Abstract
The prevalence of second primary malignancies (SPMs) in the western world is continually increasing with the risk of a new primary cancer in patients with previously diagnosed carcinoma at about 20%. The aim of this retrospective analysis is to identify SPMs in colorectal cancer patients in a single-institution cohort, describe the most frequent SPMs in colorectal cancer patients, and discover the time period to occurrence of second primary tumors. We identified 1174 patients diagnosed with colorectal cancer in the period 2003–2013, with follow-up till 31.12.2018, and median follow-up of 10.1 years, (median age 63 years, 724 men). A second primary neoplasm was diagnosed in 234 patients (19.9%). Older age patients, those with early-stage disease and those with no relapse have a higher risk of secondary cancer development. The median time from cancer diagnosis to development of CRC was 8.9 years for breast cancer and 3.4 years for prostate cancer. For the most common cancer diagnosis after primary CRC, the median time to development was 0–5.2 years, depending on the type of malignancy. Patients with a diagnosis of breast, prostate, or kidney cancer, or melanoma should be regularly screened for CRC. CRC patients should also be screened for additional CRC as well as cancers of the breast, prostate, kidney, and bladder. The screening of cancer patients for the most frequent malignancies along with systematic patient education in this field should be the standard of surveillance for colorectal cancer patients.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death in the United States. It is estimated that in 2020 there will be 147,950 patients diagnosed and 53,200 will die from the disease1. The prevalence of second primary malignancies in the western world is continually increasing2. Due to screening programs and the success of personalized therapy, the mortality rate from this disease has decreased. In 2015, CRC prevalence in the Czech Republic (population 10.5 mil in 2015) reached 64,126 patients (6107/mil inhabitants). This is in comparison with 2005 (population 10.2 mil in 2005), there were 46,053 patients (4515/mil inhabitants) which was an increase of almost 40%3.
As the survival rates of cancer patients improves, those patients are more likely to develop SPMs. The type of SPMs and the frequency of occurrence is important in the field of cancer surveillance. The risk of a new primary cancer in patients with previously diagnosed carcinoma is about 20%, and more than one other cancer is diagnosed in approximately 30% of cancer survivors aged > 60 years4,5. As the number of cancer survivors increases, the occurrence of multiple primary cancers is also likely to rise. The most common subsequent cancers in the western world are nonmelanoma skin cancer, colorectal cancer, and breast cancer2. Primary and secondary malignancies are associated with lifestyle, environmental risk factors, host factors and hereditary susceptibility6. In secondary tumors, the late toxicity from previous anticancer therapy is also significant. Patients with SPMs after primary CRC have a worse prognosis than those with only a CRC diagnosis7.
Follow-up of CRC survivors was developed on the basis of limited resources, irrespective of the higher incidence of secondary malignancies. High-quality surveillance with the determination of duration, frequency, and method for the screening of SPMs is still missing.
The aim of this retrospective analysis is to identify SPMs in colorectal cancer patients in a single-institution cohort, describe the most frequent SPMs in colorectal cancer patients, and discover the time period to occurrence of second primary tumors.
Material and methods
Patient selection
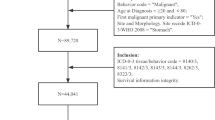
After approval by the ethics committee (number 2019/1827/MOU), patients with CRC diagnosed in the period 2003–2013 and followed-up by the end of 2018 at the Masaryk Memorial Cancer Institute (MMCI) in Brno, Czech Republic, were screened for eligibility after gaining their written informed consent for dealing with personal data in regard to the research. Data of those meeting the following criteria were retrieved from electronic medical records: adult patients aged ≥ 18 years with a CRC diagnosis confirmed by positive histology. Exclusion criteria were: (1) CRC diagnosed at autopsy, (2) patients lost to follow-up, (3) patients with a high risk of development SPMs due to hereditary cancer syndrome (e.g. BRCA 1, 2, Lynch syndrome, familial adenomatous polyposis (FAP)). We included cases of carcinoma in situ and clinically localized, regionally advanced, and metastatic disease.
