HRQoL30 and reduction in ESS scores31. However, a randomised controlled trial of CPAP, for 6 months in people with NAFLD and OSA did not demonstrate regression of hepatic steatosis or fibrosis32 suggesting that mechanisms beyond hypoxaemia and fibrosis regression influence HRQoL in patients with NAFLD and OSA. In addition, further work is needed to assess the impact of daytime sleepiness on key lifestyle determinants, such as exercise capacity, mood, appetite, eating patterns and other activities or daily living, which may reduce the ability of people with NAFLD to make substantive lifestyle changes. Daytime sleepiness in people with NAFLD without obesity was high in our study (26.7% of all participants with ESS score > 10 did not have obesity), in keeping with similar findings in a non-morbidly obese Italian NAFLD cohort33. Future studies with polysomnography to confirm OSA are warranted in this subpopulation of NAFLD with high ESS scores.
To date, the majority of studies assessing HRQoL in NAFLD have been conducted in the United States with preponderance of White ethnicity (> 73%)8,9,10,26,27. In patients with advanced NASH fibrosis enrolled in the multinational phase 3 STELLAR trials of selonsertib, there were significant differences in HRQoL scores between White, Asian and Black groups of participants, although only 1.5% of the total cohort were of Black ethnicities9. We previously reported that patients of South Asian ethnicity have a greater risk of developing NAFLD at a younger age (by approximately a decade), with a lower BMI compared to White patients with comparable disease stages34. This is mirrored in our current study by the reduction in physical health scores despite lower BMI, and together, this signifies that South Asian patients with NAFLD suffer physical impairment from a much younger age compared to White patients. The underlying causes for this are likely to be multifactorial, though higher rates of diabetes were observed in South Asian people and Chawla et al. reported a significant reduction in physical health scores in people with NASH and diabetes compared to non-diabetic NASH patients25. Further work is required to understand the effect of South Asian ethnicity on the natural history of NAFLD and why it impacts HRQoL at younger ages. While higher rates of diabetes may play a role, other factors may include diet, genetics, cultural health behaviours and social deprivation, which were not explored in this study. Our collective work highlights the importance of ethnicity when evaluating NAFLD severity from a clinician as well as patient perspective.
This study has both strengths and limitations. Firstly, the patients enrolled reflect real world NAFLD in clinical practice, not restricted by stringent clinical trial criteria. Secondly, our study population is more ethnically diverse compared to previous studies, with significant proportion of South Asian patients. Thirdly, SF-36v2 questionnaire was used to evaluate HRQoL in this study. Although this questionnaire is not specifically designed to evaluate HRQoL in chronic liver disease, unlike the Chronic Liver Disease Questionnaire (CLDQ), it is a widely used HRQoL assessment tool validated in different populations globally that allows for comparisons with different disease states and the general population, which is not possible with CLDQ. Furthermore CLDQ was developed and validated using SF-36 as the gold standard25. ESS alone has modest discriminatory ability as a screening tool for OSA (sensitivity ranging from 38 to 66% and specificity ranging from 48 to 79% with ESS score > 10 as threshold)35,36. Although the focus of our study was to elicit the symptoms of daytime sleepiness rather than to formally diagnose OSA, one limitation of our study is that polysomnography was not used to confirm those suspected of OSA based on the ESS scores. High ESS score reflects a state of fatigue, which is also 1 of 8 major components measured in SF-36 questionnaire. A limitation in our study is the absence of control group(s) to assess whether liver disease or other associated factors contribute to fatigue in patients with NAFLD, but again, this was not an aim of our study. Finally, the cross-sectional nature of this study prevents causal conclusions to be drawn from the reported correlations. Very few longitudinal HRQoL studies have been conducted to date in NAFLD. The PIVENS trial for NASH reported no significant changes in SF-36 scores between the study arms after 96 weeks37. In patients with NASH F2 – 3 fibrosis treated for 24 weeks with selonsertib in a phase 2 clinical trial, patients in whom there was improvement in a surrogate marker of fibrosis (percentage collagen) but not histological NAFLD activity score also recorded improvement in physical health scores38. Future prospective longitudinal studies are warranted to investigate strategies that can improve physical impairment in people with NAFLD of different ethnicities.
Conclusion
In this study, we demonstrate that people with NAFLD from an ethnically diverse UK population report lower quality of life compared to the general population. Physical health in particular is significantly reduced. Our work also highlights the importance of ethnicity in HRQoL outcomes in NAFLD. South Asian people with NAFLD report physical health impairments from a significantly younger age compared to White people with NAFLD despite more favourable BMI. We identify undiagnosed daytime sleepiness as a major component in NAFLD HRQoL which should be considered in clinical practice and when evaluating patient-related outcomes in clinical trials through the use of tools such as the Epworth Sleepiness Scale.
Data availability
All anonymised data available by request directly to corresponding author.
References
Li, W. & Alazawi, W. Non-alcoholic fatty liver disease. Clin. Med. (Northfield. Il) 20, 509–512 (2020).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Research, U. S. D. of H. and H. S. F. D. A. C. for D. E. and, Research, U. S. D. of H. and H. S. F. D. A. C. for B. E. and & Health, U. S. D. of H. and H. S. F. D. A. C. for D. and R. Guidance for industry: patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 4, 79 (2006).
Jenkinson, C., Stewart-Brown, S., Petersen, S. & Paice, C. Assessment of the SF-36 version 2 in the United Kingdom. J. Epidemiol. Community Health 53, 46–50 (1999).
