Abstract
Nonalcoholic fatty liver disease (NAFLD) is related to subclinical atherosclerosis. However, whether the severity of the disease (or which histopathological component) is associated with subclinical atherosclerosis remains controversial. This study aimed to investigate the association between the histopathological severity of NAFLD and carotid intima-media thickness (CIMT) in Japanese patients with liver biopsy-proven NAFLD. Maximum-CIMT (max-CIMT) was measured as an index of carotid atherosclerosis in 195 biopsy-proven NAFLD patients. A significant association was observed between the severity of fibrosis (but not steatosis, inflammation, and ballooning) and max-CIMT. Older age, male gender, hypertension, and advanced fibrosis were independently linked to max-CIMT ≥ 1.2 mm. The prevalence of max-CIMT ≥ 1.2 mm was significantly higher in the advanced fibrosis group than in the non-advanced fibrosis group (75.4% versus 44.0%; p < 0.01). Non-invasive liver fibrosis markers and scoring systems, including fibrosis-4 index, NAFLD fibrosis score, hyaluronic acid, and Wisteria floribunda agglutinin positive Mac-2-binding protein, demonstrated that the diagnostic performance for max-CIMT ≥ 1.2 mm was similar to that of biopsy-based fibrosis staging. In conclusion, advanced fibrosis is significantly and independently associated with high-risk CIMT. Non-invasive fibrosis markers and scoring systems could help estimate the risk of atherosclerosis progression in patients with NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. The global prevalence of NAFLD has been increasing and has reached approximately 25% of the adult population1. Among patients with NAFLD, liver fibrosis often occurs and progresses from steatohepatitis to liver cirrhosis, and other complications, including liver failure and hepatocellular carcinoma, also occurs2,3. Furthermore, as NAFLD is considered to be the hepatic phenotype of metabolic syndrome with multi-organ involvement, patients with NAFLD frequently have impaired cardiovascular, endocrine, renal, and bone systems. It has been recently suggested that NAFLD is a key driver of multi-organ syndrome, including cardiovascular disease (CVD), atherosclerosis, chronic kidney disease, type 2 diabetes mellitus, and osteoporosis4,5. From this viewpoint, hepatic involvement is just one aspect of NAFLD, and attention should be paid to other organ disorders in addition to the liver. Indeed, the most common causes of mortality among patients with NAFLD are CVD, followed by extrahepatic malignancies, and then liver-related complications6,7,8.
It is worth noting that NAFLD increases the risk of subclinical atherosclerosis and coronary artery calcification, independent of conventional risk factors, such as older age, type 2 diabetes mellitus, hypertension, and dyslipidemia9,10,11. Moreover, advanced liver fibrosis has been reported to have a substantial effect on the incidence and mortality of CVD in patients with NAFLD8,12. Some studies demonstrated that liver fibrosis assessed by non-invasive fibrosis scoring systems such as fibrosis-4 (FIB-4) index13 and NAFLD fibrosis score (NFS)13,14,15, could be associated with carotid intima-media thickness (CIMT), which is a risk index of CVD16,17. Hyaluronic acid18 and Wisteria floribunda agglutinin positive Mac-2-binding protein (WFA+-M2BP)19,20,21, which have been reported as useful liver fibrosis markers in NAFLD, have recently attracted attention as indicators of early atherosclerosis22,23,24, albeit they remain to be investigated in association with carotid atherosclerosis in NAFLD. Meanwhile, other studies have reported that advanced fibrosis evaluated via transient elastography was not associated with CIMT25. Therefore, the association between the severity of liver fibrosis and carotid atherosclerosis remains controversial. We have recently reported that advanced fibrosis is associated with brachial-ankle pulse wave velocity independent of conventional risk factors in 153 Japanese patients with liver biopsy-proven NAFLD26. However, there have been only two studies that verified the association between the histopathological severity of liver fibrosis and CIMT in patients with liver biopsy-proven NAFLD27,28. Moreover, CIMT is different in different races29,30, and both of these studies were conducted in Italy27,28. This study aimed to investigate the association between the histopathological severity of NAFLD and CIMT in Japanese patients with liver biopsy-proven NAFLD. We also asked whether non-invasive fibrosis markers and scoring systems could be useful for predicting the risk value of CIMT.
