Abstract
Traumatic brain injury (TBI) can be associated with long-term neurobehavioral symptoms. Here, we examined levels of neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in extracellular vesicles isolated from blood, and their relationship with TBI severity and neurobehavioral symptom reporting. Participants were 218 service members and veterans who sustained uncomplicated mild TBIs (mTBI, n = 107); complicated mild, moderate, or severe TBIs (smcTBI, n = 66); or Injured controls (IC, orthopedic injury without TBI, n = 45). Within one year after injury, but not after, NfL was higher in the smcTBI group than mTBI (p = 0.001, d = 0.66) and IC (p = 0.001, d = 0.35) groups, which remained after controlling for demographics and injury characteristics. NfL also discriminated the smcTBI group from IC (AUC:77.5%, p < 0.001) and mTBI (AUC:76.1%, p < 0.001) groups. No other group differences were observed for NfL or GFAP at either timepoint. NfL correlated with post-concussion symptoms (rs = − 0.38, p = 0.04) in the mTBI group, and with PTSD symptoms in mTBI (rs = − 0.43, p = 0.021) and smcTBI groups (rs = − 0.40, p = 0.024) within one year after injury, which was not confirmed in regression models. Our results suggest the potential of NfL, a protein previously linked to axonal damage, as a diagnostic biomarker that distinguishes TBI severity within the first year after injury.
Similar content being viewed by others
Introduction
Over the last decade, almost 250,000 US service members and veterans (SMVs) have sustained a traumatic brain injury (TBI), with the vast majority classified as mild TBI (mTBI)1. Many SMVs who suffered mTBIs recover to premorbid functioning, however, an increasing percentage of SMVs report neurobehavioral symptoms many months or years following injury2. Determining blood-based biomarkers that can identify injury severity, and are related to long-term neurobehavioral symptom reporting, may aid in the identification of pathological mechanisms and the development of preventive interventions.
TBI neuropathology consists of a primary injury that results of the traumatic insult, and secondary processes that may lead to glial and neuronal changes, and also increased risk for later-in-life neurodegenerative conditions3. Biomarker studies in TBI have focused on circulating levels of proteins associated with pathological processes within the brain, including neurofilament light chain (NfL), a neurofilament protein highly expressed in large-caliber myelinated axons, and glial fibrillary acidic protein (GFAP), a component of the cytoskeleton of astrocytes4,5,6,7. GFAP is a promising acute TBI biomarker8, but a recent study also suggests its informative potential at more chronic timepoints9. GFAP levels in serum were suggested to decrease during the first 6 months after TBI, but increase at later timepoints, with elevated levels in patients with moderate or severe TBIs when compared to controls for up to 5 years after injury9. NfL has gained increasing attention as a marker of chronic neurodegeneration and poor outcomes in neurological diseases including TBI10,11,12,13. After TBIs, higher blood NfL has been linked to postconcussive symptoms (PCSx) lasting beyond one year in hockey players14, and long-term neurobehavioral symptoms in military populations15. In addition, studies have also shown acute and subacute elevations of NfL in moderate and severe TBIs16,17. In severe TBI patients, acute NfL levels predicted poor 12-month clinical outcome18.
Extracellular vesicles (EVs), such as exosomes, are released by cells throughout the body, including neurons and glia19,20. EVs are considered a promising source of biomarkers as they can cross the brain-blood-barrier and their content, which is protected from degradation by a lipid bilayer, reflects the environment of the cell of origin21,22,23. EVs can be isolated from the peripheral circulation, providing an opportunity to investigate brain-specific processes23,24. Moreover, levels of proteins within EVs may be related to the biological underpinnings of recovery from a TBI15,23,25. EVs play a role in cell signaling and in the removal of unwanted proteins of the brain, and their cargo can functionally change the recipient cells25,26. They have also been linked to the pathology of age-related neurodegenerative conditions such as Alzheimer's Disease (AD)27,28,29.
Previous studies have investigated EV levels of NfL15,30,31,32,33 and GFAP32,33 in TBI with variable findings. Our group has reported that EV NfL is chronically elevated in SMVs who sustained repetitive mTBIs (3 or more), and associated with severity of neurobehavioral symptoms15. Elevated EV levels of GFAP, but not NfL, have been observed in a civilian population with history of moderate or severe TBI one year post-injury33. Importantly, mTBIs are heterogenous according to clinical presentation and neuroimaging findings34,35. Uncomplicated and complicated mTBIs are characterized, respectively, by the absence or presence of intracranial abnormalities34. To the best of our knowledge, no study has evaluated EV NfL or EV GFAP levels across TBI severities (i.e., mild, moderate, and severe) at distinct chronic timepoints after TBI (i.e., ≤ 1 year, and > 1 year). Similarly, no study has evaluated the potential of EV biomarkers in the stratification of mild injuries in uncomplicated and complicated mTBIs.