Second primary malignancies
Multiple primaries are defined as more than one synchronous or metachronous cancer in the same individual. For epidemiological studies, tumors are considered multiple primary malignancies if they arise in different sites and/or are of a different histology or morphological group8. For the definition of site of the tumor in our study, criteria according to the SEER definition of multiple primary tumors was used, i.e. multiple primaries are: (1) tumors with ICD-O-3 histology codes that are different to the first, second or third number; (2) tumors with ICD-O-3 topography codes that are different at the second and/or third characters9.
Synchronicity was qualified according to the rules of the International Agency for Research on Cancer (IARC) which suggest the registration of synchronous tumours diagnosed in an interval of fewer than 6 months (or metachronous if more than 6 months) if arising in different sites10.
Statistical analysis
Comparisons of basic characteristics between the patients with SPM and the patients without SPM were summarized with counts and frequencies and tested with the Fisher exact test and Mann–Whitney test in case of categorical characteristics and continuous characteristics, respectively. Considering the sidedness of CRC, the International Classification of Diseases for Oncology coding scheme was used to categorize by the primary site as either: right colon (cecum, ascending colon, hepatic flexure), left colon (splenic flexure, descending colon, sigmoid colon), or rectum (rectosigmoid, rectum). The transverse colon (C18.4) was excluded from the laterality assessment (45 patients), as it was only possible by ICD-O-3 topography codes to define assignment to the right or left colon.
Logistic regression models were used to determine predictors of occurrence of SPM in patients with CRC. The following covariates were examined: gender, age at CRC diagnosis, clinical stage, status of relapse and sidedness of CRC. Grade and KRAS status were not examined due to the large number of missing records. Patients with an unknown clinical stage and a diagnosis of transverse colon (C18.4) were removed from the analysis (91 patients). Each covariate was fit univariately in separate logistic regression models. One overall multivariate logistic model including all covariates was used to assess independent effects.
Occurrences of SPMs by the site of diagnosis were described by counts and frequencies. SPMs with an unknown date of diagnosis were not included in this analysis (7 SPMs). The national cancer registry of the Czech Republic (NOR)11 was used to compare the frequencies of sites of diagnosis in our study with the frequencies in the entire Czech population.
The time from the diagnosis of previous neoplasm to the diagnosis of the first colorectal cancer and the time from the diagnosis of the first colorectal cancer to the diagnosis of subsequent neoplasm were described by mean and median. SPMs with an unknown date of diagnosis were not included in this analysis (7 SPMs).
Kaplan‐Meier curves were utilized to display the survival of the patients with colorectal cancer stratified by the occurrence of an SPM. The primary endpoint used was 15-year survival. Observations with 15 or more years of follow-up were censored at 15 years. The Breslow test was used to compare the differences in survival between defined groups of patients with respect to the occurrence of a SPM. The hazard ratio (HR) with corresponding 95% confidence interval was determined based on the Cox proportional hazards model, adjusted to gender, age at diagnosis, clinical stage, status of relapse and sidedness of CRC. The relationship between the occurrence of SPMs and presence of radiotherapy/chemotherapy was tested by the Fisher exact test.
Ethics approval
We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. All authors have contributed significantly and are in agreement with the content of the manuscript. This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional review Board of Masaryk Memorial Cancer Institute approved this study (number 2019/1827/MOU).
Results
Patients selection
In total, 1174 CRC patients diagnosed in the period from 1.1.2003 till 31.12.2013 were identified and enrolled in this study. Follow-up was continued till 31.12.2018, with median follow-up of 10.1 years, (median age 63 years, 724 men). The baseline patient characteristics are summarized in Table 1.
Second primary malignancies
We did not find any statistically significant difference between patients with and without an SPM with respect to gender, the grade of the tumor, or KRAS status. However, for age at diagnosis, clinical stage, and status of relapse significant differences were revealed (Table 1). Considering sidedness of CRC, it was evident that patients with rectal cancer are less likely to have SPMs than patients with colon cancer, however, the p value for sidedness did not reach statistical significance. NRAS as well as BRAF status was not assessed as this information was missed in the majority of patients due to the evaluated time period.
