Abstract
Faba bean (Vicia faba L.), a globally important grain legume providing a stable source of dietary protein, was one of the earliest plant cytogenetic models. However, the lack of draft genome annotations and unclear structural information on mRNA transcripts have impeded its genetic improvement. To address this, we sequenced faba bean leaf transcriptome using the PacBio single-molecule long-read isoform sequencing platform. We identified 28,569 nonredundant unigenes, ranging from 108 to 9669 bp, with a total length of 94.5 Mb. Many unigenes (3597, 12.5%) had 2–20 isoforms, indicating a highly complex transcriptome. Approximately 96.5% of the unigenes matched sequences in public databases. The predicted proteins and transcription factors included NB-ARC, Myb_domain, C3H, bHLH, and heat shock proteins, implying that this genome has an abundance of stress resistance genes. To validate our results, we selected WCOR413-15785, DHN2-12403, DHN2-14197, DHN2-14797, COR15-14478, and HVA22-15 unigenes from the ICE-CBF-COR pathway to analyze their expression patterns in cold-treated samples via qRT-PCR. The expression of dehydrin-related genes was induced by cold stress. The assembled data provide the first insights into the deep sequencing of full-length RNA from faba bean at the single-molecule level. This study provides an important foundation to improve gene modeling and protein prediction.
Similar content being viewed by others
Introduction
In addition to cereals, grain legumes are economically valuable crops for smallholder farmers. The reasons for cultivating pulses rather than cereals include the fact that they are a rich source of proteins and carbohydrates essential for the human diet, and that there is substantial demand for them as animal feed1,2. Similar to other leguminous species, pulses can fix atmospheric nitrogen into biologically useful ammonia, promoting natural soil nitrogen fertilization3. Indeed, the increasing demand for organic food should favor legume-based crops in crop rotation with cereals and oilseeds.
Faba bean (Vicia faba) is the fourth most widely grown temperate legume and the only edible pulse crop among Vicia species4. Compared with other legumes, faba bean is distinguished by its high protein content and balanced amino acid profile5. Moreover, its low fat content and relatively low endogenous lipoxygenase activity make faba bean less prone to developing off-flavors than soybean and pea, enabling it to be incorporated into daily diets6. Faba bean can also adapt to diverse climatic and soil conditions, providing another advantage over other legumes7,8,9. However, similar to other major legumes, the yield of faba bean remains relatively unstable, which has limited its competitiveness as a commercial crop. This inherent yield instability can be attributed to profligate flowering habits that result in fertilized ovule abortion or insufficient pollination.
Faba bean is also susceptible to various biotic and abiotic stresses, contributing to environment-dependent losses10. Although winter-type faba beans have higher yield and protein content than spring-type ones, to increase the yield in cool-temperate regions, faba beans are mainly sown as a spring crop because of the insufficient winter hardiness of the available winter-type cultivars11. Several studies have revealed the cold/frost tolerance of faba bean12,13,14. However, investigations of the genes conferring cold tolerance have been hampered by the insufficient genomic information and long-read sequencing transcriptome data for faba bean, which has a relatively large genome (approximately 13 Gb distributed over six chromosomes).
Although genetic variability among plant species is quite large, especially within primitive forms and landraces, and significant progress has been made through conventional breeding, accelerated genetic gains through genomics and associated biotechnology-related research have been limited15. There are many reasons why new breeding technologies have not been used for faba bean to the same level as for other crop species. For example, the phenotypic and genetic variability of this species is quite large but remains unexplored, and the method for characterizing its germplasm has not been updated16. Furthermore, establishing an efficient faba bean regeneration protocol has been challenging, which has restricted the use of cutting-edge biotechnology-based methods17. However, one of the most limiting factors is the considerable size of the faba bean genome, with more than 85% of the genome comprising repetitive sequences18.
Moreover, the faba bean is a partially allogamous diploid species with six pairs of extremely large chromosomes19,20. Accordingly, the biological and computational analyses required have made it difficult to assemble and annotate such a large genome. Despite these issues, the application of next-generation sequencing (NGS) technology has increased the transcriptome data available for faba bean and enriched the genomic resources for this legume21,22,23.
Transcriptome analysis via NGS is relatively inexpensive and provides sufficient data for clarifying transcriptional and post-transcriptional gene regulation, single-nucleotide variations, and transcript rearrangements. Thus, it is useful for nonmodel crops lacking an available reference genome24. However, NGS technology is not ideal for identifying full-length splicing isoforms because the generated reads are short and the associated computational analyses are complicated. Full-length splicing isoforms can produce multiple transcripts for most genes; this increases the protein-coding potential of the genome and increases the transcriptome complexity and flexibility25,26.
Isoform sequencing (Iso-seq) using SMRT (single-molecule real-time) technology developed by PacBio (Pacific Biosciences of California Inc., Menlo Park, CA, USA) is an alternative approach for generating single sequence reads of a typical gene or gene family without the need for sequence assembly. Unlike other NGS techniques, the long reads generated by Iso-seq can produce complex assemblies with fully or partially closed gaps with minimal structural errors and accurate gene annotations. Hence, Iso-seq has been applied for comprehensive transcriptome analyses of major crops with large and small genomes27,28.
