Abstract
In the agricultural industry, advances in optical imaging technologies based on rapid and non-destructive approaches have contributed to increase food production for the growing population. The present study employed autofluorescence-spectral imaging and machine learning algorithms to develop distinct models for classification of soybean seeds differing in physiological quality after artificial aging. Autofluorescence signals from the 365/400 nm excitation-emission combination (that exhibited a perfect correlation with the total phenols in the embryo) were efficiently able to segregate treatments. Furthermore, it was also possible to demonstrate a strong correlation between autofluorescence-spectral data and several quality indicators, such as early germination and seed tolerance to stressful conditions. The machine learning models developed based on artificial neural network, support vector machine or linear discriminant analysis showed high performance (0.99 accuracy) for classifying seeds with different quality levels. Taken together, our study shows that the physiological potential of soybean seeds is reduced accompanied by changes in the concentration and, probably in the structure of autofluorescent compounds. In addition, altering the autofluorescent properties in seeds impact the photosynthesis apparatus in seedlings. From the practical point of view, autofluorescence-based imaging can be used to check modifications in the optical properties of soybean seed tissues and to consistently discriminate high-and low-vigor seeds.
Similar content being viewed by others
Introduction
Seeds are important sources of food for human and livestock, in addition to be considered the best natural method for protection of genetic material variability. Soybean [Glycine max (L.) Merril] is one of the most important oil and protein crop worldwide. The soybean yield depends on the use of high-vigor seeds because they guarantee a rapid and uniform establishment of plants under a variety of environmental conditions1,2,3. Nowadays, the seed industry uses standardized germination and vigor tests to predict field performance of seedlots. These tests provide valuable information on seed physiological potential; however, they are relatively time-consuming with subjective results that depend on specialized analysts, being difficult to reproduce the results4. Hence, there is a great interest for exploring advanced and innovative methods to further refine the current seed testing methods.
In the last few years, fluorescence-based methods are providing significant advances in many areas of life science, including medical diagnostics5, forensics6, food quality7,8, plant physiology9 and genetic analysis10. Traditional fluorescence imaging is mainly based on fluorescence spectroscopy (e.g. FTIR or Raman microspectroscopy) that can be applied to determine autofluorescent compounds, or non-fluorescent compounds can be measured by using multiple fluorescent tracers to highlight molecular, physiological or anatomical features11,12. However, this technique assesses only a small part of an object (i.e., a “spot measurement”), so they do not provide spatial information that it is important for many seed inspection applications. In this context, advanced autofluorescence-spectral imaging techniques can be a great alternative to overcome such a limitation. Autofluorescence-spectral imaging is a modern optical technology that combine both spatial and spectral information in a single measuring protocol. It provides a high-resolution optical spectrum for each image pixel and yields a set of images of the same object, in which each image is represented by a specific wavelength. In seed technology, autofluorescence-spectral imaging technique can be applied on fluorescent chemical compounds—the so-called fluorophores—with important roles in seed biology, including pigments (e.g. chlorophylls) and structural components of cell wall such as lignin13,14.
When fluorescent compounds are stimulated by light, they are raised to an excited state as a result of photon absorption, and the radiation light re-emitted is measured by autofluorescence-spectral imaging sensors. Photon absorption and fluorescence emission occur simultaneously15. Excitation is induced in the ultraviolet and visible regions, and a charge-coupled device chip (CCD-chip) combined with different filters at longer wavelengths detect the light emission (fluorescence) as seed compounds relax to lower energy levels. Furthermore, combining autofluorescence-spectral imaging technology with chemometric approaches may allow better characterization of seed quality in a non-destructive way. For instance, machine learning algorithms such as artificial neural network (ANN), support vector machine with either linear (SVM-l) or radial basis (SVM-r) kernel, and linear discriminant analysis (LDA) have been proven to be effective for solving problems in many research fields16,17,18,19,20. In the present study, we tested the use of these machine learning algorithms combined with autofluorescence-spectral imaging for classification of soybean seeds based on their physiological quality levels.
Results
Physiological status of seeds
The germination percentage decreased as seed aged, especially at 5 days after sowing (Fig. 1). While the electrical conductivity (an indirect indicator of seed membrane integrity) increased in seeds aged for 24 h and 48 h (Fig. 1), the saturated-salt accelerated aging (SSAA) separated seeds into two groups, i.e., seeds aged for 0 and 12 h vs seeds aged for 24 and 48 h. Meanwhile, the traditional artificial aging (AA) further sliced non-aged seeds from the aged ones. The seedling emergence test was unable to differ seed classes (Fig. 1).
Biochemical changes in seeds
The total carotenoids and phenols in the seed coat and embryo (embryonic axis plus cotyledons) did not differ in aged seeds compared to non-aged class (Fig. 2). The malonaldehyde (MDA) content was markedly decreased in aged seeds in comparison to non-aged seeds (42.33–71.33%) (Fig. 2). In addition, there was a high amount of hydrogen peroxide (H2O2) in non-aged seeds, and a maximum level in seeds aged for 12 h (Fig. 2). Further biochemical analyses indicated that the content of lignin, chlorophyll a and b (Fig. 2) remained at similar levels for 0 (non-aged seeds), 12 and 24 h of aging; however, increasing the age time to 48 h reduced lignin content (by 14.23% compared to non-aged seeds). At the same time, seeds aged for 48 h had a substantial increment in chlorophyll a and b (108.09% and 86.50%, respectively).