Based on univariate logistic models it was shown that patients aged 65 and over are approximately two times more likely to develop SPM than patients under 45 years (Table 2). Similarly, increased odds were detected in patients without relapse. In contrast, a significantly lower chance of SPM was demonstrated for clinical stage IV compared to stage I (OR = 0.49, p = 0.006) and localization in rectum compared to right colon (OR = 0.66, p = 0.039). In the multivariate model, after considering the effect of all variables analyzed, a statistically increased chance of SPM was reported only for patients without relapse (OR = 1.79; p = 0.004). Age at diagnosis over 65 years and clinical stage IV did not reach statistical significance in the multivariate model, however, the detected OR values were still very different from 1.
A second primary neoplasm was diagnosed in 234 patients (Table 3), one secondary neoplasm was found overall in 190 (16.2%), 36 (3.1%) patients suffered from two SPMs, and 8 (0.7%) were treated with three SPMs. Among SPMs, colorectal cancer (21.1%), breast cancer (17.6%) and prostate cancer (10.0%) were the most represented diagnoses (Table 4). Considering the relatively high proportion of men (62%) in our study, the incidence of prostate cancer is only slightly increased compared to general population, while the incidence of breast cancer is even more pronounced and indicates a significant risk of CRC occurance. The description of the occurrence of multiple primary neoplasms diagnosed before, synchronously, or after diagnosis of CRC is listed in Table 3. The majority of breast cancer and almost half of melanoma cases preceded the CRC diagnosis as well as a diagnosis of prostate cancer, where the distribution of patients over time is more homogenous. Renal cancer was diagnosed predominantly synchronously and after CRC diagnosis, as well as bladder cancer, where two-thirds of cases were diagnosed after CRC (Table 4; Fig. 1). The total number of secondary tumors was homogeneously stratified over time, approximately one third of the secondary tumors were diagnosed before, 1/3 synchronously, and 1/3 after the diagnosis of CRC. Table 5 summarizes the time from the previous neoplasm to the diagnosis of the SPMs. The median time from diagnosis to the development of CRC is 8.9 years for breast cancer and 3.4 years for prostate cancer. The time to second colorectal cancer is shown only for the site of diagnosis with the number of previous primary neoplasms greater than 10.
Occurrence of second primary malignancies by the time of diagnosis. Only SPMs with known date of diagnosis were considered (date of diagnosis was not available for 7 SPMs). Only sites of diagnosis with total SPMs greater than 10 are shown. 1Diagnosed 6 or more months before the first CRC in the patient. 2Diagnosed within 6 months before or after the first CRC in the patient. 3Diagnosed 6 or more months after the first CRC in the patient. SPM, multiple primary neoplasm; CRC, colorectal cancer.
As indicated in Table 5, the most common cancer diagnosis was found to be a median of 0–5.2 years after primary CRC. As well as the previous, the time to subsequent colorectal cancer is shown only for the site of diagnosis with the number of subsequent primary neoplasms greater than 10, for the reason of possible bias using small numbers of patients in a particular diagnosis.
The Kaplan–Meier curves of 15-year survival among colorectal cancer patients stratified by the occurrence of multiple primary neoplasms show better OS for patients with SPMs in the first 6 years and therefore OS was lower (Fig. 2), but this difference is not statistically significant. The differences in the clinical stages are shown in Fig. 3. Patients with SPM showed significantly worse survival in earlier clinical stages (stages I and II) compared with patients without SPM. In contrast, in advanced metastatic disease (grade IV), patients with SPM showed better survival than patients without SPM. In stage III, survival was comparable between the two groups of patients. Patients with early stages of CRC stay alive longer and have a greater chance of developing SPM, their prognosis is limited by SPM, not by the diagnosis of CRC, in contrast to stage IV, where is limiting CRC, not SPM. According to the Cox proportional hazards model adjusted to gender, age at diagnosis, clinical stage, status of relapse and sidedness, the risk of death from CRC with SPM was significantly higher than that from CRC without SPM (HR: 1.24, 95% CI: 1.02–1.51, p = 0.029).