In this study, we evaluated SMVs with history of uncomplicated mild, complicated mild, moderate, or severe TBI. We aimed to expand on previous findings and examine whether EV proteins can distinguish TBI patients from controls and TBI severity, as well as their relationship with PCSx and post-traumatic stress disorder (PTSD) symptoms.
Results
Demographic and clinical characteristics
Participants (n = 218) were predominately male (94%), white (76%), with a median age of 32 (IQR = 26–42) (Table 1). No significant group differences on demographics were observed, except for time since injury (TSI, months, p = 0.012), which was higher in the mTBI group (median = 35, IQR = 6–92) than in the smcTBI (median = 10.5, IQR = 5–60, p = 0.025) and IC groups (median = 9, IQR = 3–35, p = 0.003). Clinical characteristics, neurobehavioral measures, and biomarker concentrations per TBI severity are provided in Supplemental Table 1 and Supplemental Table 2.
NfL discriminates TBI patients within one year after TBI
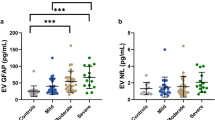
Participants were divided according to their TSI (months). Within one year after injury (n = 114, median = 5 months, IQR = 3–8), there were significant group differences for EV NfL (p < 0.001), but not EV GFAP (p = 0.081) when comparing smcTBI (n = 41), mTBI (n = 43), and IC (n = 30) groups (Fig. 1). EV NfL levels were higher in the smcTBI group when compared to IC (p = 0.001, d = 0.66) and mTBI (p = 0.001, d = 0.35) groups, which was confirmed in logistic regression models controlling for demographics and TSI (Table 2). Univariate under the receiver-operating characteristic curve (AUC) analysis revealed that NfL distinguished smcTBI group from IC (AUC = 77.5%, 95% CI 65.3–89.7%, p < 0.001) and mTBI groups (AUC = 76.1%, 95% CI 64.2–88.0%, p < 0.001), but not mTBI from the IC group (AUC = 50.84%, 95% CI 35.31–66.36%, p = 0.546) (Fig. 2). Similarly, EV GFAP distinguished smcTBI group from IC (AUC = 63.08%, 95% CI 49.89–76.27% p = 0.031) and mTBI groups (AUC = 62.14%, 95% CI 49.59–74.69%, p = 0.029), but not mTBI and IC groups (AUC = 50.79%, 95% CI 36.87–64.72%, p = 0.457). AUC values for logistic regression models comparing TBI groups were also calculated (Table 2). One or more years after injury (n = 104, median = 61, IQR = 36–120), we found no significant differences in biomarker levels when comparing smcTBI (n = 25), mTBI (n = 64), and IC (n = 15) groups, for EV NfL (p = 0.659) and GFAP (p = 0.567) (Fig. 1). Logistic regression models also confirmed these findings (Table 2). Neither EV NfL nor EV GFAP discriminated smcTBI from IC (AUC = 53.9%, 95% CI 29.78–78.01% for NfL; AUC = 54.4%, 95% CI 35.19–73.61%, for GFAP) or mTBI groups (AUC = 44.82%, 95% CI 27.41–62.23%, for NfL; AUC = 48.81%; 95% CI 33.71–63.91%, for GFAP) one or more years after the injury. No statistically significant differences in biomarker levels between IC and mTBI groups were observed at either timepoint. In addition, neither GFAP nor NfL distinguished mTBI and IC groups. Biomarker levels across TBI severity at each timepoint are also reported in Supplemental Table 3 and Supplemental Table 4.
EV levels of biomarkers under one year (a,c) and one or more years after injury (b, d). Participants were divided into 3 groups at each timepoint: one or more years after injury (IC, n = 30; mTBI, n = 43; and smcTBI, n = 41) and one more years after injury (IC, n = 15; mTBI, n = 64; and smcTBI, n = 25). Biomarker concentrations are represented as median ± IQR. P values refer to non-parametric pairwise group comparisons adjusted for multiple comparisons ((a Dunn’s test correcting for repetitive pairwise comparisons using Bonferroni method) when overall group comparison was statistically significant, or overall group comparison significance (b, c, d Kruskal–Wallis test). IC, injured controls; mTBI, uncomplicated mild TBI; smcTBI, complicated mTBI, moderate TBI, and severe TBI combined; TBI, traumatic brain injury; NfL, neurofilament light chain; GFAP, glial fibrillary acidic protein.
Univariate area under the receiver operating characteristic curves (AUCs) for EV NfL (blue) and EV GFAP (red) within one year after injury comparing smcTBI versus IC (a), smcTBI versus mTBI (b), and mTBI versus IC (c). Grey diagonal line represents reference line (AUC = 0.5). TSI, time since injury; GFAP, Glial fibrillary acidic protein; NfL, Neurofilament light chain; IC, injured controls; mTBI, uncomplicated mild TBI; smcTBI, complicated mTBI, moderate TBI, and severe TBI combined.