Total carotenoids, total phenols in the seed coat and embryo, malondialdehyde (MDA), hydrogen peroxide (H2O2), lignin in the seed coat, chlorophyll a and b of non-aged soybean seeds and seeds aged for 12, 24 and 48 h. Means (± standard deviation) followed by the same letter are not significantly different according to Tukey test (P < 0.05).
Autofluorescence-spectral markers
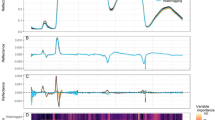
The average autofluorescence spectra of the seeds were higher at excitation wavelengths from 365 to 540 nm (with exception of 470 nm) (Fig. 3a). Non-aged seeds were efficiently separated from aged classes at 365/400 nm, 405/500 nm and 630/700 nm excitation-emission combinations; among these combinations, 365/400 nm provided the most refined discrimination of the seed classes (Fig. 3a). The highest correlation coefficients between bandwidths were: 1.00 (515/600 nm vs 540/600 nm), 0.99 (430/500 nm vs 450/500 nm; 630/700 nm vs 645/700 nm), and 0.97 (405/500 nm vs 430/500; 645/700 nm vs 660/700 nm) (Fig. 3b).
Comparison between classes of non-aged soybean seeds and seeds aged for 12, 24 and 48 h using autofluorescence-spectral data from different excitation-emission combinations; means followed by the same letter are not significantly different according to Tukey test (P < 0.05) (a). Pearson’s correlation coefficients between all bandwidths used for autofluorescence-spectral imaging of soybean seeds (n = 200 seeds) (b).
Using nCDA algorithm, the pixel values of the autofluorescence images acquired with the combination of 365/400 nm were transformed by removing the outlier observations, which allowed to calculate the trimmed mean. This technique is useful to eliminate the influence of outliers that can affect the mean, and thus providing a more realistic image. The autofluorescence intensity was displayed in the images using pixel-to-pixel mapping that was created using false color codes ranging from red (0) to blue (256), where higher pixel values represent tissues with higher autofluorescence (Fig. 4). Although all classes showed similar RGB images, there was a different autofluorescent pattern when the images were captured in the presence of the seed coat (Fig. 4a), in which seeds aged for 24 and 48 h exhibited the lowest autofluorescence signals (lower pixel values). However, removing the seed coat, all seed classes presented a similar autofluorescent pattern, regardless of the aging period (Fig. 4b). In the germination test at 8 days, the lower the seed autofluorescence signal in the presence of the seed coat the lower the seedling performance (Fig. 4c).
Raw RGB images from soybean seeds for classes of non-aged seeds and seeds aged for 12, 24 and 48 h, and corresponding autofluorescence images (grayscale and nCDA) captured at 365/400 nm excitation-emission combination, showing autofluorescence patterns in the presence (a) and absence (b) of the seed coat. Germination test at 8 days after sowing using seeds with different autofluorescence patterns in the nCDA images (c). In the nCDA images, the pixel values (autofluorescence intensity) is calculated based on 10% trimmed mean to provide a more realistic image.
In the linear discriminant analysis (LDA) the first two discriminant components (LD1 and LD2) explained 91.19% of the total variation between autofluorescence-spectral data (Fig. 5a). Autofluorescence data obtained from 365/400 nm combination contributed most to the LDA model (Fig. 5b), in which higher vigor seeds (i.e., non-aged seeds and seeds aged for 12 h) showed the highest fluorescence values (Fig. 5c).
Linear discriminant analysis (LDA) for autofluorescence-spectral data in soybean seeds using different excitation-emission combinations (a). Importance of each excitation-emission combination to discriminate non-aged seeds from seeds aged for 12 h, 24 h and 48 h (b). Scatter plot with the representation of the values of each variable based on autofluorescence data using 365/400 nm combination (c) (n = 200 seeds).
Seed quality classification based on different machine learning algorithms
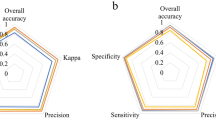
The algorithms (i.e., ANN, SVM-l, and LDA) presented excellent performance for discrimination of seeds from different aged groups based on autofluorescence-spectral data (Table 1). In addition, the models developed showed very high accuracy (0.99), kappa (≥ 0.98), precision (0.99), recall (0.99), and F1 (0.99) in both cross and external validations (Table 1). The ANN algorithm showed slightly superior performance for kappa coefficient in cross-validation test.
