Abstract
To explore the effects of nitrogen deficiency in burley tobacco, two varieties were cultivated and subjected to conditions of sufficient and deficient nitrogen. The natural characteristics of varieties TN90 and TN86 during tobacco cultivation were similar for nitrogen metabolism. Both carbon and nitrogen metabolism were significantly affected by reducing amounts of applied nitrogen. Under nitrogen-deficient conditions, average leaf biomass, root weight, photosynthetic rate (Pn), pigment levels, total nitrogen, and nitrate content of TN86 and TN90 were significantly decreased by 52.88%, 69.19%, 22.65%, 46.80%, 37.42%, and 79.15%, respectively (p < 0.01). Nicotine and soluble reducing sugar contents were significantly decreased by 96.67% and 95.12%, respectively, in TN86 roots (p < 0.01), which was consistent with the reductions in root surf area, average diameter, and root volume. Nitrogen deficiency induced 6318 differentially expressed genes in both TN90 and TN86, which were highly expressed. In total, 428 upregulated genes were analysed and found to be mainly enriched in the MAPK signalling pathway, sesquiterpenoid and triterpenoid biosynthesis, and arginine and proline metabolism. Meanwhile, 213 downregulated genes were analysed and found to be mainly enriched in photosynthesis, nitrogen metabolism, and amino acid biosynthesis. Reduced pigment content and Pn may result in low carbohydrate formation and decreased leaf biomass in burley tobacco under nitrogen-deficient conditions.
Similar content being viewed by others
Introduction
Burley tobacco is a well-known yellow-green leaf tobacco with low chlorophyll content and carbohydrate that accumulates in its leaves1,2. Mutations at the Yellow Burley 1 (YB1) and Yellow Burley 2 (YB2) loci have been reported, resulting in a double homozygous recessive genotype and chlorophyll deficiency. The changes may also result in low efficiency of nitrogen use and the accumulation of high levels of nitrate, which can increase tobacco-specific nitrosamines (TSNAs) in the tobacco3,4.
Nitrogen is a micromineral in plants and is essential for plant development and yield5. Nitrogen is absorbed in the roots and transported to the stem and leaves where it used as a carbon and energy source in the biosynthesis of amino acids and proteins in the cells6. To obtain high crop yield and increase revenue, farmers use excessive nitrogen fertiliser in tobacco cultivation7. Nitrogen fertiliser is applied approximately 3–5 times higher on burley tobacco than on flue-cured tobacco, but the biomass between them is similar8,9. Overuse of nitrogen fertiliser in tobacco cultivation may lead to environmental problems as a result of leaching and can increase nitrate accumulation in the plant tissues10, which negatively affects the quality of burley tobacco3. Nitrate levels have been found to be dozens to several hundred times greater in burley tobacco compared to that in other tobacco types11. The response of burley tobacco to reducing application levels of nitrogen is not completely clear. Burley tobacco varieties TN86 and TN90 are the most commonly cultivated tobacco cultivars in China, and their characteristics regarding nitrogen fertiliser application, yield, and value are similar 12, but their responses to conditions of nitrogen deficiency at the transcriptome level are unclear.
Nitrogen fertilisation has been reported to significantly influence plants growth traits13 and reducing the application of nitrogen fertiliser can effectively improve the efficiency of nitrogen use and reduce nitrogen losses14. Nitrogen fertilisation increases the leaf area index, leaf area duration, and leaf biomass accumulation of tobacco, while lower application of nitrogen significantly decreases nicotine, alkaloid content, and root density15,16,17. Furthermore, nitrogen deficiency has been reported to significantly affect carbon and nitrogen metabolism in crops, including rice, maize, and wheat18,19,20. However, there are currently few reports on the effects of nitrogen deficiency in burley tobacco at the transcriptome level (Supplementary Information 1).