Results
Patients
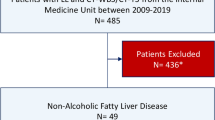
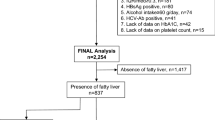
Of the 357 patients with suspected NAFLD, 195 biopsy-proven NAFLD patients underwent carotid artery ultrasonography within 3 months of liver biopsy and included in the study (Supplementary Fig. 1). Baseline characteristics of the 195 patients with liver biopsy-proven NAFLD are presented in Table 1. There were 90 males and 105 females, with a median age of 59 years (range, 18–84 years). The prevalence of hypertension was 51.8% (101/195), and that of type 2 diabetes mellitus was 40.0% (78/195). Of the 78 NAFLD patients with type 2 diabetes mellitus, 64 (82.1%) had been receiving diabetes medications. On the pathological examination of liver biopsy specimens, the fibrosis stage was F0 for 29 (14.9%) patients, F1 for 62 (31.8%), F2 for 43 (22.1%), F3 for 49 (25.1%), and F4 for 12 (6.2%). Advanced fibrosis (F3–4) was present in 61 (31.3%) patients. The median maximum-CIMT (max-CIMT) value was 1.2 mm (range, 0.4–4.4 mm). The prevalence of max-CIMT ≥ 1.2 mm was 53.8% (105/195).
Correlation between max-CIMT and histopathological severity of NAFLD
As shown in Fig. 1, no correlation with max-CIMT was observed for steatosis (median max-CIMT for grade 1 = 1.3 mm, grade 2 = 1.2 mm, and grade 3 = 0.9 mm; p = 0.09), inflammation (median max-CIMT for grade 0–1 = 1.2 mm and grade 2–3 = 1.2 mm; p = 0.94), and ballooning (median max-CIMT for grade 0 = 1.4 mm, grade 1 = 1.2 mm, and grade 2 = 1.2 mm; p = 0.61). In contrast, max-CIMT significantly differed among the fibrosis stages (p < 0.01), and the median values and interquartile ranges increased as the severity of liver fibrosis increased (median max-CIMT for F0–1 = 1.0 mm, F2 = 1.2 mm, F3 = 1.3 mm, and F4 = 2.1 mm). Patients with F3 had significantly higher max-CIMT than those with F0–1 (p < 0.05). Of note, patients with F4 had significantly higher max-CIMT than those with F0–1 (p < 0.01), F2 (p < 0.01), and F3 (p < 0.05). Based on their fibrosis stage, the patients were assigned to either non-advanced (F0–2) or advanced (F3–4) fibrosis groups. The max-CIMT was found to be significantly higher in the advanced fibrosis group than in the non-advanced fibrosis group (p < 0.01) (Fig. 2A). Regarding the NAFLD activity score (NAS), no significant difference was noted in max-CIMT between the two groups (NAS ≤ 4 versus ≥ 5; p = 0.95) (Fig. 2B).
Factors associated with max-CIMT ≥ 1.2 mm
In the univariate analysis, older age (≥ 65 years), low eGFR (< 60 mL/min/1.73 m2), low total cholesterol (< 220 mg/dL), low low-density lipoprotein (LDL) cholesterol (< 140 mg/dL), type 2 diabetes mellitus, hypertension, and advanced fibrosis were significantly associated with max-CIMT ≥ 1.2 mm (Table 2). Multivariate analysis demonstrated that the following four variables were independently associated with max-CIMT ≥ 1.2 mm (Table 2): older age (odds ratio [OR] 5.46; 95% confidence interval [CI] 2.66–11.19; p < 0.01), male gender (OR 2.36; 95% CI 1.18–4.73; p < 0.05), hypertension (OR 2.24; 95% CI 1.13–4.44; p < 0.05), and advanced fibrosis (OR 2.24; 95% CI 1.03–4.86; p < 0.05).
Prevalence of max-CIMT ≥ 1.2 mm in relation to liver fibrosis and conventional risk factors
The prevalence of max-CIMT ≥ 1.2 mm in the advanced fibrosis group (75.4%; 46/61) was significantly higher than that in the non-advanced fibrosis group (44.0%; 59/134) (p < 0.01; Fig. 3A). In each group, the prevalence of max-CIMT ≥ 1.2 mm was analyzed based on the number of the aforementioned risk factors (i.e., older age, male gender, and hypertension). In both groups, max-CIMT significantly increased stepwise as the number of risk factors increased (Fig. 3B,C). Intriguingly, in the advanced fibrosis group, even if the number of risk factors was low (0 or 1), the prevalence of max-CIMT ≥ 1.2 mm was 50% or greater. Meanwhile, in the non-advanced fibrosis group, the number of risk factors more strongly affected the prevalence of max-CIMT ≥ 1.2 mm.