Correlation between autofluorescence measurements and seed quality indicators
The Pearson’s correlation analysis (Fig. 6) showed a perfect negative correlation between 365/400 nm combination and the total phenols in the embryo (− 1.00), and a perfect positive correlation between 660/700 nm combination and the SSAA test (1.00). High correlation coefficients were also noticed for other bandwidths: 0.99 (either 515/600 nm or 540/600 nm vs lignin), − 0.99 (630/700 nm vs electrical conductivity; 645/700 nm vs total phenols in the embryo), 0.98 (430/500 nm vs lignin), 0.97 (405/500 nm vs lignin), − 0.97 (570/600 nm vs either chlorophyll a or chlorophyll b), 0.96 (450/500 nm vs lignin) and 0.95 (470/500 nm vs lignin).
Seedings grown from aged and non-aged seeds
Although seedlings from seeds aged for 48 h reached a germination percentage similar to non-aged seeds at 8 days after sowing (Fig. 1), with a remarkably enhanced chlorophyll a index in the resulting seedlings on the 16th day (Fig. 7), they exhibited less efficient photochemical apparatus than those from non-aged seeds as highlighted by the quantum yield of photosystem II (FV/FM) (Fig. 7). Higher chlorophyll fluorescence was closely related to aged class, particularly in seedlings grown from seeds aged for very short period (12 h) (Fig. 7).
Chlorophyll a index, chlorophyll a fluorescence, and maximum quantum yield of photosystem II based on FV/FM in soybean seedlings grown from non-aged seeds and seeds aged for 12, 24 and 48 h on the 16th day after sowing. Means (± standard deviation) followed by the same letter are not significantly different according to Tukey test (P < 0.05).
Discussion
The vigor of seeds depends essentially on their ability to withstand the deleterious effects of aging, which induces the deterioration of tissues through consumption of reserves and damages to genetic material21. Artificial seed aging has been widely used to study the physiological and biochemical mechanisms associated with decreased seed vigor22,23,24, particularly to predict the seed performance during the entire storage period. Seed storage starts in the field when the seeds reach physiological maturity and ends at the beginning of the germination process25. For instance, at the point of physiological maturity, soybean seeds have a relatively high water content, and therefore seed quality can be highly affected by environmental conditions until harvest maturity, i.e., when the seeds reach a moisture content level that permits an efficient harvest. In the present study, we used an advanced autofluorescence-spectral imaging and machine learning models to efficiently classify the physiological potential of soybean seeds.
The artificial aging promoted deterioration to the soybean seed tissues as revealed by reduced physiological performance (Fig. 1) and biochemical changes (Fig. 2). For instance, lower seed germination was accompanied by loss of membrane integrity over time (as shown by progressive increase in electrical conductivity through the electrolyte leakage—Fig. 1). Another interesting result was the strong accumulation of H2O2 in the first aging period (12 h) (Fig. 2). Despite the fact that H2O2 can be a toxic byproduct of aerobic metabolism, depending on the concentration its role as a signaling molecule during oxidative stress is well-known26. Perhaps, as a consequence of H2O2 signaling, there was less damages to membrane polyunsaturated fatty acids27 with decreased MDA levels (a toxic aldehydic end product of lipid peroxidation of polyunsaturated fatty acids) during aging, as shown in Fig. 2. In this context, H2O2 content can be used as an indicative of “metabolic health” in soybean seeds. Accordingly, autofluorescence signals emitted in the excitation-emission combinations of 365/400 nm and 660/700 nm showed a very strong positive correlation with H2O2 levels (r2 = 0.91 and 0.93, respectively), which were two of the four bandwidth combinations strongly correlated with early germination test (r2 = 0.91 and 0.92, respectively). In addition, the combination of 365/400 nm was perfectly correlated with the accumulation of the total phenols in the seed embryo (r2 = − 1.00), which efficiently protect soybean seeds from the oxidative stress of various origins 28. Meanwhile, the combination of 660/700 nm reached a perfect positive correlation with seed tolerance to the stressful conditions of the SSAA test (r2 = 1.00).
The nature of the autofluorescence intensity at excitation wavelength of 365 and 660 nm is mainly attributed to chlorophylls29,30, which absorb light in a broader range of the spectrum. Therefore, chlorophyll fluorescence appeared to be an important indicator of soybean seed quality for agricultural industry; however, besides chlorophyll, other autofluorescent molecules can also be excited at 365 nm such as lignin and ferulic acid (weaker signal) present in the cell wall29,31. Curiously, the combination of 365/400 nm provided a better separation of the seed classes, in which the autofluorescence decreased as the aging time increased (Figs. 3, 4, 5). It is important to emphasize that such discrimination was more efficient than the early germination test, for example (Fig. 1).
Additionally, our results indicated that depending on the seed region and the aging period, the aging process can (i) enhances chlorophyll synthesis (Fig. 2), (ii) increases chlorophyll degradation (Fig. 4) or (iii) induces these two events in seeds. As shown in the autofluorescence images (Fig. 4), the deleterious effects of deterioration predominantly affected the autofluorescent compounds present in the superficial regions of the seeds. Donaldson and Williams31 have demonstrated that chlorophyll fluorescence is highly reduced in deteriorated tissues due to the loss of chlorophylls, whereas these regions generally show a substantial increase in green fluorescence associated with browning. In recent studies, Medeiros et al.16 investigated soybean seed images obtained in the visible spectrum, and these authors also found a strong correlation between seed coat patterns and seed vigor levels.