In the current study, we performed RNA sequencing (RNA-Seq) based comparative transcriptome analysis of TN90 and TN86 grown under nitrogen-sufficient (N-sufficient) and nitrogen-deficient (N-deficient) conditions to gain an understanding of the pathways modulating carbon and nitrogen metabolism at low nitrogen levels. Pigment levels, photosynthesis rates (Pn), amounts of carbon (C) and nitrogen (N) compounds, activity of nitrogen-assimilating enzymes, and nicotine concentrations in tobacco leaves were also determined to evaluate the response of burley tobacco to low nitrogen. The results of this study will enable in-depth analyses of the effects of nitrogen deficiency in burley tobacco and help identify promising genes associated with nitrogen deficiency. The results may be useful in engineering burley tobacco plants that allow a reduction in the amount of chemical nitrogen fertiliser required while maintaining high crop yields.
Results
Leaf biomass and RNA-Seq analyses under N-deficient conditions
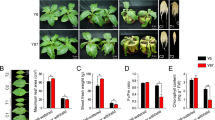
Nitrogen deficiency significantly decreased burley tobacco leaf biomass by 52.88% (p < 0.01) (Fig. 1A). To evaluate the effect of nitrogen deficiency on gene expression in burley tobacco, twelve complementary DNA (cDNA) libraries (two varieties × two nitrogen application levels × three biological replicates) were analysed using RNA-Seq-based technology. Principal component analysis (PCA) results revealed there were significant differences between treatments (Fig. 1B). In total, 72.56 million reads were obtained after removing sequencing adaptors and low-quality reads. Based on the average of TN90 and TN86, 84.98% of the reads were mapped to the reference genome.
RNA-Seq analysis of burley tobacco response to nitrogen deficiency. (A) Leaf biomass; (B) PCA; (C) RNA-Seq results confirmed by quantitative qRT-PCR; (D) Venn diagram of upregulated DEGs under nitrogen-deficient conditions vs nitrogen-sufficient conditions in burley tobacco varieties TN90 and TN86; (E) KEGG enrichment in common upregulated DEGs in TN90 and TN86; (F) GO enrichment in common upregulated DEGs in TN90 and TN86; (G) Venn diagram of downregulated DEGs under nitrogen-deficient conditions vs nitrogen-sufficient conditions in TN90 and TN86; (H) KEGG enrichment in common downregulated DEGs in TN90 and TN86; (I) GO enrichment in common downregulated DEGs in TN90 and TN86. Nd-TN90 TN90 cultivated under nitrogen-deficient conditions, Ns-TN90 TN90 cultivated under nitrogen-sufficient conditions, Nd-TN86 TN86 cultivated under nitrogen-deficient conditions, Ns-TN86 TN86 cultivated under nitrogen-sufficient conditions. Error bars of leaf biomass indicate standard error of the means (n = 3). Error bars of qRT-PCR indicate standard error of the means (n = 6). Symbol ** indicates a statistically significant differences between the nitrogen-deficient and nitrogen-sufficient nitrogen at p < 0.01.
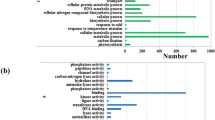
A total of 6318 differentially expressed genes (DEGs) induced by nitrogen deficiency in both TN90 and TN86 were analysed (Fig. 1). Among the DEGs, 428 upregulated genes and 213 downregulated genes were observed (Fig. 1D–G). Approximately 12.85% of the upregulated genes were involved in defence response (GO:0050832, GO:0042742, and GO:0031347; Fig. 1F) and 15.96% of the downregulated genes were associated with photosynthesis (GO:0009765 and GO:0015979; Fig. 1I). Genes annotated to gene ontology cellular component (GO-CC) were highly expressed in photosystem I (GO:0009522), photosystem II (GO:0009523), chloroplast thylakoid membrane (GO:0009535), and cytosol (GO:0005829). Genes annotated to gene ontology molecular function (GO-MF) included chlorophyll binding (GO:0016168) and glutamate synthase (NADH) activity (GO:0016040). The results indicated that genes in burley tobacco involved in photosynthesis were suppressed under conditions of reduced nitrogen application, which was consistent with the decreased accumulation of leaf biomass.
Eight DEGs were assayed by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) to validate the accuracy of the RNA-Seq data (Fig. 1C). Expression patterns of the selected genes were similar in the qRT-PCR and RNA-Seq analyses, indicating the RNA-Seq results were reliable.