(A) The prevalence of max-CIMT ≥ 1.2 mm according to fibrosis stage. The prevalence of max-CIMT ≥ 1.2 mm according to the number of risk factors (older age, male gender, and hypertension) in the non-advanced group (B) and the advanced group (C). CIMT, carotid intima-media thickness. The non-advance group had fibrosis stage of F0–2, while the advanced fibrosis had fibrosis stage of F3–4.
Diagnostic performance of biopsy-based liver fibrosis staging and non-invasive tests for max-CIMT ≥ 1.2 mm
We evaluated the diagnostic performance of biopsy-based liver fibrosis staging and non-invasive tests for max-CIMT ≥ 1.2 mm: the area under the receiver operating characteristics (ROC) curve (AUC) and cutoff values were 0.672 and ≥ F3 for biopsy-based fibrosis staging, 0.668 and 2.06 for FIB-4 index, 0.658 and − 0.674 for NFS, 0.630 and 57.1 ng/ml for hyaluronic acid, and 0.620 and 1.23 C.O.I. for WFA+-M2BP, respectively (Supplementary Fig. 2). No significant differences were observed in the AUC between biopsy-based fibrosis staging and non-invasive tests (p = 0.90 for FIB-4 index, 0.72 for NFS, 0.35 for hyaluronic acid, and 0.19 for WFA+-M2BP). Next, we divided the patients into two groups using the cutoff value corresponding to each fibrosis marker or scoring system and compared the prevalence of max-CIMT ≥ 1.2 mm between the two groups. The prevalence of max-CIMT ≥ 1.2 mm in the high fibrosis marker/scoring system groups was significantly higher than that in the low fibrosis marker/scoring system groups (Supplementary Fig. 3). Finally, we investigated the prevalence of max-CIMT ≥ 1.2 mm according to the number of the aforementioned risk factors in the high and low groups for each fibrosis marker or scoring system. The prevalence of max-CIMT ≥ 1.2 mm significantly increased in a stepwise manner as the number of risk factors increased in both groups for all fibrosis markers and scoring systems (Supplementary Fig. 4). Similar to the results in the biopsy-based fibrosis staging, the prevalence of max-CIMT ≥ 1.2 mm was relatively low in patients with a low number of risk factors (approximately 10% for 0 and around 30% for 1) in the low fibrosis marker/scoring system groups, whereas it was relatively high even in patients with a low number of risk factors (approximately 30%–40% for 0 and 50%–60% for 1) in the high fibrosis marker/scoring system groups. When patients had two or more risk factors, the prevalence of max-CIMT ≥ 1.2 mm was approximately 70%–80% in both high and low fibrosis marker/scoring system groups.
Discussion
This study demonstrated that advanced liver fibrosis, in addition to conventional risk factors for cardiovascular events (older age, male gender, and hypertension), was significantly and independently associated with a high-risk level of CIMT (surrogate measure of atherosclerosis) in 195 Japanese patients with NAFLD. The meta-analyses and systematic reviews have indicated that there is a positive and significant association between NAFLD and CIMT, irrespective of conventional risk factors9,10,11. Zhang et al. reconfirmed that NAFLD is significantly and independently associated with CIMT in patients with type 1 (but not type 2) diabetes31. In the WELCOME study cohort, decreased liver fat (as assessed by magnetic resonance spectroscopy) and reduced necroinflammation (serum cytokeratin-18 level) were found to be associated with suppressed CIMT progression32. However, in most of these previous studies, NAFLD was diagnosed by ultrasonography, and not by liver biopsy, which is the gold standard method for diagnosing and staging NAFLD. These data confirmed the association between NAFLD and CIMT, but the role of histological severity of NAFLD and individual histological features requires further studies. Some previous small-scale or pilot studies have reported that 23–57 patients with biopsy-proven NAFLD had significantly higher CIMT than 21–30 control subjects33,34,35. However, due to the limited reports available on the impact of histopathology of NAFLD on CIMT, the consensus on whether the severity of the disease (or a specific histopathological component) could be associated with the CIMT level remains controversial. For instance, in a NAFLD rat model, the hepatic inflammation scores were correlated with CIMT by high-fat diet36. To the best of our knowledge, only two studies from Italy have so far reported on how the histopathological severity of liver fibrosis in patients with biopsy-proven NAFLD could be related to carotid atherosclerosis27,28. Targher et al. reported that liver steatosis, inflammation, and fibrosis were significantly correlated with CIMT in 85 patients27. Petta et al. reported that liver inflammation and fibrosis were associated with carotid artery plaque formation in 162 patients, though not significant in multivariate analysis28. However, CIMT values or progression of atherosclerosis may differ according to the racial/ethnic cohorts and lifestyle habits. The CIMT values in Japanese are reportedly lower than those in Caucasian American (even after adjusting for metabolic risk factors)29 and Korean populations (although both Japanese and Korean are Asian)30. This is the first report to clarify a significant association between the histopathological severity of fibrosis (but not steatosis, inflammation, and ballooning) and the high-risk CIMT level in Japanese patients with biopsy-proven NAFLD.