In the current study, the greatest autofluorescence-spectral signals shown in non-aged soybean seeds corroborates previous studies of Li et al.32 who verified that the average spectral fluorescence of viable soybean seeds (by 365 nm excitation) was higher in relation to non-viable seeds. Moreover, we developed models based on ANN, SVM-l and LDA algorithms and full excitation-emission combinations, and the models presented an excellent performance for soybean seed quality classification (0.99 accuracy). The ANN method was implemented with the multilayer perceptron (MLP) algorithm that trains using Backpropagation. Neural networks have been successfully applied to identify contaminants in seeds through image analysis and the topology of the multilayer perceptron (MLP) also proved to be the best in recognizing classes of triticale seeds33. The SVM-l algorithm showed to be efficient in discriminating high-and low-vigor soybean seeds compared to LDA models34. However, the LDA approach has achieved the highest training speed, with high potential to classify the quality of beans35 and oat19 seeds from multispectral sources.
Other interesting results were: (1) a direct relationship between very low autofluorescence in seeds at 365/400 nm combination (aging for 48 h—Fig. 3a) and lower FV/FM in the resulting seedlings (Fig. 7); (2) increased synthesis of chlorophyll a and chlorophyll b in seeds (aging for 48 h—Fig. 2) and greater accumulation of chlorophyll a in seedlings (Fig. 7). Considering that the percentage of emerged seedlings was significantly similar between non-aged and aged seeds (Fig. 1), our data suggest a possible compensatory mechanism during seedling development to outstand aging side effects that were detected at early stage of germination. Such mechanism may be related to an increase in chlorophyll a and b in aged seeds and higher amount of chlorophyll a in the resulting seedlings to improve the conversion of light to chemical energy—although this was not sufficient to improve the photochemical activity of the photosystem II as revealed by FV/FM values. In fact, chlorophyll plays an essential role in the physiological performance of mature seeds36,37, and plant responses to abiotic stress conditions38.
Taken together, seed aging impacted photon-emitting substances that were detected by autofluorescence-spectral imaging, which can be used as a new marker for qualitative analysis of soybean seeds. Soybean seed is very sensitive to environmental stress, leading to both nutritious and economic losses. Moreover, in a seed bank context this approach may improve the accuracy of quality tests, providing novel insights into the storage and use of soybean seed sources.
Conclusions
Autofluorescence-spectral imaging based on fluorescent properties of seed tissues can be a potential tool for characterization of soybean seed physiological potential. This approach can rapidly assess soybean seed quality using artificial-aging treatment by applying artificial neural network, support vector machine or linear discriminant analysis, with an excellent performance (0.99 accuracy). The autofluorescence emitted in the excitation-emission combination of 365/400 nm (that exhibited a perfect correlation with the total phenols in the embryo) enables a clear separation of seed classes.
Furthermore, there is a strong correlation between autofluorescence-spectral data and several quality indicators, including early germination and seed tolerance to stressful conditions. In addition, altering the autofluorescent compounds in seeds impact the photosynthesis apparatus in seedlings. To further advance development, we strongly support in depth-studies with a larger number of crop cultivars to detect seed quality traits and evaluate differences among different genotypes.
We would like to emphasize that the artificial-aging method used in the present work was previously used by other authors, with excellent results in the development of new seed quality methods based on non-destructive techniques39,40,41. However, another important future study will be to compare the results obtained from artificially-aged seeds, as done in the current work, with those of naturally-aged seeds (see Rajjou et al.42). Indeed, it is known that the physiology of seed deterioration during storage is largely influenced by the physical state of the cytoplasm43, which depends on equilibrium RH during storage. Under dry conditions (as in commercial and genebank storage) this cytoplasm is glassy with very low molecular mobility, while under high humidity aging conditions (as employed in the current study) the cytoplasm is liquid with high molecular mobility. Despite these limitations we anticipate that autofluorescence-spectral imaging based on fluorescent properties of seed tissues will provide a very potent and convenient tool for characterization of soybean seed physiological quality.
Materials and methods
Plant material
The soybean seeds (M5705 IPRO cultivar) used in this study were kindly provided by Dr. Raquel Márcia Modena Wutzki, Seed Quality Manager at the Lagoa Bonita Company, in Itaberá city, São Paulo State, Brazil. Seeds were produced in the Fazenda Redomona (field 194S18), Arapoti city, Paraná State, Brazil during 2018/2019 crop season. All required approvals were obtained for the study, which complied with all relevant regulations. Seeds with an initial moisture content of 7.9% were artificially aged for 0, 12, 24 and 48 h. For aging, seeds were distributed over a single layer on a wire mesh screen suspended inside a covered box (11.0 cm × 11.0 cm × 3.5 cm) containing 40 mL of distilled water at 42 °C and 98 ± 2% relative humidity (RH). Subsequently, seeds were dried at 20 °C for 24 h to bring them back to their initial pre-aged moisture content before measurements.