TBI characteristics and biomarker levels
We analyzed correlations between biomarker levels and TSI (months). Within one year after TBI, the median TSI was 4.0 (IQR = 3.0–8.0) in the mTBI group and 5.0 in the smcTBI group (IQR = 3.0–8.0). EV GFAP levels positively correlated with TSI (rs = 0.35 , p = 0.024) in the mTBI group, while NfL levels negatively correlated with TSI in the smcTBI group (rs = − 0.51, p = 0.002). Concentrations of EV NfL and EV GFAP in relation to TSI are graphically represented in Fig. 3. One or more years after injury, the median TSI was 66.5 (IQR = 36–120) in the mTBI group and 118 (IQR = 60–120) in the smcTBI group, but the difference was not statistically significant (p = 0.149). No significant correlations between TSI and biomarker levels were observed. In the mTBI group, the correlation between EV NfL levels and number of TBIs was marginally significant (rs = 0.29, p = 0.064).
Correlations between biomarker levels and time since injury (TSI, months) or behavioral scores within one year of injury in the mTBI group (a–c) and smcTBI group (d,e). For each correlation, rho (rs) and p values are provided (Spearman rank correlations). Regression line is shown in blue. One influential data point was removed from the mTBI group as it greatly affected the slope of the regression line involving either NfL or GFAP. Concentrations of NfL and GFAP for the data point were higher than the median plus 3 median absolute deviations. GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; TSI, time since injury (months); PCL-C, PTSD Checklist-civilian Version; NSI, Neurobehavioral Symptom Inventory.
Biomarker levels and behavioral symptoms
Within one year after injury, EV NfL levels correlated with NSI (rs = − 0.38, p = 0.044) in the mTBI group but not in the smcTBI group (rs = − 0.32, p = 0.074) (Fig. 3). EV NfL levels also correlated with PCL-C in the mTBI (rs = − 0.44, p = 0.021) and smcTBI groups (rs = − 0.40, p = 0.024). However, associations between EV NfL levels and symptoms were not confirmed in regression models including demographics, TSI, and number of lifetime TBIs. No other associations between biomarkers and NSI or PCL-C scores were observed.
Discussion
Our main finding suggests a dose–response relationship between TBI severity and concentrations of NfL in EVs within one year after TBI. Specifically, participants who had sustained a complicated mTBI, moderate or severe TBI had higher levels of blood EV NfL than those with uncomplicated mTBI or injured controls. Additionally, correlation analysis suggests an increase with time in levels of EV GFAP in participants with uncomplicated mTBI, and a decrease in EV NfL in more severe injuries, within the first year after TBI. Our findings support the potential of EV NfL as a diagnostic biomarker in TBI and as a means to discriminate TBI severity, shedding light on underlying pathology mechanisms.
NfL is a component of the neuronal cytoskeleton and a marker of axonal injury and degeneration36,37. Several recent studies have indicated the prognostic and predictive value of measuring concentrations of NfL in the peripheral blood in chronic neurological diseases including amyotrophic lateral sclerosis (ALS)38, multiple sclerosis (MS)39, AD40, Parkinson’s Disease (PD)13, as well as peripheral neuropathies41. In PD patients, NfL has shown potential as a predictive biomarker of disease severity and progression based on motor and cognitive measures13,42. Higher plasma NfL has also been linked to higher risks of developing dementia in a non-demented population40. In TBI, chronically elevated levels of NfL in individuals with multiple mTBIs have also been shown, in association with more severe neurobehavioral symptoms in athletes and military populations14,15,36,43,44,45. Other studies have observed early increases in the NfL levels after moderate and severe TBIs16,17. A recent study has shown that serum NfL levels decreased in a linear fashion over the first 5-years after mild to moderate TBI9. NfL distinguished participants with moderate to severe TBI from controls with higher AUCs at 30, 90, and 180 day time points. NfL also distinguished mTBI from controls at the 30-day time point9. Nevertheless, the temporal profile of NfL release in the blood after TBIs is not completely understood. GFAP is an important component of the cytoskeleton of astrocytes and has been considered a biomarker of astroglial injury46. Changes in astrocyte function have been associated with aging and neurological diseases47,48. In TBI, several studies have provide strong indications of the value of GFAP as an acute biomarker, but links between changes in astrocyte function and TBI pathology are only beginning to be understood4,5,49,50,51,52.
EVs have a complex biogenesis that is yet to be completely understood, and are heterogenous in size, content and origin23,53. They play a role in local and systemic intercellular signaling54,55,56. In addition, EVs have been linked to the pathology of age-related neurodegenerative conditions such as AD with a role in packaging and spread of misfolded proteins27,28,57,58. TBI pathology shares similarities with progressive neurological conditions, and is also associated with neurogenerative processes, suggesting the potential of EV NfL as a biomarker in chronic phases after the TBI.