It is unclear whether the mechanism for liver fibrogenesis affects the progression of atherosclerosis in patients with NAFLD. The association between liver fibrosis and atherosclerosis may be partially explained by a high prevalence of conventional risk factors, such as type 2 diabetes mellitus, dyslipidemia, and hypertension, in NAFLD patients with advanced liver fibrosis37,38. In this study, advanced liver fibrosis was significantly associated with higher CIMT values, independent of these risk factors. This suggested the involvement of another unidentified mechanism. Various theories have been proposed for the common mechanism underlying the progression of liver fibrosis and atherosclerosis. Oxidative stress and subclinical inflammation, which play important roles in the progression of NAFLD pathology, induce endothelial dysfunction, which may lead to systemic vascular sclerosis39,40. The severity of NAFLD increases the PAI-1 levels, thereby increasing the risk of atherothrombosis41. TGF-β, a pro-fibrogenic cytokine, plays a key role in the progression of liver fibrosis and induces arterial intima thickening42,43. Various factors are likely involved in complex pathways/networks that could develop and progress both NAFLD and atherosclerosis. Further study is needed to uncover the common or interrelated mechanisms underlying the pathology of NAFLD and atherosclerosis.
In this study, we demonstrated that NAFLD patients with advanced fibrosis had significantly higher max-CIMT values and prevalence of max-CIMT ≥ 1.2 mm compared to those with non-advanced fibrosis. More importantly, advanced fibrosis was a significant and independent factor associated with max-CIMT ≥ 1.2 mm, irrespective of conventional risk factors. The prevalence of max-CIMT ≥ 1.2 mm increased in a stepwise manner with the number of risk factors in both non-advanced and advanced fibrosis groups. Intriguingly, the prevalence of max-CIMT ≥ 1.2 mm was relatively low in patients with non-advanced fibrosis and a low number of risk factors. Thus, attention should be paid to the onset of cardiovascular events, especially when patients with non-advanced fibrosis have two or more conventional risk factors. Meanwhile, patients with advanced fibrosis should be examined for atherosclerosis, irrespective of the absence or number of conventional risk factors.
Although liver biopsy is the gold standard for diagnosis and staging of fibrosis in NAFLD, it offers several limitations, such as the sampling error, cost/effort, and the risk of complications. The FIB-4 index and NFS are highly useful as non-invasive alternatives to liver biopsy44,45,46. In particular, their practical usefulness in patients with NAFLD chiefly includes the ability to exclude advanced fibrosis and avoid liver biopsy47. Recently, hyaluronic acid and WFA+-M2BP have attracted attention as indicators of early arteriosclerosis as well as markers of liver fibrosis22,23,24. We demonstrated that the diagnostic performance of non-invasive fibrosis markers and scoring systems for max-CIMT ≥ 1.2 mm was similar to that of fibrosis staging via invasive biopsy. Among these non-invasive tests, the FIB-4 index and NFS exhibited relatively high diagnostic performance, presumably because these formulas include age factors that predispose individuals to arteriosclerosis. In fact, FIB4-index and NFS have been reported to be important predictors of cardiovascular mortality, which is the main cause of death in patients with NAFLD48,49. Specifically, patients with advanced liver fibrosis should be closely monitored for the development of arteriosclerosis and the risk of cardiovascular events, as well as liver function itself and liver-related complications.