Autofluorescence-spectral imaging
Multispectral fluorescence images were captured from seeds (four 9-cm glass Petri dishes with 50 seeds per class) using a VideometerLab4™ device (Videometer A/S, Herlev, Denmark). This system is integrated with a CCD-chip, providing autofluorescence-spectral images (2192 × 2192 pixels; 40 μm/pixel; 32 bits/pixel) in a few seconds, requiring no sample preparation. Long-Pass (LP) filters were combined with different excitation wavelengths, providing the following excitation-emission combinations: 365/400 nm, 405/500 nm, 430/500 nm, 450/500 nm, 470/500 nm, 515/600 nm, 540/600 nm, 570/600 nm, 630/700 nm, 645/700 nm, 660/700 nm. RGB images were also captured by the same sensor, in which each individual pixel is associated with the values for red, green and blue channels.
Autofluorescences-spectral data were extracted using the VideometerLab™ software version 3.14.9 (Videometer A/S, Herlev, Denmark). It was built a mask to segment the seeds and then a normalized canonical discriminant analysis (nCDA) algorithm was applied to highlight the autofluorescence signals pixel-to-pixel. This algorithm uses 10% trimmed mean, eliminating the influence of outliers (the lowest 10% and the highest 10% of the data).
To verify whether artificial aging affects primarily the fluorophores present in the superficial regions of the seed tissues, autofluorescence images of all samples were also generated without seed coat using the same procedures described above, and they were used for illustration only.
Germination tests
Following the imaging, seeds were distributed on a wire mesh screen suspended inside a box (11.0 cm × 11.0 cm × 3.5 cm) containing 40 mL of distilled water. The boxes were covered with lids and maintained at 25 °C for 16 h to provide a lower uptake of water. Subsequently, four repetitions of 50 seeds were placed into rolled paper towels moistened with distilled water (1: 2.5, g: mL) and kept at 25 °C with 8/16 h (day/night). The percentage of germinated seeds were based on the number of normal seedlings on the 5th and 8th days after sowing.
Vigor tests
Four repetitions with 50 seeds per class were evaluated for electrical conductivity, SSAA, AA, and seedling emergence tests. The electrical conductivity (μS cm−1 g−1) was measured in samples previously weighed and placed in plastic containers with 75 mL of distilled water at 25 ºC for 24 h. In the aging tests, seeds were distributed over a single layer on a wire mesh screen suspended inside a box (11.0 cm × 11.0 cm × 3.5 cm) containing either 40 mL of distilled water (AA test) or 40 mL of saturated NaCl solution (i.e., 40 g of NaCl in 100 mL of water) (SSAA test), providing 98 ± 2% RH (AA test) or 76 ± 2% RH (SSAA test) at 42 °C for 72 h. The rate of normal seedlings was recorded at 5 days. The seedling emergence test was conducted in plastic boxes (32.0 cm × 28.0 cm × 10.0 cm) at room temperature (25–30 °C) for 8 days.
Chlorophylls and carotenoids in seeds
Chlorophyll a, chlorophyll b and the total carotenoids were extracted from 5 g of seeds tissue in 10 mL of 80% acetone. After 24 h the material was filtered and centrifuged at 500×g for 10 min. The Chlorophylls and carotenoids were determined by the absorbance at 470, 662 and 645 nm44,45.
Lipid peroxidation in seeds
Lipid peroxidation or MDA was measured according to the method described by Heath and Packer46. Samples with 400 mg of macerated material were mixed with 4 mL of 0.1% (w/v) trichloroacetic acid (TCA) and then centrifuged at 10,000 rpm for 15 min at 4 °C. The reaction was started by adding 250 μL of supernatant and 1 mL of 20% trichloroacetic acid + 0.5% thiobarbituric acid solution. All samples were kept in a dry bath at 95 °C for 30 min. The material was centrifuged at 10,000 rpm for 10 min, and the absorbance was read at 535 and 600 nm. The concentration of H2O2 was determined according to Alexieva et al.47. Approximately 400 mg of macerated material was mixed with 4 mL of 0.1% (w/v) trichloroacetic acid (TCA). The material was homogenized and centrifuged at 10,000 rpm for 15 min at 4 °C. Subsequently, 200 μL of supernatant was combined with 200 μL of 100 mM potassium phosphate buffer and 800 μL of 1 M potassium iodide solution. The absorbance was read at 390 nm.
Lignin in the seed coat
Lignin was determined using acetyl bromide procedure as described by Fukushima and Kerley48. Cell wall extraction was performed with 10 mg of seed material combined with 1 mL water at 98 °C for 30 min. The samples were centrifuged for 5 min at 14,000 rpm, and then the supernatant was mixed with 1 mL ethanol and incubated at 76 °C for 30 min. Next, the samples with 1 mL of chloroform were incubated at 59 °C for 30 min. Finally, it was added 1 mL of acetone and the samples were incubated at 54 °C for 30 min. After centrifuging for 5 min at 14,000 rpm, the supernatant was removed and the pellet was dried for 45 min using a SpeedVac vacuum. The purified cell wall was used for lignin quantification using acetyl bromide.