Our group has previously reported higher EV NfL in SMVs with repetitive mTBI in comparison to those with 1 or 2 TBIs in a cohort in average 8 years after the last injury, with a positive correlation between NfL and TSI15. In the same study, we also found links between higher levels of EV NfL and more severe PCSx and PTSD symptoms. Here, correlations between the severity of symptoms and biomarker levels were observed within the first year of injury, but these findings were not confirmed in regression models. We observed no significant correlations between NfL, PCSx and PTSD symptoms after one year following the injury in the mTBI group as we have previously reported. Moreover, correlations between number of TBIs and concentrations of EV NfL in the mTBI group were only marginally significant. These differences between studies might be due to a higher number of participants with repetitive mTBI and lifetime mTBIs in the previous study, where participants could have up to 6 lifetime TBIs, compared to a maximum number of 3 in the present study. We hypothesize that a single mTBI may not induce severe enough injury to the brain to result in chronic elevation of peripheral levels of NfL, and that higher blood NfL in those with repetitive mTBI and in the smcTBI group might reflect more severe axonal degeneration.
Biological relevance and time course of EV release after TBI are still unclear, but they might depend at least partially on the mechanism of injury32. Our group has previously reported that moderate-to-severe TBI patients with diffuse injury displayed higher levels of EV NFL and EV GFAP in serum than those with focal lesions at acute timepoints after injury32. Moreover, EVs play a role in maintenance of central nervous system homeostasis and in the removal of unwanted proteins of the brain59,60,61, and it is plausible that EVs function as a mechanism to remove products of TBI-induced damage and pathological processes from the brain.
In this study, within one year after injury, EV NfL was significantly higher in participants with more severe injuries when compared to those with uncomplicated mTBI and controls. Median levels of GFAP were also higher in more severe injuries, but group differences were only marginally significant. EV NfL also discriminated those in the smcTBI group from mTBI and IC groups, especially when controlling for demographics and injury characteristics, outperforming GFAP. No group differences in either NfL or GFAP were observed after one year of injury. Interestingly, a recent study has shown significantly higher levels of EV GFAP in moderate and severe TBIs when compared to controls, but not EV NfL, in a civilian cohort at one year after the injury33. It is possible that these discrepancies are due in part to differences in the relative number of severe TBI cases and to a decrease in blood concentrations of EV NfL within the first year after the TBI. Accordingly, we observed in this study a negative correlation between concentration of EV NfL and time in months within the first year after injury. Here, we also observed an increase in the levels of EV GFAP with time in the mTBI groups, which could be an indication of the development of chronic pathological processes involving astrocytes. Importantly, we had a smaller sample size in the group with one or more years since the injury, with a lower percentage of participants with more severe injuries, than in the group within one year after injury. These differences between groups could be a potential confounder, and should be considered when interpreting our results. Future studies with larger samples sizes are warranted to confirm our findings and to further evaluate the prognostic potential of EV NfL and EV GFAP at distinct timepoints after injury across TBI severities. Moreover, temporal profiles of EV biomarkers should be further investigated, addressing a possible biphasic release of EV NfL and EV GFAP, with a decrease within the first year after injury followed by increased levels at more chronic timepoints.
Strengths of this study include the rigorous categorization of TBIs and symptom validation by using the MMPI-2-RF. Nevertheless, this study has limitations, which include the use of cross-sectional data, variability in time since injury at each timepoint, as well as self-reported behavioral symptoms. In addition, the relatively small sample size reduced the sensitivity of our analysis. The smaller number of participants with one or more years after TBI than within the first year after TBI, especially in the group with more severe injury, might be a confounding factor in this study. Moreover, our cohort was predominantly white and male, which reduces the generalizability of our results to other populations. Sex may be linked to differences in the biological response to injuries as well as brain repair processes and recovery62,63,64,65. Additionally, sociocultural factors in association with conspicuous disparities in healthcare access and utilization may influence recovery and clinical outcomes after TBI in more racially/ethnically diverse cohorts66,67,68. Future efforts are warranted to further explore the biomarker utility of EV proteins as biomarkers in TBI in more representative samples, as well as effects of sex and sociocultural differences in TBI pathology and recovery. Moreover, NfL cannot discriminate neuro-axonal injury of the central and peripheral nervous system. Thus, NfL levels might also reflect peripheral nerve damage caused by body injury. Our findings support the potential of EV NfL as a diagnostic biomarker in TBI and as a means to discriminate TBI severity. Increased levels of NfL in TBI ranging from complicated mild to severe suggest that persistent axonal degeneration or remodeling occur in the first year after the TBI. Additional studies are warranted to validate our results, examining longitudinal changes in EV NfL and EV GFAP.