Sinha, R., van den Heuvel, W. A. & Arokiasamy, P. Validity and reliability of MOS short form health survey (SF-36) for use in India. Indian J. Community Med. 38, 22 (2013).
Atif, M. et al. Health-related quality of life and depression among medical sales representatives in Pakistan. Springerplus 5, 1048 (2016).
Feroz, A. H. M. et al. The Bengali Short Form-36 was acceptable, reliable, and valid in patients with rheumatoid arthritis. J. Clin. Epidemiol. 65, 1227–1235 (2012).
David, K. et al. Quality of life in adults with nonalcoholic fatty liver disease: Baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology 49, 1904–1912 (2009).
Younossi, Z. M. et al. Reduced patient-reported outcome scores associate with level of fibrosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 17, 2552-2560.e10 (2019).
Samala, N. et al. Decreased quality of life is significantly associated with body composition in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2020.04.046 (2020).
Younossi, Z. M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 70, 531–544 (2019).
Quintero, M. et al. The effects of intermittent hypoxia on redox status, NF-κB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic. Biol. Med. 65, 1143–1154 (2013).
Hnin, K. et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med. Rev. 41, 78–86 (2018).
Johns, M. W. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: Failure of the MSLT as a gold standard. J. Sleep Res. 9, 5–11 (2000).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004).
Chen, X. et al. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep https://doi.org/10.5665/sleep.4732 (2015).
Gupta, S. & Wang, Z. Predictors of sleep disorders among patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 10, 213–220 (2016).
Fatima, Y. et al. Sleep trajectories and mediators of poor sleep: findings from the longitudinal analysis of 41,094 participants of the UK Biobank cohort. Sleep Med. 76, 120–127 (2020).
Roopa, M., Deepa, M., Indulekha, K. & Mohan, V. Prevalence of sleep abnormalities and their association with metabolic syndrome among Asian Indians: Chennai Urban Rural Epidemiology Study (CURES-67). J. Diabetes Sci. Technol. 4, 1524–1531 (2010).
Chirakalwasan, N. et al. Comparison of polysomnographic and clinical presentations and predictors for cardiovascular-related diseases between non-obese and obese obstructive sleep apnea among Asians. J. Clin. Sleep Med. 09, 553–557 (2013).
Barfield, R. et al. Epigenome-wide association analysis of daytime sleepiness in the Multi-Ethnic Study of Atherosclerosis reveals African-American-specific associations. Sleep https://doi.org/10.1093/sleep/zsz101 (2019).
Ito, D., Akashiba, T., Yamamoto, H., Kosaka, N. & Horie, T. Craniofacial abnormalities in Japanese patients with severe obstructive sleep apnoea syndrome. Respirology 6, 157–161 (2001).
Sakakibara, H. et al. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur. Respir. J. 13, 403–410 (1999).
Chawla, K. S. et al. Reliability and validity of the Chronic Liver Disease Questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 3, e000069 (2016).
Younossi, Z. M. et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health-related quality of life. Am. J. Gastroenterol. 114, 1636–1641 (2019).
Sayiner, M. et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 3, e000106 (2016).
Newton, J. L. et al. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut 57, 807–813 (2008).
Bernsmeier, C. et al. Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in non-alcoholic fatty liver disease: A comparison with healthy controls. PLoS ONE 10, e0143293 (2015).
Wimms, A. J. et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): A multicentre, randomised controlled trial. Lancet Respir. Med. 8, 349–358 (2020).
Patel, S. R., White, D. P., Malhotra, A., Stanchina, M. L. & Ayas, N. T. Continuous positive airway pressure therapy for treating gess in a diverse population with obstructive sleep apnea. Arch. Intern. Med. 163, 565 (2003).
Ng, S. S. et al. CPAP did not improve nonalcoholic fatty liver disease in patients with obstructive sleep apnea: A randomized clinical trial. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.202005-1868OC (2020).
Pulixi, E. A. et al. Risk of obstructive sleep apnea with daytime sleepiness is associated with liver damage in non-morbidly obese patients with nonalcoholic fatty liver disease. PLoS ONE 9, e96349 (2014).
Alazawi, W. et al. Ethnicity and the diagnosis gap in liver disease: A population-based study. Br. J. Gen. Pract. 64, e694–e702 (2014).
Rosenthal, L. D. & Dolan, D. C. The Epworth Sleepiness Scale in the identification of obstructive sleep apnea. J. Nerv. Ment. Dis. 196, 429–431 (2008).
Puretic, H. et al. The Epworth sleepiness scale 23 years after: Is daytime sleepiness still a valid screening tool for sleep apnea. Eur. Respir. J. 44, P2286 (2014).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Younossi, Z. M. et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 38, 1849–1859 (2018).
Acknowledgements
We are grateful to Salma Samsuddin, Victoria Berryman, Johanna Preston, Melanie Pattrick, Sanjay Agrawal for facilitating patient recruitment for this study.
Funding
Grant from the Diabetes Wellness and Research Foundation (WA, WKS). WA was supported by a New Investigator Research Grant from the Medical Research Council.
Author information
Authors and Affiliations
Contributions
All authors reviewed and agreed manuscript concept and design. Data acquisition and analysis: W.L., B.K.K. and J.H.B. Initial drafting of manuscript: W.L. Critical review and adaptation of the manuscript: B.K.K., J.H.B., J.L., G.H., W.K.S. and W.A. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, W., Kadler, B.K., Brindley, J.H. et al. The contribution of daytime sleepiness to impaired quality of life in NAFLD in an ethnically diverse population. Sci Rep 12, 5123 (2022). https://doi.org/10.1038/s41598-022-08358-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08358-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.