Photosynthesis, carbohydrate metabolism, and carbon metabolism responses to N-deficiency
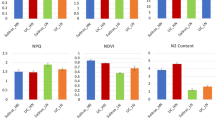
Carbon metabolism in TN90 and TN86 burley tobacco was reduced by N-deficiency (Fig. 2A–F). Among the DEGs downregulated by N-deficient conditions, 11 were associated with the photosynthesis pathway, indicating photosynthesis in burley tobacco would be decreased by reducing the application of nitrogen. Pigment concentration and Pn were decreased in TN90 and TN86 under N-deficient conditions (pigment: 51.71% and 39.85%, p < 0.01, respectively; Pn: 23.53%, p < 0.05 and 21.55%, p > 0.05, respectively). Among the DEGs upregulated under N-deficient conditions, 11 were associated with starch and sucrose metabolism pathways. Furthermore, soluble reducing sugar content in TN90 and TN86 was significantly increased by 54.28% and 69.17%, (p < 0.01), respectively, indicating carbohydrate biosynthesis in burley tobacco was increased in response to reducing the application of nitrogen.
Carbon metabolism of burley tobacco under N-deficient conditions. (A) Primary transcripts associated with carbon metabolism; (B) expression pattern of genes related to photosynthesis; (C) expression pattern of genes related to starch and sucrose metabolism; (D) pigment levels; (E) photosynthesis rate (Pn); (F) soluble reducing sugar content. Symbol * and ** indicate a statistically significant differences between the nitrogen-deficient and nitrogen-sufficient conditions at p < 0.05 and p < 0.01, respectively. ns indicates no statistically significant difference between nitrogen-deficient and nitrogen-sufficient conditions at p < 0.05 or p < 0.01, respectively. Nd-TN90 TN90 cultivated under nitrogen-deficient conditions, Ns-TN90 TN90 cultivated under nitrogen-sufficient conditions, Nd-TN86 TN86 cultivated under nitrogen-deficient conditions, Ns-TN86 TN86 cultivated under nitrogen-sufficient conditions. Error bars of leaf biomass indicate standard error of the means (n = 3).
Nitrogen metabolism response to N-deficiency
Nitrogen metabolism in burley tobacco was suppressed under N-deficient conditions (Fig. 3A–F). DEGs downregulated by reducing the application of nitrogen included 10 that were associated with nitrogen assimilation. In contrast, genes associated with nitrate response and nitrate transporters were upregulated. Total nitrogen content, NO3-N content, and glutamine synthetase (GSA) activity under N-deficient conditions were significantly decreased by 45.37%, 80.85%, and 52.60%, respectively, in TN90 and 29.53%, 77.72%, and 48.39%, respectively, in TN86 (p < 0.01). Soluble protein content was decreased by 7.59% in TN90 and 6.15% in TN86, indicating nitrogen assimilation was suppressed and nitrate transport was enhanced when the application of nitrogen was reduced.
Nitrogen metabolism of burley tobacco under N-deficiency conditions. (A) Primary transcripts associated with nitrogen metabolism; (B) expression pattern of genes related to nitrogen metabolism; (C) total nitrogen content; (D) NO3-N content; (E) GSA activity; (F) soluble protein content. Symbol ** indicate a statistically significant differences between nitrogen-deficient and nitrogen-sufficient conditions at p < 0.01. ns indicates no statistically significant difference between nitrogen-deficient and nitrogen-sufficient conditions at p < 0.05 and p < 0.01, respectively. Error bars of leaf biomass indicate standard error of the means (n = 3).
Root development and chemical content in response to N-deficiency
Nicotine levels in the roots and leaves of burley tobacco were decreased under N-deficient conditions, especially in the roots (Fig. 4A–C). Soluble reducing sugar content and root weight were significantly decreased by reducing the application of nitrogen (p < 0.01). Root length, root surface, root diameter, and root volume in TN86 under N-deficient conditions were decreased by 16.63%, 52.82%, 42.22%, and 73.56%, respectively (Table 1), indicating that root morphology was suppressed and energy production was insufficient, which would lead to decreased nicotine biosynthesis. Among the DEGs upregulated by N-deficiency, 15 were annotated to alkaloid transporters. The alkaloid transport-related genes were mainly enriched in ABC transporters, multidrug and toxic compound extrusion (MATE), and purine permease (PUP) families.