Methods
Study design and population
Participants were 215 SMVs enrolled in the 15‐Year Longitudinal TBI Study (Defense and Veterans Brain Injury Center [DVBIC]/Traumatic Brain Injury Center of Excellence[TBICoE]), recruited from inpatient and outpatient clinics at the Walter Reed National Military Medical Center (WRNMMC; Bethesda, MD) from 2011 to 2019. Participants were active-duty service members or other Defense Enrollment Eligibility Reporting System-eligible veteran; and 18 years of age or older who could read and understand English. Exclusion criteria included: a lack of proficiency in conversational English, or a history of significant neurological or psychiatric condition(s) unrelated to the injury event or deployment (e.g., Meningioma, Bipolar Disorder). Determination of TBI diagnosis and severity was based on medical records and an interview as previously described2,69. Participants were divided into three injury groups: Injury Controls (IC, orthopedic injury without TBI, n = 45), mTBI (uncomplicated TBIs only, n = 107) and smcTBI (complicated mTBI, moderate, or severe TBIs, n = 66). All participants provided written informed consent to participate. This study was approved by the WRNMMC Institutional Review Board in Bethesda, MD and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Determination of TBI severity and orthopedic injury
TBI severity was classified as follows: [1] uncomplicated mild TBI: (i) Glasgow Coma Scale score (GCS) = 13–15, post-traumatic amnesia (PTA) < 24 h, loss of consciousness (LOC < 30 min), and/or alteration of consciousness/mental state (AOC) present, and (ii) no trauma-related intracranial abnormality (ICA) on computed tomography (CT) or structural magnetic resonance imaging (MRI); [2] complicated mild TBI: (i) GCS = 13–15, PTA < 24 h, LOC < 30 min, and/or AOC present, and (ii) trauma-related ICA on CT or MRI; [3] moderate TBI: LOC 30 min–24 h, PTA 1–7 days, and ICA present or absent; and [4] severe TBI: LOC > 24 h, PTA > 7 days, and ICA present or absent. Participants were classified into the Injured Control group if the following was observed: (i) they experienced an orthopedic injury event, (ii) no evidence of an altered state of consciousness as a result of the injury (e.g., GCS < 15; AOC, LOC, and PTA present) or ICA, and (iii) no history of TBI. Neuroimage evaluation was performed at the National Intrepid Center of Excellence (NICoE), which is located on the campus of Walter Reed National Military Medical Center (WRNMMC). All participants received a CT or MRI to evaluate possible of intracranial abnormalities as part of a two-day evaluation that also included clinical interviews and a variety of neurobehavioral measures.
Assessment of PCSx and PTSD symptoms
Participants completed a 2.5‐hour battery of self‐report neurobehavioral measures. PTSD Checklist–Civilian version (PCL-C)70 and Neurobehavioral Symptom Inventory (NSI)71 were used to evaluate PTSD symptoms and PCSx, respectively. Higher scores in PCL-C and NSI indicate more severe symptoms. Participants’ responses on the PCL-C were used to create two PTSD categories based on DSM-IV-TR symptom criteria for PTSD. A participant was classified into the PTSD-Present category based on the endorsement of moderate or higher symptoms for (a) one or more Criterion B symptoms, (b) three or more Criterion C symptoms, and (c) two or more Criterion D symptoms. Similarly, participants’ responses on the NSI were also used to classify DSM-IV-TR research criteria for Postconcussional Disorder (PCD). A participant was classified into the PCD-Present category based on the endorsement of moderate or higher symptoms for (a) three or more Category C symptoms, and (b) subjective complaints of attention or memory problems (i.e., Category B; note that Category B criteria require objective evidence of cognitive impairment in attention or memory. For the purposes of this study, subjective reports of these cognitive complaints were used as a proxy). Participants also completed the Minnesota Multiphasic Personality Inventory-2, Restructured Form (MMPI-2-RF)72 to evaluate symptom validity and were not included in the behavioral analysis if they scored above the recommended cutoffs on the validity scales (i.e., Infrequent Responses [F-r] ≥ 100 T or Infrequent Psychopathology Responses [Fp-r] ≥ 90 T or Infrequent Somatic Responses [Fs] ≥ 100 T or Symptom Validity [FBS-r] ≥ 100 T or Response Bias Scale [RBS43] ≥ 100 T). The number of participants who scored above the cutoff on MMPI-2-RF in the IC, mTBI, and smcTBI groups were 5 (11%), 15 (14%), and 7 (11%), respectively. Additionally, the validity data was missing for one participant in the IC group, and one participant in the smcTBI group.
Blood sampling and EV isolation from serum
Non-fasting blood samples were collected using serum separator tubes (SST) and processed using standard protocols73. Serum was aliquoted, stored at − 80 °C until EV isolation. EVs were isolated from 0.8 ml of frozen serum. After thawing the serum, samples were centrifuged at 3000 × g for 15 min at 4 °C to remove debris and the supernatant was transferred into a clean tube. ExoQuick solution (System Biosciences Inc., Mountainview, CA) was added to the samples according to the manufacturer instructions. Samples were incubated for 1 h at 4 °C and centrifuged at 1500 g for 30 min. After the centrifugation, the supernatant was aspirated and the pellet was resuspended in 0.8 ml of Dulbecco’s calcium- and magnesium free salt solution (Sigma-Aldrich, St. Louis, MO, USA). Resuspended pellets containing EVs were stored at − 80 °C and later used to measure total EV protein content and particle characterization. For particle characterization, samples were analyzed using MACSPlex Exosome Kit (130-108-813, Miltenyi Biotec, Bergisch Gladbach, Germany) and nanoparticle tracking analysis (NTA) software (Malvern Instruments, Malvern, United Kingdom) to determine the mean diameter (nm) and concentration (particles/mL) of EVs.