In this study, type 2 diabetes mellitus, a critical risk factor for cardiovascular events50,51, was not identified as an independent factor associated with CIMT in multivariate analysis. Considering that treatment for type 2 diabetes mellitus improves CIMT52,53,54,55, possible explanations for the lack of independent association include variations in the duration and severity of type 2 diabetes mellitus and diabetes treatment, such as diet, exercise, and drug therapy. A part of the diabetic patients in this study cohort was treated by diabetologists shortly after the diagnosis of diabetes. Most of them had been receiving diabetes medication and were experiencing good control in terms of their hemoglobin A1c levels. Therefore, the results of this study should be verified in a larger NAFLD cohort or the general population.
This study has several limitations. First, this was a retrospective and hospital-based study, which involves a potential for patient selection bias. Second, the number of patients was relatively small, especially those with advanced fibrosis. A large cohort including many patients with advanced fibrosis is required to verify the association between severe atherosclerosis and more advanced fibrosis stages. Third, as aforementioned, the community-based general population (serving as a control) was not taken into consideration, suggesting that the influence of risk factors, including type 2 diabetes mellitus, on CIMT was not accurately evaluated in patients with NAFLD.
In conclusion, this study demonstrated that advanced fibrosis, as well as conventional risk factors (older age, male gender, and hypertension), is significantly and independently associated with the high-risk CIMT level for cardiovascular events in Japanese patients with biopsy-proven NAFLD. Non-invasive fibrosis markers and scoring systems could be helpful when estimating the risk of atherosclerosis progression in patients with NAFLD. Specifically, patients with NAFLD accompanied by advanced live fibrosis should be examined and monitored for atherosclerosis and the risk of cardiovascular events.
Methods
Patients
Among the patients who presented to Nippon Medical School Hospital, Nippon Medical School Chiba Hokusoh Hospital, and Shinmatsudo Central General Hospital between July 2011 and December 2020, 357 patients with suspected NAFLD underwent liver biopsy. Among the 357 patients, 195 who met the inclusion criteria, did not fill the exclusion criteria, and underwent carotid artery ultrasonography within 3 months of liver biopsy were enrolled in this study (Supplementary Fig. 1). The inclusion criteria were as follows: (1) age ≥ 18 years; (2) undergoing liver biopsy with consent; and (3) the diagnosis of NAFLD based on histopathological evaluation according to the guidelines of the European Association for the Study of the Liver56,57,58. The exclusion criteria were as follows: (1) daily alcohol consumption ≥ 30 g for men and ≥ 20 g for women; (2) other chronic liver diseases, such as viral hepatitis B or C, autoimmune hepatitis, Wilson disease, or hemochromatosis; and (3) secondary causes of steatosis, including use of drugs (e.g., amiodarone and tamoxifen), total parenteral nutrition, and inborn errors related to metabolism. A careful interview and clinical, laboratory, and imaging examinations were conducted at the time of liver biopsy in all the patients.
The study protocol complied with the ethical guidelines established following the 2013 Declaration of Helsinki. The Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital approved this study (approval number, 603). All patients provided written informed consent before their participation.
Clinical evaluation and laboratory data
Clinical evaluations and laboratory data were collected at the time of liver biopsy. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Blood pressure was measured in a seated position, at least twice and several minutes apart, and the mean value was calculated and utilized for analysis. Hypertension was diagnosed as systolic blood pressure ≥ 135 mmHg or diastolic blood pressure ≥ 85 mmHg, or when the patients were being treated with an antihypertensive drug59. Dyslipidemia was diagnosed when the total cholesterol level was ≥ 220 mg/dL, high-density lipoprotein (HDL) cholesterol level was < 40 mg/dL, triglycerides level was ≥ 150 mg/dL or LDL cholesterol level was ≥ 140 mg/dL, or when the patients were being treated with an antihyperlipidemic drug60. Type 2 diabetes mellitus was diagnosed with reference to the 2006 World Health Organization criteria or when the patients reported having been receiving treatment with an oral hypoglycemic agent and/or insulin.