Effects of N-deficient conditions on alkaloid transporters, nicotine, soluble reducing sugar, and weight of tobacco roots. (A) Expression pattern of genes related to alkaloid transports; (B) nicotine content; (C) soluble reducing sugar content and root weight. Symbol ** indicates a statistically significant differences between nitrogen-deficient and nitrogen-sufficient conditions at p < 0.01. ns indicates no statistically significant difference between nitrogen-deficient and nitrogen-sufficient conditions at p < 0.05 and p < 0.01, respectively. Error bars of leaf biomass indicate standard error of the means (n = 3).
Signalling response to N-deficiency
Clustering analysis was performed to evaluate the differentially expressed genes involved in signalling in the two burley tobacco varieties under N-deficient conditions and the results are demonstrated in the heatmap shown in Fig. 5. A total of 25 DEGs were annotated to plant hormone signal transduction with the MAPK signalling pathway, NF-kappa B signalling pathway, and FoxO signalling pathway being upregulated by N-deficiency. Moreover, there were more differentially expressed genes in TN86 compared to those in TN90. Expression of At2g29380, ATL21A, and LOCX2 were upregulated in both TN90 and TN86 under N-deficient conditions, indicating N-deficient conditions promoted the expression of genes associated with serine-threonine protein phosphatase and putative ring-H2 finger protein.
Expression pattern of genes related to nitrogen deficiency responses. Nd-TN90 TN90 cultivated under nitrogen-deficient conditions, Ns-TN90 TN90 cultivated under nitrogen-sufficient conditions, Nd-TN86 TN86 cultivated under nitrogen-deficient conditions, Ns-TN86 TN86 cultivated under nitrogen-sufficient conditions.
Discussion
To gain the same leaf biomass for burley tobacco as that for other tobacco types requires almost a six-fold higher application of nitrogen21, which demonstrates that nitrogen utilization efficiency in burley tobacco is low. To explore the effects of nitrogen deficiency on burley tobacco, the transcriptome, physiology, and biochemistry of two burley varieties were evaluated under N-sufficient and N-deficient conditions. The carbohydrate and nitrogenous compound content, nicotine, nitrogen, and carbon metabolism, alkaloid translation, response to nitrogen deficiency, and root architecture were all analysed.
In our study, the leaf biomass of burley tobacco plants was significantly decreased by nitrogen deficiency (p < 0.01). Nitrogen is an essential factor that can influence the development and biomass of plants22,23. We found that total nitrogen and nitrate contents were significantly decreased in the two burley varieties under N-deficient conditions (p < 0.01), indicating the accumulation of total nitrogen and available nitrogen in burley tobacco was not high under N-deficient conditions. Burley tobacco is characterised by high nitrogenous content and low carbohydrate content in its leaves24. In our previous work, soluble protein content was much lower in burley tobacco compared with that in other tobacco types24. However, nitrogen metabolism and the amount of nitrogen in TN90 and TN86 were similar under the same nitrogen conditions. In the current study, soluble protein content was reduced in response to nitrogen deficiency, but not significantly. This may be a natural characteristic of burley tobacco24. NTR2.5 (a high-affinity nitrogen translator), GDHB (the gene encoding glutamate dehydrogenase B), and GLT1 (a gene involved in the ammonium assimilation pathway) are related to nitrogen translation and nitrogen assimilation25,26,27,28. The expression of NRT2.5, GDHB, and GLT1 was increased in the current study in response to nitrogen deficiency, indicating the ability of nitrogen translation and assimilation in burley tobacco leaves may be enhanced under nitrogen deficiency conditions.