We did not find prone to develop secondary malignancies in rectosigmoid and rectal cancer patients treated by radiotherapy (Table 6), neither chemotherapy administration in our cohort of patients (Table 6) (patients diagnosed before and synchronously with the first CRC were not included).
Discussion
The screening and personalized therapy of CRC leads to prolonged survival of these patients; however, it carries a higher chance for the development of SPMs. In this analysis, we have presented a large cohort of CRC patients treated in a single institution, with extended follow-up.
Different types of second primary malignancies in a particular type of tumor have been described12. Cancer of the lung, head and neck, and the genitourinary tract is associated with NSCLC13. Anal cancer has been increasingly associated with tumors of the oral cavity and pharynx, rectum and anal canal, larynx, lung and bronchus, ovary, vagina, and vulva, Kaposi's sarcoma and hematological malignancies14. Gastric cancer patients suffer more malignant tumors of the head and neck, esophagus, colon and rectum, bones and soft tissues, ovaries, bladder, or kidneys, as well as non-Hodgkin’s lymphoma15. Patients with bladder carcinoma are the most frequently diagnosed with SPMs and the most described second malignancy is lung cancer16. Compared to the general population, patients with CRC have a higher incidence of a second CRC17, as described in this study. Breast cancer is among the most common type of second primary malignancy in our cohort of CRC patients. As previously described, patients with breast cancer are at higher risk of developing colorectal cancer18,19, and they should be frequently screened for CRC, as well as patients with prostate cancer20,21 and malignant melanoma, where the high incidence of CRC after malignant melanoma was described by Caini et al.22. The incidence of gynecological cancer was similar in our patient cohort compared to the general Czech population, contrary to a Korean study23, but the total numbers are small overall.
For most cancers, the main risk period for the development of secondary malignancies is during the 3 years after initial diagnosis of the first tumor24. The highest risk of SPMs after Hodgkin´s lymphoma treatment is at 5–10 years25, but in solid tumors this period has not been well described. In a large Swiss study, 40% of patients developed SPMs between 1 and 5 years after the first cancer, and approximately one-third of them were diagnosed 5–10 years later12. In our analysis, the median time period to develop CRC after breast cancer diagnosis was 8.9 years and 3.4 years after prostate cancer diagnosis. In our data set, the median time to development of subsequent tumors was 1.7–5.2 years for the most frequent malignancies, depending on the specific diagnosis. It seems that after the first cancer diagnosis, patients should be screened for at least 5–10 years for SPMs, but this period remains unclear.
The influence of sidedness of CRC on SPMs was described in Jia et al. and Liu et al.17,26. The difference in the incidence of SPMs between the right and left colon was supported by Broman et al.27. The prognosis is better for left-sided than right-sided colon cancer28, and if patients survive longer, the probability of SPMs is higher for the left-side of the colon. Even so, we did not find any difference between right and left colon cancer. The high incidence of SPMs in colon cancer is in contrast with rectal cancer. According to our results, patients with rectal cancer are less likely to develop SPM than patients with right colon. This is probably not related to survival, although the prognosis of colon cancer patients is better at the early stage, but survival at advanced stages of rectal cancer is longer than colon cancer29.
Rectal and rectosigmoid cancer patients are often treated by radiotherapy, which has been described as a risk factor for SPMs, particularly in the pelvis, but it was not a cause of a higher incidence of SPMs in our cohort of patients30,31. We have not found any relationship in the occurrence of SPMs and adjuvant or neoadjuvant radiotherapy. Chemotherapy is described as a persistent risk factor for carcinogenesis32,33. Nevertheless, in our study, its administration was not associated with the risk of development of a SPM and patients with development of SPMs before chemotherapy/radiotherapy, and synchronously, were excluded.