Laboratory evaluation included complete blood count, routine liver (aspartate aminotransferase, alanine aminotransferase [ALT], total bilirubin, albumin, alkaline phosphatase, and gamma-glutamyl transpeptidase) and renal (urea nitrogen, creatinine, and estimated glomerular filtration rate [eGFR]) biochemistry tests, fasting lipid profiles (total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol), and diabetes-related tests (fasting plasma glucose, hemoglobin A1c, and immunoreactive insulin). As an index of insulin resistance, the homeostasis model assessment-insulin resistance (HOMA-IR) was calculated as follows: fasting immunoreactive insulin (μU/mL) × fasting plasma glucose (mg/dL)/40561. Hyaluronic acid18 and WFA+-M2BP19,20,21, both of which have been reported as liver fibrosis markers in NAFLD, were also determined. In addition, the FIB-4 index and NFS were calculated to estimate the degree of liver fibrosis, as reported elsewhere44,45,46.
Carotid ultrasound
Carotid artery ultrasonography was conducted using a B-mode ultrasound system with a 7.5-MHz linear transducer within 3 months of liver biopsy. According to the guidelines of the Japan Society of Ultrasonics in Medicine62, all scans were performed by skilled laboratory technicians who were blinded to the patient information. The examination included the near and far walls of the common carotid arteries (CCAs), carotid bulbs (CBs), and internal carotid arteries (ICAs). The thickest point, including plaque lesions in the entire scanned intra-media thickness of the CCAs, CBs, and ICAs, was defined as max-CIMT. With reference to a report on the general Japanese papulation, patients with max-CIMT ≥ 1.2 mm were considered to be at a high risk for cardiovascular events63.
Histopathological evaluation
Of the patients with suspected NAFLD based on ultrasound and increased ALT levels, some consented to liver biopsy, which is the gold standard for diagnosis and staging of fibrosis in patients with NAFLD. Histopathological evaluation was conducted by at least two experienced pathologists from each facility who were blinded to the patient’s data. In case of a disagreement related to the diagnosis, the pathologists discussed until they reached a consensus. NAFLD was diagnosed when the lipid droplet deposition was observed in ≥ 5% of hepatocytes. Steatosis, lobular inflammation, ballooning, and liver fibrosis were semi-quantitatively evaluated according to the NASH Clinical Research Network scoring system64. Steatosis was graded as 0–3 based on the percent of hepatocytes with steatosis on biopsy specimens (0: < 5%, 1: 5–33%, 2: 33–66%, 3: > 66%). Lobular inflammation was graded as 0–3 based on the number of inflammatory foci per 200 × field (0: no foci, 1: < 2 foci, 2: 2–4 foci, 3: > 4 foci). Ballooning was graded as 0–2 based on the number of hepatocytes with this change (0: none, 1: few cells, 2: many cells or prominent ballooning). The NAS was considered as the unweighted sum of steatosis, lobular inflammation, and ballooning scores. NAS of ≥ 5 was strongly correlated with the diagnosis of NASH64. The stage of fibrosis was classified as follows: F0 = no fibrosis, F1 = perisinusoidal or periportal fibrosis, F2 = perisinusoidal and portal/periportal fibrosis, F3 = bridging fibrosis, and F4 = cirrhosis. F3–4 was provisionally designated as advanced fibrosis.
Statistical analysis
Continuous variables are presented as medians and ranges. Categorical variables were presented as numbers and percentages. Continuous variables with skewed distributions were compared among or between the groups using the Kruskal–Wallis test or the Mann–Whitney U-test, respectively. The Steel–Dwass test was performed when the Kruskal–Wallis test indicated a significant difference among the groups. Categorical variables were compared using Fisher’s exact test. The variables associated with max-CIMT ≥ 1.2 mm on univariate analysis (probability threshold, p < 0.10) were included in multiple logistic regression. The Cochran–Armitage trend test was employed to evaluate increases in the prevalence of max-CIMT ≥ 1.2 mm in relation to increases in the number of risk factors, such as older age, male gender, and hypertension. An ROC curve was generated to determine the values of non-invasive markers and scoring systems that most rationally predicted max-CIMT ≥ 1.2 mm. The cutoff value for each non-invasive test was determined corresponding to the point of the ROC curve, which was closest to the upper-left corner of the plot (i.e. point [0, 1]). The DeLong’s test was applied to compare the AUC values of non-invasive fibrosis markers and scoring systems with those of biopsy-based fibrosis staging. All statistical analyses were performed using the IBM SPSS version 17.0 (IBM Japan, Tokyo, Japan). The level of statistical significance was set at p < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Farrell, G. C. & Larter, C. Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43, S99–S112 (2006).