Protein quantification
For each participant, 100 µl of the EV sample were used to measure total protein content. Each sample received the same volume of M-PER mammalian protein extraction reagent to lyse EV (Thermo Scientific, Inc., Rockford, IL), containing three times the suggested concentrations of protease and phosphatase inhibitors (EASYpack Protease Inhibitor Cocktail, catalog number 5892791001, Millipore Sigma). These mixtures were analyzed using a site-specific Simoa HD-1 analyzer (Quanterix, Lexington, MA). EV concentrations NfL, GFAP, Tau and Ubiquitin C-Terminal Hydrolase L1 (UCHL1) were measured using an ultrasensitive paramagnetic bead-based enzyme-linked immunosorbent assay (Neurology 4-plex A, item 102,153, Quanterix), according to the manufacturer’s protocol. Samples were randomized over plates, run in duplicate, with laboratory scientists blinded to participant groups. The accepted coefficients of variation (CVs) of analyzed samples were no higher than 30% for all analytes. Results for UCHL1 and tau did not meet our quality standards as a large percentage of samples (71.6% and 77.5% for tau and UCHL1, respectively) had non-detectable levels of protein or CVs higher than 30%. Thus, we only report here results for NfL and GFAP. Average CVs were 11% (SD = 7.5) and 3% (SD = 2.5%) for NfL and GFAP, respectively.
Statistical analysis
Comparison of demographic and clinical characteristics between groups were conducted using Chi-square test (χ2), Mann–Whitney U tests or Kruskal–Wallis test. Biomarker comparisons among TBI groups were performed by using Kruskal–Wallis test followed by Dunn’s test correcting for repetitive pairwise comparisons using Bonferroni method. In addition, we performed logistic regression analysis, and calculated the AUC for each model. Univariate AUCs for each biomarker were also calculated. Logistic regression models included demographics (age, gender, race) and time since injury when comparing a TBI group and the injured control group. When comparing two TBI groups, number of TBIs was also included in the model. Confidence intervals were calculated by using DeLong’s method. Cohen’s d was calculated for biomarker pairwise group comparison. Spearman correlations and regressions analysis (negative binomial generalized linear models, GLMs) were used to examine relationships between biomarkers and PCSx and PTSD symptoms. The GLMs included age, gender, race, time since injury, and number of TBIs. Outcomes were either PCL-C or NSI total scores. All data were analyzed using R version 4.0.2. R and GraphPad Prism version 7.04 were used to produce graphs.
Data availability
Aggregate data that support the findings of this study are available upon reasonable request to the corresponding author.
References
DoD Worldwide Numbers for TBI | DVBIC. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi.
Lippa, S. M., French, L. M., Bell, R. S., Brickell, T. A. & Lange, R. T. United States military service members demonstrate substantial and heterogeneous long-term neuropsychological dysfunction after moderate, severe, and penetrating traumatic brain injury. J. Neurotrauma 37, 608–617 (2020).
Mckee, A. C. & Daneshvar, D. H. The neuropathology of traumatic brain injury. in Handbook of Clinical Neurology vol. 127 45–66 (Elsevier B.V., 2015).
Bazarian, J. J. et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 17, (2018).
Gill, J. et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 91, e1385–e1389 (2018).
Ljungqvist, J., Zetterberg, H., Mitsis, M., Blennow, K. & Skoglund, T. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J. Neurotrauma 34, 1124–1127 (2017).
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019).
Bazarian, J. J. et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 17, 782–789 (2018).
Shahim, P. et al. Time course and diagnostic utility of NfL, tau, GFAp, and UCH-L1 in subacute and chronic TBI. (2020). https://doi.org/10.1212/WNL.0000000000009985.
Werner, J. K. et al. Poor sleep correlates with biomarkers of neurodegeneration in mild traumatic brain injury patients: a CENC study. Sleep 2020, 1–8 (2020).
van der Ende, E. L. & van Swieten, J. C. Fluid biomarkers of frontotemporal lobar degeneration. Adv. Exp. Med. Biol. 1281, 123–139 (2021).
Guedes, V. A. et al. Exosomal neurofilament light. Neurology https://doi.org/10.1212/WNL.0000000000009577 (2020).
Lin, C. H. et al. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology 93, e1104–e1111 (2019).
Shahim, P. et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology 95, e610–e622 (2020).
Guedes, V. A. et al. Exosomal neurofilament light: A prognostic biomarker for remote symptoms after mild traumatic brain injury?. Neurology 94, e2412–e2423 (2020).
Maas, A. I. R. et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 (2017).
Shahim, P. et al. Neurofilament Light as a Biomarker in Traumatic Brain Injury. https://doi.org/10.1212/WNL.0000000000009983 (2020).
Shahim, P. et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 1–9 (2016).
Krämer-Albers, E. M. et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons?. Proteomics - Clin. Appl. 1, 1446–1461 (2007).