Carbon metabolism and nitrogen metabolism in plants are very closely related29. Nitrogen metabolism supplies amino acids, enzyme proteins, and photosynthetic pigments to carbon metabolism, while carbon metabolism supplies carbon atoms and energy to nitrogen metabolism29,30. Expression of genes related to carbon metabolism and photosynthesis decreased under nitrogen-deficient conditions. The Pn and total sugar content (not shown) were significantly decreased in TN90 under N-deficiency conditions (p < 0.05), but not in TN86. This may have been due to the lower pigment levels in TN86 compared to that in TN90. In general, low nitrogen can accelerate senescence by significantly reducing pigment content, Pn, and total sugar content in plant leaves31,32,33, the Pn of TN86 did not significantly decrease in our study, indicating carbon metabolism in TN86 may be complex34,35. In our previous work, an exogenous carbon source sprayed onto the leaf of burley tobacco enhanced nitrogen translation and assimilation21. The amount of soluble reducing sugar supplying energy to nitrogen translation and assimilation in the two burley tobacco plant varieties was significantly decreased under N-deficient conditions. This indicated that no excess soluble reducing sugar was available to accumulate, which had a negative effect on leaf biomass formation and accrual. Expression of trehalose phosphatase/synthase 11 (TPS11), trehalose-phosphatase/synthase 7 (TPS7), beta-fructofuranosidase (INV2), the leucine-rich receptor-like protein kinase family protein β-amylase 1 (BAM1), and ADP-glucose pyrophosphorylase small subunit CagpS2 (AGPS2) were decreased, which may have been due to decreased soluble reducing sugar content.
Nicotine biosynthesis in roots is an important parameter for evaluating the quality of tobacco. Root growth and weight are significantly suppressed under low nitrogen conditions36. Nicotine levels were decreased in burley tobacco under N-deficient conditions, especially in the roots, which may have been a result of low soluble reducing sugar content or smaller surface area, average diameter, and root volume of the roots. In contrast, the expression in leaves of genes involved in alkaloid transport was increased under N-deficient conditions, which reduced the impact of decreasing nicotine in the aboveground parts of burley tobacco plants.
In conclusion, nitrogen deficiency significantly affected carbon and nitrogen metabolism in burley tobacco and expression of genes related to carbon and nitrogen metabolism were decreased. Leaf biomass and total nitrogen, nitrate, soluble reducing sugar, and nicotine content were all significantly decreased under N-deficient conditions (p < 0.01). Carbon metabolism was more complex in TN86 than that in TN90. Finally, nitrogen deficiency negatively affected the development of root morphology, but was positive for nitrogen, nitrate, and nicotine transporters.
Material and methods
Plant material and treatments
Two varieties of burley tobacco (TN86 and TN90) and four treatment groups were used in this study. The treatment groups were as follows: (1) TN90 with 24 mmol/L nitrogen; (2) TN86 with 24 mmol/L nitrogen; (3) TN90 with 4 mmol/L nitrogen; and (4) TN86 with 4 mmol/L nitrogen. A nitrogen concentration of 24 mmol/L was considered N-sufficient and a concentration of 4 mmol/L was considered N-deficient. Plants for the experiments were prepared on substrate culture in a greenhouse maintained at a temperature of 23 ± 2 °C. Seeds were sterilised with 2% (v/v) sodium hypochlorite and then sown in a floating system. After 40 days, the seedlings were transplanted into 25 cm diameter × 30 cm depth plastic pots and placed onto trays. Specimens were screened and selected from three independent test plots. The composition of the nutrient solution was determined using the method described by Hoagland21. The ratio of NO3- to NH4+ was 1:3 in both the N-sufficient and N-deficient treatments. The nutrient solution decanted onto the trays was replaced daily.
Biomass accumulation and root morphology assessment
The plant specimens were dissected into leaves, stems, and roots and cleaned with distilled water. Fresh leaves were fixed for 20 min at 105 °C and then dried for 48 h at 60 °C. Roots were kept moist with deionised water at 4 °C and then scanned using an Epson Expression 10000XL digital scanner (Epson Electronics Inc., San Jose, CA, USA). The images were analysed using WinRHIZO 2012b root analysis software (Regent Instrument Inc. Canada) to quantify the main root parameters. Root length, root superficial area, average diameter, root volume, and root tip were calculated using the data as previously described37.
Measurement of GSA activity, Pn, and nitrate, pigment, total nitrogen, and soluble reducing sugar content
Fresh lamina tissues without veins were cut into 2 mm × 5 mm pieces. GSA activity was determined according to the method described by O’Neal and Joy38. Nitrate content was determined according to the method described by Cataldo39. Pigment content was determined using 95% ethanol as previously described40. Pn was measured at 9:00–11:00 a.m. using a 6400XT portable photosynthesis system (LI-COR Biotechnology, Lincoln, NE, USA) as described by Liu41. Total nitrogen, nicotine, and soluble reducing sugar content were determined according to the modified Chinese Tobacco industry standard (YC/T 161, 159-2002)24.