Total phenols in the seed coat and embryo
The total phenols in the seed coat and embryo was determined following the procedures described by Swain and Hillis49. The measurements were conducted in 100 mg of seed material mixed with 2.0 mL of ethanol (70%) at 70 °C water bath for 30 min. After centrifuging for 5 min at 13,000 rpm, the supernatant was combined with the Folin–Ciocalteau reagent and a sodium carbonate solution. The absorbance was measured at 725 nm.
Photosynthesis and chlorophyll a in seedlings
Measurements were conducted on 32 pots per class (two seedlings per pot) kept at 25 °C, 8/16 h (day/night), and 50–70% RH. Light was provided by LED lamps (900 mm, 13 W) (County Ilum., São Paulo, Brazil) with a photosynthetically active radiation (PAR) of 200 μmols m−2 s−1. At 16 days, seedlings were adapted in the dark for 30 min, and then they were illuminated with high intensity amber LEDs (620 nm peak) with a saturating light intensity of 6,320 μmol m−2 s−1 in a short time interval (≈ 0.8 s). A fast chlorophyll fluorescence method based on the Kautsky induction curve was used to measure the initial fluorescence (F0), maximum fluorescence (FM), variable fluorescence (FV) and FV/FM50 using a SeedReporter™ equipment (PhenoVation B.V., Wageningen, Netherlands). A CCD-chip provided a fast screening of the chlorophyll fluorescence emission at 730 nm, generating images with a spatial dimension of 2,448 × 2,448 pixels (3.69 μm/pixel). The FV/FM was measured based on the following formula:
Chlorophyll a index was measured as described by Gitelson et al.51 based on reflectance at 710 and 770 nm, according to the following formula:
where ρ represents the spectral reflectance for each wavelength.
Data analysis
The main procedures for extracting and analyzing autofluorescence-spectral data are shown in Fig. 8. The autofluorescence values were compared by Tukey’s test (P < 0.05) and the Pearson’s correlation coefficient was measured to investigate the relationship among different excitation-emission combinations. A LDA was applied to rank the importance of bandwidths. Different models were developed for seed quality classification based on ANN (solver: Stochastic Gradient Descent; hidden layer sizes: two layers with 25 neurons in each; Activation function: Tanh; Learning rate: adaptive; maximum number of interactions: 5000), SVM-l, and LDA algorithms. Data from 680 seeds were used to train and test the models in cross-validation (K-fold = 5). An external data set with 120 seeds was used to perform external validation. The performance of the models was evaluated using five metrics—accuracy, Cohen’s Kappa coefficient, precision, recall, F1 (documentation can be found at https://scikit-learn.org/stable/modules/model_evaluation.html). The Pearson’s correlation coefficient was measured to investigate the relationship between the autofluorescence measurements and the traditional seed quality tests. All the statistical analyses were performed using R 4.0.0 software program52.
References
Caverzan, A. et al. How does seed vigor affect soybean yield componentes? Agron. J. 110, 1318–1327. https://doi.org/10.2134/agronj2017.11.0670 (2018).
Hao, Q. et al. Evaluation of seed vigor in soybean germplasms from different eco-regions. Oil Crop Sci. 5, 22–25. https://doi.org/10.1016/j.ocsci.2020.03.006 (2020).
Ebone, L. A. et al. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy 10, 545. https://doi.org/10.3390/agronomy10040545 (2020).
ElMasry, G., Mandour, N., Al-Rejaie, S., Belin, E. & Rousseau, D. Recent applications of multispectral imaging in seed phenotyping and quality monitoring—An overview. Sensors 19, 1–32. https://doi.org/10.3390/s19051090 (2019).
Leavesley, S. J. et al. Hyperspectral imaging fluorescence excitation scanning for colon cancer detection. J. Biomed. Opt. 21, 1–10. https://doi.org/10.1117/1.JBO.21.10.104003 (2016).
Denny, S. E. et al. Forensic application of fluorescence spectroscopy: An efficient technique to predict the presence of human saliva. J. Lumin. 203, 696–701. https://doi.org/10.1117/1.JBO.21.10.104003 (2018).
Noh, H. K. & Lu, R. Hyperspectral laser-induced fluorescence imaging for assessing apple fruit quality. Postharvest Biol. Technol. 43, 193–201. https://doi.org/10.1016/j.postharvbio.2006.09.006 (2007).
Delwiche, S. R., Stommel, J. R., Kim, M. S., Vinyard, B. T. & Esquerre, C. Hyperspectral fluorescence imaging for shelf life evaluation of fresh-cut Bell and Jalapeno Pepper. Sci. Hortic. 246, 749–758. https://doi.org/10.1016/j.scienta.2018.11.061 (2019).
Gorbe, E. & Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 138, 24–35. https://doi.org/10.1016/j.scienta.2012.02.002 (2012).