Our results concord with Jia et al. which demonstrated that CRC patients with SPMs have better overall survival (OS) in the first 10 years and thereafter, have worse survival than patients without SPMs. In our study, OS was better in the first 6 years for CRC patients with SPMs, and thereafter was worse than in CRC patients without SPM. Nevertheless, the relationship between the year from CRC diagnosis and the occurrence of SPM was not statistically significant according to the logistic model (p = 0.306). The addition of an adjustment for the year of diagnosis to the Cox regression model also did not show significant changes in the results. An explanation of cross curve in survival analysis can also be found in the prognosis of the underlying CRC disease. Patients with a better prognosis have a higher probability of SPMs than patients with a worse prognosis, but finding the differences in OS of these patients require longer follow-up. Recently, an online competing-risk nomogram was released: Predicting Probabilities of Developing a Second Primary Malignancy for Colorectal Cancer Patients (http://biostat.fudan.edu.cn/crc)26.
An inherent limitation of this study is related to its retrospective nature, which is similar to all other studies dealing with this issue. The same reason limits availability of some other data which may be related to the risk of a SPM such as obesity, which increases the risk of malignancy34 as well as information on smoking, alcohol use, diet, sports activity and lifestyle35,36, which have a significant impact on cancer development, and data about these were not available for the majority of our patient cohort.
The strengths of our study include the use of a large population-based cohort of CRC patients, the patients' characteristics and treatment, detailed information on the incidence of SPMs in CRC patients from source documentation, review of medical charts, and long follow-up.
Previous studies have indicated no effect of more frequent specialized follow-up on the survival of CRC patients, but for some patients their prognosis could be limited by the occurrence of SPMs. The screening of cancer patients for the most frequent malignancies and their systematic education about risk reduction strategies should be standard in surveillance for all cancer patients, not just colorectal cancer patients.
We realize that the results from this analysis should be interpreted with caution and further studies in other centers are needed to confirm our outcomes. Understanding the risk of patients with a history of colorectal cancer would help to identify appropriate prevention strategies. Early detection of a second primary tumor should be the focus of healthcare providers as well as health insurance companies. It is imperative that professionals note that 20% of all cancer patients develop during their lives second primary tumors37.
Conclusion
Patients with a diagnosis of breast cancer, prostate cancer, kidney cancer, or melanoma should be regularly screened for CRC. As well, colorectal cancer patients should also be screened for additional cancers, namely colon, breast, prostate, kidney, and bladder cancer. We recommend that CRC patients in the early stages should be screened for second primary malignancies more often than the standard population, the duration of the screening should be at least 5–10 years though intervals remain unclear. Inexpensive and noninvasive methods should be used for early detection of the most frequent SPMs. Using standard screening methods for the general population (colonoscopy or fecal occult blood test, mammography, low-dose CT of the chest under certain conditions), enriched with abdominal ultrasound, and clinical examination, we can detect the early-stage of a secondary malignancy and hopefully prolong the overall survival of CRC patients.
The early detection of cancer, whether primary or second primary, leads to lives being saved as well as economic cost savings for healthcare systems. Our goal, as professionals in healthcare is to create a screening process for SPMs that will identify the most frequent primary tumors and will be focused on the most frequent second primary malignancies bound to specific tumors, and which can prolong survival of not only colorectal cancer patients, but all cancer patients.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70(3), 145–164. https://doi.org/10.3322/caac.21601 (2020).
Liu, L. et al. Prevalence of multiple malignancies in the Netherlands in 2007. Int. J. Cancer 128(7), 1659–1667. https://doi.org/10.1002/ijc.25480 (2011).
Dusek, L. et al. Estimating cancer incidence, prevalence, and the number of cancer patients treated with antitumor therapy in 2015 and 2020—Analysis of the Czech National Cancer Registry. Klin. Onkol. 28(1), 30–43. https://doi.org/10.14735/amko201530 (2015).
Soerjomataram, I. & Coebergh, J. W. Epidemiology of multiple primary cancers. Methods Mol. Biol. 471, 85–105. https://doi.org/10.1007/978-1-59745-416-2_5 (2009).
Koubková, L., Hrstka, R., Dobes, P., Vojtesek, B. & Vyzula, R. Second primary cancers—Causes, incidence and the future. Klin. Onkol. 27(1), 11–17. https://doi.org/10.14735/amko201411 (2014).
Anand, P. et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 25(9), 2097–2116. https://doi.org/10.1007/s11095-008-9661-9 (2008).