RNA extraction and sequencing
RNA was extracted from each sample using the methods described previously21. Samples with an RNA integrity number (RIN) ≥ 7 were subjected to subsequent analysis. The sequencing format was 125 paired-end or 150 paired-end. After removal of low-quality data42, the clean reads with Q20 percentage of 94.07% were mapped to the Populus trichocarpa reference genome using bowtie2 or Tophat (http://tophat.cbcb.umd.edu/)43,44.
RNA-Seq, GO, and KEGG pathway enrichment analyses of DEGs
Transcript profiles of the RNA-Seq data were analysed by calculating the read fragments per kilobase per million mapped reads (FPKM). The FPKM value of each gene was calculated using cufflinks and the read counts of each gene obtained using htseq-count45,46. DEGs were identified using the DESeq (2012 update) function to estimate size factors and nbinomTest to test for differences between base means47. A p value < 0.05 and fold change > 2 or fold change < 0.5 was set as the threshold for significantly differential expression. Gene function was annotated based on data from databases, including the National Center for Biotechnology Information (NCBI) non-redundant protein sequences NR database (ftp://ftp.ncbi.nlm.nih.gov/blast/db/), the KOG database of Clusters of Orthologous Groups of proteins (http://www.ncbi.nlm.nih.gov/COG/)48, the manually annotated and reviewed protein sequence database Swiss-Prot (http://web.expasy.org/docs/swiss-prot_guideline.html), the Kyoto Encyclopedia of Genes and Genomes (KEGG) Ortholog (KO) database (http://www.genome.jp/kegg/), and Gene Ontology (GO; http://www.geneontology.org)49,50,51 (https://www.ncbi.nlm.nih.gov/). GO enrichment analysis (https://bioconductor.org/packages/release/data/annotation/html/GO.db.html) and KEGG pathway enrichment analysis (https://bioconductor.org/packages/release/data/annotation/html/KEGG.db.html) of DEGs were performed based on hypergeometric distribution using R. PCA and Heatmap analysis of DEGs were performed using R, version 3.4.1 (https ://www.r-proje ct.org/; Lucent Technologies, Murray Hill, NJ, USA)52. Box plots were plotted using Bioconductor affycomp (https://bioconductor.org/packages/release/bioc/html/affycomp.html) according to the methods of Jin53.
Validation of RNA-Seq using qRT-PCR analysis
Eight genes obtained in the RNA-Seq results were randomly selected and validated by qRT-PCR. Quantification was performed using a two-step reaction process of reverse transcription (RT) and PCR amplification. Reactions were incubated in a 384-well optical plate (Roche, Basel, Switzerland) at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The primer sequences were designed in house based on the mRNA sequences obtained from the NCBI database and synthesised by Generay Biotech (Shanghai, China). The mRNA expression levels were normalised and calculated using the 2−ΔΔCt method54.
Statistical analysis
The figures were prepared using Origin Pro 9.0 software (Origin Lab Corporation, Northampton, MA, USA). Variance analysis was performed using IBM SPSS version 20.0 software (IBM, Palo Alto, CA, USA) at a level of 0.05 with the least significant difference (LSD) multiple range test (p < 0.05).
Ethics statement
The tobacco varieties were supplied by China National Infrastructure for Crop Germplasm Resources (Tobacco, Qingdao), including TN90 and TN86 varieties. Experimental research and field studies on plants in this work, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
References
Henica, F. S. The inheritance of the White Burley character in tobacco. Jpn. J. Crop Sci. 4, 281–282 (1932).
Edwards, K. D. et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genom. 18, 14 (2017).
Lewis, R. S. et al. Impact of alleles at the yellow burley (Yb) loci and nitrogen fertilization rate on nitrogen utilization efficiency and tobacco-specific nitrosamine (TSNA) formation in air-cured tobacco. J. Agric. Food Chem. 60(25), 6454–6461 (2012).
Shi, H. et al. Changes in TSNA contents during tobacco storage and the effect of temperature and nitrate level on TSNA formation. J. Agric. Food Chem. 61(47), 11588–11594 (2013).