In this study, we used the SMRT Iso-seq protocol for long-read sequencing analysis of faba bean. Furthermore, we identified stress-related transcripts and validated the expression of a few genes involved in cold stress responses. We aimed to characterize and functionally annotate the transcripts to produce data useful for improving gene identification. The generated data may help elucidate the mechanism underlying the tolerance of faba bean to cold stress.
Results
Overview of the transcriptome sequencing with SMRT analysis
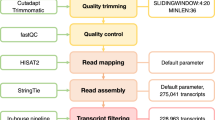
To obtain a full-length representative transcriptome for faba bean, we sequenced and analyzed the total RNA using the PacBio Sequel System. To avoid biases, both long and short transcripts were selected (< 3 kb and ≥ 3 kb). A total of 957,010 polymerase reads (20.1 Gb) were obtained, from which a dataset of clean reads was generated (Supplementary Table S1). Additionally, 35,930,187 subreads were generated, with an average length of 4981 bp and an N50 of 5153 bp (Supplementary Table S2). To produce more accurate information, 847,930 circular consensus sequences (CCS) were obtained using SMRTAnalysis 5.0 software (Supplementary Fig. S1). The mean length of the CCS reads was 5542 bp, with an average of 94 passes (Supplementary Table S3). Approximately 89% (754,713) of the reads were classified as full length (containing the 5′ primer, 3′ primer, and poly-A tail), while 730,301 reads were identified as non-full-length non-chimeric (FLNC) reads with few artificial concatemers (Table 1). Finally, redundant transcripts were collapsed using Cogent, after which the Cupcake ToFU pipeline generated 28,569 clustered transcripts (unigenes), with a total length of 94,401,312 bp, and a final gene set comprising 26,321 genes. The sequences in the dataset ranged from 108 to 9666 bp, with an average length of 2786 bp and an N50 of 3425 bp (Table 2). Furthermore, we characterized and assigned isoform information to full-length cDNA. We detected alternative splicing in 3597 unigenes with 2–20 isoforms per transcript derived from alternative transcription sites, alternative polyadenylation, or alternative splicing events, with isoforms ranging from 270 to 9666 bp (Fig. 1). The basic workflow for the data analysis is summarized in Supplementary Fig. S2.
Functional annotation of faba bean unigenes
To obtain information regarding transcript function, coverage, and quality, we performed a BLASTX analysis29. A total of 27,580 unigenes (96.5%) were aligned to sequences in protein databases, including the NR, Pfam, EggNOG, NT, TAIR, and UniProt databases (Table 3). We analyzed homologous species by comparing unigenes to the sequences in the NR database, which revealed that the unigenes mainly matched sequences from Medicago truncatula (41.06%), Cicer arietinum (20.55%), and Trifolium pratense (13.6%) (Fig. 2). To functionally characterize the orthologous proteins, we performed a BLASTX analysis using EggNOG (version 4.5). On the basis of the NOG analysis, 33,635 unigenes were assigned to 25 functional clusters. Excluding the functionally unknown class (14,659 unigenes, 43.58%), “cellular processing and signaling” was the largest class (7937 unigenes), with “post-translational modification” and “signal transduction mechanisms” representing the dominant functional groups. A total of 6189 unigenes (18.40%) belonged to the “metabolism” class and were involved in “energy production and conversion” and “carbohydrate transport and metabolism.” Additionally, 4850 unigenes (14.41%) belonged to the “information storage and processing” class and contributed to “translation, ribosomal structure, and biogenesis” and “transcription” (Fig. 3). The PacBio data were used to predict transcription factors (TFs). A total of 901 putative TFs from 50 families were identified. The major families were associated with C3H-, ARF-, and MYB-related TFs, all of which are considered to regulate metabolism and secondary metabolite biosynthesis in green plants (Fig. 4).
On the basis of the similarity to sequences in the NR database, unigenes were annotated with GO terms. The GO classification assigned unigenes to 62 functional groups in the three main categories as follows: cellular component (12,406, 36.62%), molecular function (9858, 29.1%), and biological process (9844, 29.06%). Approximately 5.25% of the unigenes were not annotated. In the biological process category, “metabolic process” (30.21%) and “cellular process” (14.81%) were the two most represented subcategories. In the molecular function category, the unigenes were primarily associated with “catalytic activity” (42.03%) and “binding” (38.19%), whereas in the cellular component category, the unigenes were mainly related to “cell part” (50.3%) and “organelle” (22.29%) (Fig. 5). The BLASTX results for the faba bean transcriptome (i.e., read length, isoforms, and annotations from all databases) are presented in Supplementary Table S4.