Chen, Q. et al. Do patients with second primary colorectal cancer hold the similar prognosis and therapeutic benefits as those with initial primary colorectal cancer?. Biomed. Res. Int. 2018(6172670), 2018. https://doi.org/10.1155/2018/6172670.eCollection (2018).
Vogt, A. et al. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2(2), e000172. https://doi.org/10.1136/esmoopen-2017-00017 (2017) (eCollection 2017).
SEER Training Modules, Multiple primary neoplasms. U. S. National Institutes of Health, National Cancer Institute. Cit 15.5.2020. https://training.seer.cancer.gov/.
Working Group Report. International rules for multiple primary cancers (ICD-0 third edition). Eur. J. Cancer Prev. 14(4), 307–308. https://doi.org/10.1097/00008469-200508000-00002 (2005).
Institute of Health Information and Statistics of the Czech Republic. National Health Information System (NHIS), Czech National Cancer Registry (CNCR). http://www.uzis.cz/en/czech-nationalcancer-registry-cncr.
Feller, A. et al. The relative risk of second primary cancers in Switzerland: A population-based retrospective cohort study. BMC Cancer 20(1), 51. https://doi.org/10.1186/s12885-019-6452-0 (2020).
Duchateau, C. S. & Stokkel, M. P. Second primary tumors involving non-small cell lung cancer: Prevalence and its influence on survival. Chest 127(4), 1152–1158. https://doi.org/10.1378/chest.127.4.1152 (2005).
Shah, B. K. & Budhathoki, N. Second primary malignancy in anal carcinoma—A US population-based study. Anticancer Res. 35(7), 4131–4134 (2015).
Chen, S. C. et al. Second primary malignancy risk among patients with gastric cancer: A nationwide population-based study in Taiwan. Gastric Cancer 19(2), 490–497. https://doi.org/10.1007/s10120-015-0482-3 (2016).
Donin, N. et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 122(19), 3075–3086. https://doi.org/10.1002/cncr.30164 (2016).
Liu, L. et al. Second primary cancers in subsites of colon and rectum in patients with previous colorectal cancer. Dis. Colon Rectum. 56(2), 158–168. https://doi.org/10.1097/DCR.0b013e318279eb30 (2013).
Soerjomataram, I. et al. Primary malignancy after primary female breast cancer in the south of the Netherlands, 1972–2001. Breast Cancer Res. Treat. 93(1), 91–95. https://doi.org/10.1007/s10549-005-4016-2 (2005).
La Francis, I. E. & Cooper, R. B. Second primary malignancies associated with primary female breast cancer: A review of the Danbury Hospital experience. Conn. Med. 56(8), 411–414 (1992).
Saltus, C. W. et al. Incidence of second primary malignancies in patients with castration-resistant prostate cancer: An observational retrospective cohort study in the United States. Prostate Cancer. 2019(4387415), 2019. https://doi.org/10.1155/2019/4387415.eCollection (2019).
Chattopadhyay, S. et al. Prostate cancer survivors: Risk and mortality in second primary cancers. Cancer Med. 7(11), 5752–5759. https://doi.org/10.1002/cam4.1764 (2018).
Caini, S. et al. The risk of developing a second primary cancer in melanoma patients: A comprehensive review of the literature and meta-analysis. J. Dermatol. Sci. 75(1), 3–9. https://doi.org/10.1016/j.jdermsci.2014.02.007 (2014).
Shin, D. W. et al. Secondary breast, ovarian, and uterine cancers after colorectal cancer: A nationwide population-based cohort study in Korea. Dis. Colon Rectum. 61(11), 1250–1257. https://doi.org/10.1097/DCR.0000000000001203 (2018).
Rasmussen, L. A. et al. Time from incident primary cancer until recurrence or second primary cancer: Risk factors and impact in general practice. Eur. J. Cancer Care (Engl.) 28(5), e13123. https://doi.org/10.1111/ecc.13123 (2019).
Schaapveld, M. et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N. Engl. J. Med. 373(26), 2499–2511. https://doi.org/10.1056/NEJMoa1505949 (2015).