Bülbül, G., Hayat, A., Mustafa, F. & Andreescu, S. DNA assay based on nanoceria as fluorescence quenchers (NanoCeracQ DNA assay). Sci. Rep. 8, 1–9. https://doi.org/10.1038/s41598-018-20659-9 (2018).
Berg, N. S., Buckle, T., Kleinjan, G. H., Poel, H. G. & Leeuwen, F. W. B. Multispectral fluorescence imaging during robot-assisted laparoscopic sentinel node biopsy: A first step towards a fluorescence-based anatomic roadmap. Eur. Urol. 72, 110–117. https://doi.org/10.1016/j.eururo.2016.06.012 (2016).
Wang, Z., Amirkhani, M., Avelar, S. A. G., Yang, D. & Taylor, A. G. Systemic uptake of fluorescent tracers by soybean (Glycine max (L.) Merr.) seed and seedlings. Agriculture 10, 1–13. https://doi.org/10.3390/agriculture10060248 (2020).
Goggin, D. E. & Steadman, K. J. Blue and green are frequently seen: Responses of seeds to short- and mid-wavelength light. Seed Sci. Res. 22, 27–35. https://doi.org/10.1017/S0960258511000444 (2012).
Donaldson, L. Autofluorescence in plants. Molecules 25, 1–20. https://doi.org/10.3390/molecules25102393 (2020).
Zheng, Q. et al. Frequency-upconverted stimulated emission by simultaneous five-photon absorption. Nat. Photonics 7, 234–239. https://doi.org/10.1038/nphoton.2012.344 (2013).
Medeiros, A. D. et al. Interactive machine learning for soybean seed and seedling quality classification. Sci. Rep. 10, 1–10. https://doi.org/10.1038/s41598-020-68273-y (2020).
Manthou, E. et al. Application of spectroscopic and multispectral imaging technologies on the assessment of ready-to-eat pineapple quality: A performance evaluation study of machine learning models generated from two commercial data analytics tools. Comput. Electron. Agric. 175, 1–10. https://doi.org/10.1016/j.compag.2020.105529 (2020).
Younas, S. et al. Multispectral imaging for predicting the water status in mushroom during hot-air dehydration. J. Food Sci. 1, 1–7. https://doi.org/10.1111/1750-3841.15081 (2020).
França-Silva, F. et al. Detection of Drechslera avenae (Eidam) Sharif [Helminthosporium avenae (Eidam)] in black oat seeds (Avena strigosa Schreb) using multispectral imaging. Sensors 20, 1–10. https://doi.org/10.3390/s20123343 (2020).
Lawson, C. E. et al. Machine learning for metabolic engineering: A review. Metab. Eng. 63, 34–60. https://doi.org/10.1016/j.ymben.2020.10.005 (2021).
Ventura, L. et al. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 60, 196–206. https://doi.org/10.1016/j.plaphy.2012.07.031 (2012).
Xin, X. et al. Reduced mitochondrial and ascorbate–glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 171, 140–147. https://doi.org/10.1016/j.jplph.2013.09.016 (2014).
Simić, A., Sredojević, S., Todorovic, M., Dukanović, L. & Radenović, C. Studies on the relationship between content of total phenolics in exudates and germination ability of maize seed during accelerated aging. Seed Sci. Technol. 32, 213–218. https://doi.org/10.15258/sst.2004.32.1.22 (2004).
ElMasry, G. et al. Utilization of computer vision and multispectral imaging techniques for classification of cowpea (Vigna unguiculata) seeds. Plant Methods 15, 1–16. https://doi.org/10.1186/s13007-019-0411-2 (2019).
FAO. Seeds toolkit—Module 6. Seed Storage (2018). http://www.fao.org/3/ca1495en/CA1495EN.pdf. Accessed 25 July 2021.
Soares, C., Carvalho, M. E. A., Azevedo, R. A. & Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 161, 4–25. https://doi.org/10.1016/j.envexpbot.2018.12.009 (2019).
Priestley, D. A. & Leopold, A. A. Absence of lipid oxidation during accelerated aging of soybean seeds. Plant Physiol. 63, 726–729. https://doi.org/10.1104/pp.63.4.726 (1979).
Cui, M. et al. Dynamic changes of phenolic compounds during artificial aging of soybean seeds identified by high-performance liquid chromatography coupled with transcript analysis. Anal. Bioanal. Chem. 411, 3091–3101. https://doi.org/10.1007/s00216-019-01767-5 (2019).
Buschmann, C., Langsdorf, G. & Lichtenthaler, H. K. Imaging of the blue, green, and red fluorescence emission of plants: An overview. Photosynthetica 38, 483–491. https://doi.org/10.1023/A:1012440903014 (2000).
Galletti, P. A. et al. Integrating optical imaging tools for rapid and non-invasive characterization of seed quality: tomato (Solanum lycopersicum L.) and carrot (Daucus carota L.) as study cases. Front. Plant Sci. 11, 577851. https://doi.org/10.3389/fpls.2020.577851 (2020).