Lundy, M. E. et al. Nitrogen fertilization reduces yield declines following no-till adoption. Field Crop Res. 183, 204–210 (2015).
RezaFarrokh, A. et al. The effect of nitrogen and potassium fertilizer on yield and mineral accumulation in flue-cured tobacco. J. Agric. Sci. 4(2), 167–178 (2012).
Postiglione, M. I. S. L. The effect of nitrogen fertilization on nitrogen use efficiency of irrigated and non-irrigated tobacco (Nicotiana tabacum L.). Plant Soil 252, 313–323 (2003).
Li, Y. et al. The analysis of the difference on nitrogen metabolism of flue-cured tobacco and burley seedling. The analysis of the difference on nitrogen metabolism of flue-cured tobacco and burley seedling. Beijing 2016, 244–244 (2016).
Chai, J. et al. Introduction trials on burley tobacco variety TN86. Chinese Tobacco Science 24(3), 42–46 (2003).
Stefanelli, D., Winkler, S. & Jones, R. Reduced nitrogen availability during growth improves quality in red oak lettuce leaves by minimizing nitrate content, and increasing antioxidant capacity and leaf mineral content. Agric. Sci. 2(2), 477–486 (2011).
Shi, H. et al. The relationships between TSNAs and their precursors in burley tobacco from different regions and varieties. J. Food Agric. Environ. 10(3–4), 1048–1052 (2012).
Shang, Z. Variety comparison study on burley tobacco. Rev. China Agric. Sci. Technol. 8(4), 23–27 (2006).
Canam, T. & Campbell, M. M. Genes and nitrogen fuel wood formation. New Phytol. 182, 783–785 (2009).
Liu, X. et al. Effect of continuous reduction of nitrogen application to a rice-wheat rotation system in the middle-lower Yangtze River region (2013–2015). Field Crop Res. 196, 348–356 (2016).
Bangemann, L.-W., Sieling, K. & Kage, H. The effect of nitrogen and late blight on crop growth, solar radiation interception and yield of two potato cultivars. Field Crop Res. 155, 56–66 (2014).
Moustakas, N. K. & Ntzanis, H. Dry matter accumulation and nutrient uptake in flue-cured tobacco (Nicotiana tabacum L.). Field Crops Res. 94(1), 1–13 (2005).
Zou, C. et al. No-tillage culture and nitrogen fertilizer management for burley tobacco production. J. Agric. Sci. 155(4), 599–612 (2017).
Mascia, M. et al. Nitrogen Starvation Differentially Influences transcriptional and uptake rate profiles in roots of two maize inbred lines with different NUE. Int. J. Mol. Sci. 20, 4856 (2019).
Lv, X. et al. Low-nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat: Roles of phytohormone signaling. J. Plant Growth Regul. 20, 10 (2020).
Song, W. et al. Increased photosynthetic capacity in response to nitrate is correlated with enhanced cytokinin levels in rice cultivar with high responsiveness to nitrogen nutrients. Plant Soil 373, 981–993 (2013).
Li, Y. et al. Metabolome and molecular basis for carbohydrate increase and nitrate reduction in burley tobacco seedlings by glycerol through upregulating carbon and nitrogen metabolism. Sci. Rep. 10, 8 (2018).
Gorret, N. et al. Bioreactor culture of oil palm (Elaeis guineensis) and effects of nitrogen source, inoculum size, and conditioned medium on biomass production. J. Biotechnol. 108(3), 253–263 (2004).
Yin, S. et al. Effects of nitrogen source and phosphate concentration on biomass and metabolites accumulation in adventitious root culture of Glycyrrhiza uralensis Fisch. Acta Physiol. Plant. 36(4), 915–921 (2013).
Li, Y. et al. Biochemical, physiological and transcriptomic comparison between burley and flue-cured tobacco seedlings in relation to carbohydrates and nitrate content. Molecules 22(12), 2126 (2017).
Li, W. et al. Dissection of the AtNRT2.1: AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 143(1), 425–433 (2007).
Schafer, M. et al. Cytokinin levels and signaling respond to wounding and the perception of herbivore elicitors in Nicotiana attenuata. J. Integr. Plant Biol. 57(2), 198–212 (2015).