Jia, H., Li, Q., Yuan, J., Sun, X. & Wu, Z. Second primary malignancies in patients with colorectal cancer: A population-based analysis. Oncologist 25(4), e644–e650. https://doi.org/10.1634/theoncologist.2019-0266 (2020).
Broman, K. K., Bailey, C. E. & Parikh, A. A. Sidedness of colorectal cancer impacts risk of second primary gastrointestinal malignancy. Ann. Surg. Oncol. 26(7), 2037–2043. https://doi.org/10.1245/s10434-019-07326-7 (2019).
Petrelli, F. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol. 3(2), 211–219. https://doi.org/10.1001/jamaoncol.2016.4227 (2017).
Lee, Y. C., Lee, Y. L., Chuang, J. P. & Lee, J. C. Differences in survival between colon and rectal cancer from SEER data. PLoS ONE 8(11), e78709. https://doi.org/10.1371/journal.pone.0078709 (2013) (eCollection 2013).
Warschkow, R. et al. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother. Oncol. 123(1), 139–146. https://doi.org/10.1016/j.radonc.2017.02.007 (2017).
Dracham, C. B., Shankar, A. & Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 36(2), 85–94. https://doi.org/10.3857/roj.2018.00290 (2018).
Liang, F., Zhang, S., Xue, H. & Chen, Q. Risk of second primary cancers in cancer patients treated with cisplatin: A systematic review and meta-analysis of randomized studies. BMC Cancer 17(1), 871. https://doi.org/10.1186/s12885-017-3902-4 (2017).
Boffetta, P. & Kaldor, J. M. Secondary malignancies following cancer chemotherapy. Acta Oncol. 33(6), 591–598. https://doi.org/10.3109/02841869409121767 (1994).
Gibson, T. M. et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: A pooled analysis of prospective cohort studies. J. Clin. Oncol. 32(35), 4004–4011. https://doi.org/10.1200/JCO.2014.56.8444 (2014).
Morais, S. et al. Second primary cancers and survival in patients with gastric cancer: Association with prediagnosis lifestyles. Eur. J. Cancer Prev. 28(3), 159–166. https://doi.org/10.1097/CEJ.0000000000000447 (2019).
Wood, M. E. et al. Second malignant neoplasms: Assessment and strategies for risk reduction. J. Clin. Oncol. 30(30), 3734–3745. https://doi.org/10.1200/JCO.2012.41.8681 (2012).
Berrington de Gonzalez, A. et al. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet Oncol. 12(4), 353–360. https://doi.org/10.1016/S1470-2045(11)70061-4 (2011).
Acknowledgements
Supported by the Ministry of Health of the Czech Republic, MZ CR—DRO (MMCI, 00209805) and by Ministry of Education, Youth and Sports, MSMT—Czech Clinical Research Infrastructure (CZECRIN) LM2018128 and BBMRI-CZ LM2018125.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.H., M.S.; Data curation, J.H., L.P.; Formal analysis, J.H., L.P., T.K., L.B.; Funding acquisition, J.H., R.D., R.G., S.K., T.K.; Investigation, J.H.; Methodology, J.H., O.S., I.K., L.P.; Project administration, J.H.; Writing—original draft, J.H., T.K., L.P.; Writing—review & editing, J.H., T.K., R.D., O.S., I.K., M.S., D.A.K. Supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halamkova, J., Kazda, T., Pehalova, L. et al. Second primary malignancies in colorectal cancer patients. Sci Rep 11, 2759 (2021). https://doi.org/10.1038/s41598-021-82248-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82248-7
This article is cited by
-
Incidence and risk outcomes of second primary malignancy of patients with post-operative colorectal cancer
International Journal of Colorectal Disease (2023)
-
A prognostic nomogram and risk classification system of elderly patients with extraosseous plasmacytoma: a SEER database analysis
Journal of Cancer Research and Clinical Oncology (2023)
-
Investigating the tissue specificity and prognostic impact of cis-regulatory cancer risk variants
Human Genetics (2023)
-
Common anti-cancer therapies induce somatic mutations in stem cells of healthy tissue
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.