Fauré, J. et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 (2006).
Mustapic, M. et al. Plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Front. Neurosci. 11, 1–12 (2017).
Peterson, M. F., Otoc, N., Sethi, J. K., Gupta, A. & Antes, T. J. Integrated systems for exosome investigation. Methods 87, 31–45 (2015).
Guedes, V. A. et al. Extracellular vesicle proteins and microRNAs as biomarkers for traumatic brain injury. Front. Neurol. 11, 663 (2020).
Li, X. et al. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 3, 011503 (2019).
Taylor, D. D. & Gercel-Taylor, C. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369, 20130503–20130503 (2014).
Zhang, Y., Liu, Y., Liu, H. & Tang, W. H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 9, 19 (2019).
Dinkins, M. B., Wang, G. & Bieberich, E. Sphingolipid-enriched extracellular vesicles and Alzheimer’s disease: a decade of research. J. Alzheimer’s Dis. 60, 757–768 (2017).
Perez-Gonzalez, R., Gauthier, S. A., Kumar, A. & Levy, E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space *. ASBMB https://doi.org/10.1074/jbc.M112.404467 (2012).
DeLeo, A. M. & Ikezu, T. Extracellular vesicle biology in Alzheimer’s disease and related tauopathy. J. Neuroimmune Pharmacol. 13, 292–308 (2018).
Winston, C. N. et al. Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front. Neurosci. 13, (2019).
Kawata, K., Mitsuhashi, M. & Aldret, R. A preliminary report on brain-derived extracellular vesicle as novel blood biomarkers for sport-related concussions. Front. Neurol. 9, 12 (2018).
Mondello, S. et al. Circulating brain injury exosomal proteins following moderate-to-severe traumatic brain injury: temporal profile, outcome prediction and therapy implications. Cells 9, 977 (2020).
Flynn, S. et al. Extracellular vesicle concentrations of glial fibrillary acidic protein and neurofilament light measured 1 year after traumatic brain injury. Sci. Rep. 11, 3896 (2021).
Lange, R. T. et al. Neuropsychological outcome from uncomplicated mild, complicated mild, and moderate traumatic brain injury in us military personnel. Arch. Clin. Neuropsychol. 27, 480–494 (2012).
Lewis, L. M. et al. Utility of serum biomarkers in the diagnosis and stratification of mild traumatic brain injury. Acad. Emerg. Med. 24, 710–720 (2017).
Shahim, P. et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. 73, 1308–1315 (2016).
Jakimovski, D. et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann. Clin. Transl. Neurol. 6, 1757–1770 (2019).
Thouvenot, E. et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur. J. Neurol. 27, 251–257 (2020).
Kuhle, J. et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92, E1007–E1015 (2019).
de Wolf, F. et al. Plasma tau, neurofilament light chain and amyloid-b levels and risk of dementia; a population-based cohort study. Brain 143, 1220–1232 (2020).
Sandelius, Å. et al. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90, e518–e524 (2018).
Fyfe, I. Neurofilament light chain—new potential for prediction and prognosis. Nat. Rev. Neurol. 15, 557 (2019).
Pattinson, C. L. et al. Elevated tau in military personnel relates to chronic symptoms following traumatic brain injury. J. Head Trauma Rehabil. https://doi.org/10.1097/HTR.0000000000000485 (2019).
Shahim, P., Tegner, Y., Marklund, N., Blennow, K. & Zetterberg, H. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 90, e1780–e1788 (2018).
Shahim, P., Zetterberg, H., Tegner, Y. & Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 88, 1788–1794 (2017).
McKeon, A. & Benarroch, E. E. Glial fibrillary acid protein: Functions and involvement in disease. Neurology 90, 925–930 (2018).
Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015).
Matias, I., Morgado, J. & Gomes, F. C. A. Astrocyte heterogeneity: impact to brain aging and disease. Front. Aging Neurosci. 11, 1–18 (2019).
Iliff, J. J. et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 (2014).
Zhou, Y. et al. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Communication and Signaling vol. 18 (2020).
Christensen, J., Wright, D. K., Yamakawa, G. R., Shultz, S. R. & Mychasiuk, R. Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci. Rep. 10, 1–9 (2020).
Diaz-Arrastia, R. et al. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-l1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 (2014).
Margolisid, L. & Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. https://doi.org/10.1371/journal.pbio.3000363.
Cypryk, W., Nyman, T. A. & Matikainen, S. From inflammasome to exosome-does extracellular vesicle secretion constitute an inflammasome-dependent immune response?. Front. Immunol. 9, 2188 (2018).
Nuriya, M. & Hirase, H. Involvement of astrocytes in neurovascular communication. Progress in Brain Research vol. 225 (Elsevier B.V., 2016).
Mustapic, M. et al. plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Front. Neurosci. 11, 278 (2017).
Thompson, A. G. et al. Extracellular vesicles in neurodegenerative disease-pathogenesis to biomarkers. Nat. Rev. Neurol. 12, 346–357 (2016).