Benhammou, J. N. et al. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci.Rep. 10, 9902. https://doi.org/10.1038/s41598-020-66507-7 (2020).
Li, A. A., Ahmed, A. & Kim, D. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gut Liver. 14, 168–178 (2020).
Rosato, V. et al. NAFLD and extra-hepatic comorbidities: Current evidence on a multi-organ metabolic syndrome. Int. J. Environ. Res. Public Health. 16, 3415 (2019).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397 (2015).
Hagström, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273 (2017).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Zhou, Y. Y. et al. Nonalcoholic fatty liver disease contributes to subclinical atherosclerosis: A systematic review and meta-analysis. Hepatol. Commun. 2, 376–392 (2018).
Ampuero, J., Gallego-Durán, R. & Romero-Gómez, M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: Meta-analysis. Rev. Esp. Enferm. Dig. 107, 10–16 (2015).
Oni, E. T. et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care?. Atherosclerosis 230, 258–267 (2013).
Henson, J. B. et al. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. 51, 728–736 (2020).
Sinn, D. H. et al. Persistent nonalcoholic fatty liver disease increases risk for carotid atherosclerosis. Gastroenterology 151, 481–488 (2016).
Chen, Y. et al. Advanced fibrosis associates with atherosclerosis in subjects with nonalcoholic fatty liver disease. Atherosclerosis 241, 145–150 (2015).
Gentili, A. et al. Non-alcoholic fatty liver disease fibrosis score and preclinical vascular damage in morbidly obese patients. Dig. Liver Dis. 48, 904–908 (2016).
Kitamura, A. et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke 35, 2788–2794 (2004).
Polak, J. F. et al. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 365, 213–221 (2011).
Suzuki, A. et al. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 25, 779–786 (2005).
Ogawa, Y. et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: Useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 33, 1795–1803 (2018).
Abe, M. et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 50, 776–784 (2015).
Atsukawa, M. et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein more reliably distinguishes liver fibrosis stages in non-alcoholic fatty liver disease than serum Mac-2 binding protein. Hepatol. Res. 48, 424–432 (2018).
Papanastasopoulou, C. et al. Cardiovascular risk and serum hyaluronic acid: A preliminary study in a healthy population of low/intermediate risk. J. Clin. Lab. Anal. 31, e22010. https://doi.org/10.1002/jcla.22010 (2017).
Sugiura, T. et al. Serum levels of Mac-2 binding protein increase with cardiovascular risk and reflect silent atherosclerosis. Atherosclerosis 251, 192–196 (2016).
Park, H., Jun, D. W., Park, H. K., Park, K. Y. & Hwang, H. S. New sequential algorithm using Mac-2 binding protein glycosylation isomer to detect advanced carotid artery atherosclerosis. Ann. Transl. Med. 9, 566 (2021).
Tan, E. C., Tai, M. S., Chan, W. K. & Mahadeva, S. Association between non-alcoholic fatty liver disease evaluated by transient elastography with extracranial carotid atherosclerosis in a multiethnic Asian community. JGH Open. 3, 117–125 (2018).
Arai, T. et al. Factors influencing subclinical atherosclerosis in patients with biopsy-proven nonalcoholic fatty liver disease. PLoS ONE 14, e0224184. https://doi.org/10.1371/journal.pone.0224184 (2019).
Targher, G. et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 29, 1325–1330 (2006).
Petta, S. et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with N. PLoS ONE 8, e74089. https://doi.org/10.1371/journal.pone.0074089 (2013).
Sekikawa, A. et al. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am. J. Epidemiol. 165, 617–624 (2007).
Choo, J. et al. Difference in carotid intima-media thickness between Korean and Japanese men. Ann. Epidemiol. 18, 310–315 (2008).
Zhang, L. et al. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness in type 1 diabetic patients. Sci. Rep. 6, 26805. https://doi.org/10.1038/srep26805 (2016).
Bhatia, L. et al. Improvement in non-alcoholic fatty liver disease severity is associated with a reduction in carotid intima-media thickness progression. Atherosclerosis 246, 13–20 (2016).
Colak, Y. et al. Relation of epicardial adipose tissue and carotid intima-media thickness in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 24, 613–618 (2012).
Colak, Y. et al. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine 43, 100–107 (2013).
Vlachopoulos, C. et al. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: A pilot study. Am. J. Hypertens. 23, 1183–1189 (2010).