Lancien, M. et al. Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant J. Cell Mol. Biol. 29(3), 347–358 (2002).
Wang, Y. et al. Overexpression of Arabidopsis Dof1, GS1 and GS2 enhanced nitrogen assimilation in transgenic tobacco grown under low-nitrogen conditions. Plant Mol. Biol. Rep. 31, 886–9000 (2013).
Shi, H. Study of Relationship Between Carbon and Nitrogen Metabolism and Tobacco Quality in flue-Cured Tobacco (Hu Nan Agriculture University, 1997).
Gao, X. et al. A review on the response of relation between carbon and nitrogen metabolism and tobacco leaf quality to nitrogen. Agric. J. 8(3), 38–40, 56 (2013).
Zhao, Z. et al. Allocation of nitrogen and sucrose in maize seedling under low nitrogen stress. J. Plant Nutr. Fertil. 26(4), 783–796 (2020).
Li, M. et al. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 229, 132–134 (2018).
Dong, Y. et al. Differences of photosynthetic characteristics in the nitrogen efficiency genotypes of eggplant under low nitrogen stress. Acta Agric. Boreali Sin. 24(1), 181–184 (2009).
Wu, Q. et al. Mapping of two white stem genes in tetraploid common tobacco (Nicotiana tabacum L.). Mol. Breed. 34(3), 1065–1074 (2014).
Wu, X. et al. A two-step mutation process in the double WS1 homologs drives the evolution of burley tobacco, a special chlorophyll-deficient mutant with abnormal chloroplast development. Planta 251(1), 10 (2020).
Wei, H. et al. Nitrogen deprivation promotes Populus root growth through global transcriptome reprogramming and activation of hierarchical genetic networks. New Phytol. 200, 483–497 (2013).
Chen, G. et al. Effects of auxin on thiophene synthesis and root morphology in Tagetes patula hairy root cultures. Plant Soil 412, 441–451 (2017).
Oneal, D. & Joy, K. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Plant Physiol. 54(5), 773–779 (1974).
Cataldo, D. A. et al. Rapid cplorimetric deternination of nitrate in plant-tissure by nitration of salicylic acid. Commun. Soil Sci. Plan. 6, 71–80 (1975).
Wintermans, J. F. G. M. & De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109, 448–453 (1965).
Liu, Y. F. et al. Regulation of calcium on peanut photosynthesis under low night temperature stress. J. Integr. Agric. 12(12), 2172–2178 (2013).
Patel, R. K. & Jain, M. NGS QC toolkit: A toolkit for quality control of next generation sequencing data. PLoS One 7(2), 7 (2012).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9(4), 357-U54 (2012).
Kim, D. et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, 4 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7(3), 562–578 (2012).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 31(2), 166–169 (2015).
Anders, S. & Huber, W. Differential expression of RNA-Seq data at the gene level—the DESeq package. Embl 20, 20 (2013).
Koonin, E. V. et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 5, 2 (2004).
Apweiler, R. et al. UniProt: The universal protein knowledgebase. nucleic Acids Res. 32, 115–119 (2004).
Kanehisa, M. et al. The KEGG resource for deciphering the genome. Nucleic Acids Res. 32, 277–280 (2004).
Ashburner, M. et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Britton, N. F. et al. RNA-seq Data Analysis—A Practical Approach (CRC Press, 2015).
Jin, J. et al. Transcriptome and functional analysis reveals hybrid vigor for oil biosynthesis in oil palm. Sci. Rep. 7, 12 (2017).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT method. Methods 25, 402–408 (2001).
Acknowledgements
This study was financially supported by the Science and Technology Plan Project of the Department of Chinese Association (Nos. 2017QNRC001, 2018410000270036, and TP2019-C4). We thank Feng Yuqing for help with sampling.
Author information
Authors and Affiliations
Contributions
Y.L., X. Z., and H.S. designed the experiment. Y.L. and D.C. analysed the data and wrote the manuscript. Y.L. and H. Y. contributed to the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Chang, D., Zhang, X. et al. RNA-Seq, physiological, and biochemical analysis of burley tobacco response to nitrogen deficiency. Sci Rep 11, 16802 (2021). https://doi.org/10.1038/s41598-021-93363-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93363-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.