Budnik, V., Ruiz-Cañada, C. & Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 (2016).
Gupta, A. & Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflammation 11, 68 (2014).
Kong, S. M. Y. et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum. Mol. Genet. 23, 2816–2833 (2014).
Emmanouilidou, E. et al. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 (2010).
Gupte, R., Brooks, W., Vukas, R., Pierce, J. & Harris, J. Sex differences in traumatic brain injury: What we know and what we should know. J. Neurotrauma 36, 3063–3091 (2019).
Villapol, S., Loane, D. J. & Burns, M. P. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 (2017).
Farin, A., Deutsch, R., Biegon, A. & Marshall, L. F. Sex-related differences in patients with severe head injury: Greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurg. 98, 32–36 (2003).
Mondello, S. et al. Sex differences in circulating T-tau trajectories after sports-concussion and correlation with outcome. Front. Neurol. 11, 651 (2020).
Kane, W. G., Wright, D. A., Fu, R. & Carlson, K. F. Racial/ethnic and insurance status disparities in discharge to posthospitalization care for patients with traumatic brain injury. J. Head Trauma Rehabil. 29, E10–E17 (2014).
Crandall, M. et al. Patterns and predictors of follow-up in patients with mild traumatic brain injury. Brain Inj. 28, 1359–1364 (2014).
Gao, S., Kumar, R. G., Wisniewski, S. R. & Fabio, A. Disparities in health care utilization of adults with traumatic brain injuries are related to insurance, race, and ethnicity: a systematic review. J. Head Trauma Rehabil. 33, E40–E50 (2018).
Lange, R. T. et al. Long-term neurobehavioural symptom reporting following mild, moderate, severe, and penetrating traumatic brain injury in U.S. military service members. Neuropsychol. Rehabil. https://doi.org/10.1080/09602011.2019.1604385 (2019).
Andrykowski, M. A., Cordova, M. J., Studts, J. L. & Miller, T. W. Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL–C) as a screening instrument. J. Consult. Clin. Psychol. 66, 586–590 (1998).
Soble, J. R. et al. Normative data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin. Neuropsychol. 28, 614–632 (2014).
Lange, R. T., Brickell, T. A., Lippa, S. M. & French, L. M. Clinical utility of the Neurobehavioral Symptom Inventory validity scales to screen for symptom exaggeration following traumatic brain injury. J. Clin. Exp. Neuropsychol. 37, 853–862 (2015).
Olivera, A. et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 72, 1109–1116 (2015).
Acknowledgements
Authors would like to acknowledge the efforts of the larger team of research coordinators, research associates, research assistants, program managers, and senior management, who contribute to the success of the DVBIC-TBICoE 15-Year Longitudinal TBI Study (Sec721 NDAA FY2007). The principal investigators, Dr. Guedes and Dr. Gill, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere.
Funding
This work was primarily supported by the Traumatic Brain Injury Center of Excellence (TBICoE; Contract HT0014-19-C-0004), and partially supported by grant funding from the Center for Neuroscience and Regenerative Medicine (HJF/USU Award number: 308811–8.01–60855, CNRM-75-9241) and the National Institutes of Health, National Institute of Nursing Research Intramural Research Program. Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the official policy or position of the Defense Health Agency, Department of Defense, or any other U.S. government agency. This work was prepared under Contract HT0014-19-C-0004 with DHA Contracting Office (CO-NCR) HT0014 and, therefore, is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact dha.TBICoEinfo@mail.mil.
Author information
Authors and Affiliations
Contributions
V.A.G had a major role in the acquisition of lab data; Interpreted the data; drafted the manuscript. R.T.L. had a major role in the acquisition of clinical data, design, and conceptualization of study, interpreted the data, drafted the manuscript. S.M.L. had a major role in the acquisition of clinical data. C.L. had a role in the acquisition of lab data; Interpreted the data. S.M. had a role in the acquisition of lab data. K.G. draft the manuscript, revised the manuscript for intellectual content. C.D. had a role in the acquisition of lab data, revised the manuscript for intellectual content. K.A.E. had a role in the acquisition of lab data. C.L.W. had a role in the acquisition of lab data. C.M. had a role in the acquisition of lab data. A.E.D. had a role in the acquisition of clinical data. M.M.W. had a role in the acquisition of clinical data. K.C.G. had a role in the acquisition of clinical data. S.M.B. had a role in the acquisition of clinical data. T.A.B. had a major role in the acquisition of clinical data. L.M.F. had a major role in design and conceptualization study, interpreted the data. J.M.G. had a major role in design and conceptualization study, interpreted the data, drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guedes, V.A., Lange, R.T., Lippa, S.M. et al. Extracellular vesicle neurofilament light is elevated within the first 12-months following traumatic brain injury in a U.S military population. Sci Rep 12, 4002 (2022). https://doi.org/10.1038/s41598-022-05772-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05772-0
This article is cited by
-
Low-intensity open-field blast exposure effects on neurovascular unit ultrastructure in mice
Acta Neuropathologica Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.