Donaldson, L. & Williams, N. Imaging and spectroscopy of natural fluorophores in pine needles. Plants 7, 10. https://doi.org/10.3390/plants7010010 (2018).
Li, Y. et al. Detection of viability of soybean seed based on fluorescence hyperspectra and CARS-SVM-AdaBoost model. J. Food Process. Preserv. 43, 1–9. https://doi.org/10.1111/jfpp.14238 (2019).
Gierz, L., Przybyl, K., Koszela, K., Duda, A. & Ostrowicz, W. The use of image analysis to detect seed contamination—A case study of triticale. Sensors 21, 151. https://doi.org/10.3390/s21010151 (2020).
Larios, G. S. et al. Laser-induced breakdown spectroscopy as a powerful tool for distinguishing high- and low-vigor soybean seed lots. Food Anal. Methods 13, 1691–1698. https://doi.org/10.1007/s12161-020-01790-8 (2020).
Rego, C. H. Q. et al. Using multispectral imaging for detecting seed-borne fungi in cowpea. Agriculture 10, 361. https://doi.org/10.3390/agriculture10080361 (2020).
Jalink, H., van der Schoor, R., Birnbaum, Y. E. & Bino, R. J. Seed chlorophyll content as an indicator for seed maturity and seed quality. Acta Hort. 504, 219–227. https://doi.org/10.17660/ActaHortic.1999.504.23 (1999).
Grulichová, M., Mendel, P., Trosssjan, V. & Vyhnanek, T. Determination of chlorophyll and carotenoids present in the seeds of the plant family Apiaceae and the impact on seed quality. Acta Univ. Agric. Silvic. Mendel. Brun. 66, 859–869. https://doi.org/10.11118/actaun201866040859 (2018).
Agathokleous, E., Feng, Z. & Penuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 726, 138637. https://doi.org/10.1016/j.scitotenv.2020.138637 (2020).
Men, S., Yan, L., Liu, J., Qian, H. & Luo, Q. A classification method for seed viability assessment with infrared thermography. Sensors 17, 845. https://doi.org/10.3390/s17040845 (2017).
Kusumaningrum, D. et al. Non-destructive technique for determining the viability of soybean (Glycine max) seeds using FT-NIR spectroscopy. J. Sci. Food Agric. 98, 1734–1742. https://doi.org/10.1002/jsfa.8646 (2018).
ElMasry, G. et al. Utilization of computer vision and multispectral imaging techniques for classification of cowpea (Vigna unguiculata) seeds. Plant Methods 15, 24. https://doi.org/10.1186/s13007-019-0411-2 (2019).
Rajjou, L. et al. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 148, 620–641. https://doi.org/10.1104/pp.108.123141 (2008).
Groot, S. P. C., Surki, A. A., de Vos, R. C. H. & Kodde, J. Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions. Ann. Bot. 100, 1149–1159. https://doi.org/10.1093/aob/mcs198 (2012).
Reis, C. O. et al. Action of N-Succinyl and N, O-Dicarboxymethyl chitosan derivatives on chlorophyll photosynthesis and fluorescence in drought-sensitive maize. J. Plant Growth Regul. 38, 619–630. https://doi.org/10.1007/s00344-018-9877-9 (2019).
Lichtenthaler, H. K. & Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 1, 1–6. https://doi.org/10.1002/0471142913.faf0402s01 (2001).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. https://doi.org/10.1016/0003-9861(68)90654-1 (1968).
Alexieva, V., Serviev, I., Mapelli, S. & Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24, 1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x (2001).
Fukushima, R. & Kerley, M. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin method. J. Agric. Food Chem. 59, 3505–3509. https://doi.org/10.1021/jf104826n (2011).
Swain, T. & Hillis, W. E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 10, 63–68. https://doi.org/10.1002/jsfa.2740100110 (1959).
Kautsky, H. & Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 19, 964. https://doi.org/10.1007/BF01516164 (1931).
Gitelson, A. A., Gritz, Y. & Merzlyak, M. N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 160, 271–282. https://doi.org/10.1078/0176-1617-00887 (2003).
R Core Team. R development core team. R Lang. Environ. Stat. Comput. 55, 275–286 (2019).
Acknowledgements
We thank the Sao Paulo Research Foundation—FAPESP (Grant Numbers# 2017/15220-7; 2018/03793-5; 2018/03802-4; 2018/01774-3) for financial support.
Author information
Authors and Affiliations
Contributions
C.B.S. generated research ideas; N.M.O., M.L.N. and A.R.R. collected data; C.B.S. wrote the first draft; A.D.M. analyzed the data; C.B.S, M.E.A.C. and A.D.M. edited the manuscript; C.B.S. supervised the project and acquired funding; C.B.S., N.M.O., M.L.N. and A.R.R. revised the manuscript for technical and scientific accuracy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barboza da Silva, C., Oliveira, N.M., de Carvalho, M.E.A. et al. Autofluorescence-spectral imaging as an innovative method for rapid, non-destructive and reliable assessing of soybean seed quality. Sci Rep 11, 17834 (2021). https://doi.org/10.1038/s41598-021-97223-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97223-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.