Wu, J., Zhang, H., Zheng, H. & Jiang, Y. Hepatic inflammation scores correlate with common carotid intima-media thickness in rats with NAFLD induced by a high-fat diet. BMC Vet. Res. 10, 162. https://doi.org/10.1186/1746-6148-10-162 (2014).
Nakahara, T. et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J. Gastroenterol. 49, 1477–1484 (2014).
Kawaguchi, T. et al. Factors associated with advanced hepatic fibrosis in patients with various internal diseases: A multicenter community-based survey. Hepatol. Res. 48, 882–892 (2018).
Targher, G., Day, C. P. & Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 363, 1341–1350 (2010).
Villanova, N. et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 42, 473–480 (2005).
Verrijken, A. et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 59, 121–129 (2014).
Kanzaki, T. et al. In vivo effect of TGF- beta 1: Enhanced intimal thickening by administration of TGF-beta 1 in rabbit arteries injured with a balloon catheter. Arterioscler. Thromb. Vasc. Biol. 15, 1951–1957 (1995).
Fleenor, B. S., Marshall, K. D., Durrant, J. R., Lesniewski, L. A. & Seals, D. R. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol. 588, 3971–3982 (2010).
Vallet-Pichard, A. et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection: Comparison with liver biopsy and fibrotest. Hepatology 46, 32–36 (2007).
Sumida, Y. et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 12, 2. https://doi.org/10.1186/1471-230X-12-2 (2012).
Angulo, P. et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854 (2007).
Kaya, E. et al. Simple noninvasive scores are clinically useful to exclude, not predict, advanced fibrosis: A study in Turkish patients with biopsy-proven nonalcoholic fatty liver disease. Gut Liver. 14, 486–491 (2020).
Kim, D., Kim, W. R., Kim, H. J. & Therneau, T. M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 57, 1357–1365 (2013).
Tada, T. et al. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol. Commun. 1, 899–910 (2017).
Robins, S. J. et al. Insulin resistance and cardiovascular events with low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care 26, 1513–1517 (2003).
Kannel, W. B. & McGee, D. L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham Study. Diabetes Care 2, 120–126 (1979).
Geng, D. F., Jin, D. M., Wu, W., Fang, C. & Wang, J. F. Effect of alpha-glucosidase inhibitors on the progression of carotid intima-media thickness: A meta-analysis of randomized controlled trials. Atherosclerosis 218, 214–219 (2011).
Langenfeld, M. R. et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation 111, 2525–2531 (2005).
Mita, T. et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: The study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care 39, 139–148 (2016).
Yokoyama, H., Katakami, N. & Yamasaki, Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke 37, 2420–2427 (2006).
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Bugianesi, E., Rosso, C. & Cortez-Pinto, H. How to diagnose NAFLD in 2016. J. Hepatol. 65, 643–644 (2016).
Nascimbeni, F. et al. From NAFLD in clinical practice to answers from guidelines. J. Hepatol. 59, 859–871 (2013).
Shimamoto, K. et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens. Res. 37, 253–390 (2014).
Teramoto, T. et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan-2012 version. J. Atheroscler. Thromb. 20, 517–523 (2013).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Terminology and Diagnostic Criteria Committee Japan Society of Ultrasonics in Medicine. Standard method for ultrasound evaluation of carotid artery lesions. J. Med. Ultrason. 36, 219–226 (2009).
Kokubo, Y. et al. Impact of intima-media thickness progression in the common carotid arteries on the risk of incident cardiovascular disease in the Suita study. J Am. Heart Assoc. 7, e007720. https://doi.org/10.1161/JAHA.117.007720 (2018).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Acknowledgements
The authors wish to thank all medical doctors from all institutions who were involved in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.A.; Analysis and interpretation, T.A., M.A., and A.T.; Acquisition of data, K.K., H.A., H.O., T.K., Y.Y., T.T., T.O., K.H., A.N.-I., N.I., C.K., K.K., N.E., M.N., K.I., and I.F.; Methodology, T.A. and M.A.; Drafting the manuscript, T.A ., M.A. and A.T.; Supervision, H.S. and K.I. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arai, T., Atsukawa, M., Tsubota, A. et al. Liver fibrosis is associated with carotid atherosclerosis in patients with liver biopsy-proven nonalcoholic fatty liver disease. Sci Rep 11, 15938 (2021). https://doi.org/10.1038/s41598-021-95581-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95581-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.