Identification of stress-related gene families
After screening the Pfam database, 25,630 unigenes were predicted to encode at least one Pfam protein domain (2723 Pfam domains in total). Protein kinase superfamily (1135), protein tyrosine kinase (517), and NB-ARC domain (355) represented most of the protein domains. The other protein families among the 10 largest protein families are listed in Table 4. We also identified genes responsive to biotic and abiotic stresses by searching the Pfam, TAIR, and PlantTFDB databases. In addition to NB-ARC gene family members, we identified 173 heat shock-related genes, 141 Myb DNA-binding domains, and 95 C3H-related domains associated with drought, heat, cold, and salinity stress tolerance (Table 5).
We further classified unigenes associated with the ICE-CBF-COR pathway, a universal pathway related to cold stress responses in plants30. These unigenes were related to ICE1-2, CBF1-3, DHN2, WCOR413, COR15a-b, and HVA22 (Table 6; Supplementary Table S5). More specifically, 96 unigenes related to the ICE-CBF-COR pathway were detected, with the largest proportion related to ICE1-2 (45 unigenes), followed by CBF1-3 (28 unigenes), WCOR413 (six unigenes), COR15a-b and DHN2 (five unigenes each), and HAV22 (one unigene). The sequence lengths in the selected gene families ranged from 482 to 3824 bp.
Expression analysis of cold-responsive genes
To validate the transcriptome data, we analyzed the expression of six unigenes (WCOR413-15785, DHN2-12403, DHN2-14197, DHN2-14797, COR15-14478, and HVA22-15) (primer information—Supplementary Table 6) by qRT-PCR (Fig. 6). In response to cold stress treatment at 4 °C, DHN2-12403 was the most highly expressed unigene in PI 469181, with an expression level approximately 9.4- and 2.3-fold higher than that under normal conditions (18 °C) and in PI 271634 at 4 °C, respectively. Additionally, during exposure to an extremely low temperature (− 7 °C), the DHN2-12403 expression level was significantly higher in PI 469181 than in PI 271634 (P < 0.01). Similarly, DHN2-14197 and HVA22-15951 expression levels were also increased by cold stress conditions (4 and 0 °C) in both accessions, but especially in PI 469181 at 0 °C (P < 0.05). In contrast, WCOR413-15795, DHN2-14797, and COR15-14478 expression levels were decreased under cold stress conditions, although the expression patterns differed between PI 469181 and PI 271634. These results indicate that DHN2-12403, DHN2-14197, and HVA22-15951 are responsive to cold stress. Moreover, the differences in their expression between the cold-tolerant accession (PI 469181) and the cold-susceptible accession (PI 271634) suggest that they may influence the cold tolerance of faba bean. Overall, the reliability of our long-read sequencing data was confirmed by the qRT-PCR analysis.
Discussion
Most previous studies on the faba bean transcriptome were performed to develop molecular markers or identify stress-related differentially expressed genes, as reviewed elsewhere31. A few studies focused on enhancing genomic resources18,22,32 and involved short-read sequencing. Although short-read sequencing is useful for quantifying and annotating transcripts in nonmodel plants, it is often unable to accurately capture the entire transcriptome’s true complexity. The faba bean genome has not been annotated, and full-length mRNA sequences with isoform information have been studied by only one research group33. Accordingly, we sequenced the faba bean leaf transcriptome using the PacBio SMRT platform to obtain complete transcripts containing untranslated regions and poly-A tails without needing to assemble short reads.
Despite the advantages of long-read sequencing, developing unified and practical approaches to analyzing the data remains a significant challenge. Previous studies applied standard Iso-seq bioinformatic pipelines to generate high-quality isoforms34,35. In the current study, we modified the standard pipeline by incorporating the Cogent tool36 and the Cupcake ToFU package to refine and reconstruct the coding genome to obtain a comprehensive faba bean transcriptome atlas. Our data included 28,569 high-quality unigenes with a total length of 94 Mb. This genome-wide transcriptome coverage data will be useful for developing molecular markers, studying repetitive elements, and as a reference for quantifying gene expression in future studies. The average length of the faba bean transcripts was 4981 bp, and more than 500 unigenes were longer than 3500 bp, which is substantially longer than the sequences revealed in earlier studies18,22. Furthermore, we identified 1349 unigenes with more than 2 and at most 20 isoforms. These results reflect the utility of the SMRT approach for capturing long transcript sequences to enrich the transcriptional information available for large and complex genomes, with implications for functional studies of important genes.
A total of 27,580 unigenes (96.5%) were annotated following an alignment with sequences in six databases. The remaining 989 unigenes lacking significant matches might represent novel genes or correspond to untranslated regions or other noncoding RNAs. BLASTX analyses revealed that more than 40% of the unigenes were significantly similar to sequences from M. truncatula, consistent with known phylogenetic relationships37 and the results of earlier transcriptome studies18,22. The systematic classification of proteins based on NOG analyses suggested that many unigenes were related to post-translational modifications, signal transduction, and transcription. The functional classification according to GO annotations indicated that most of the unigenes assigned to the cellular component category were associated with cell parts and organelles. In the molecular function category, most unigenes were related to catalytic and binding activities, gene regulation, signal transduction, and enzymatically active processes in cells. In the biological process category, unigenes contributing to metabolic and cellular processes were abundant. This functional information may be relevant for identifying genetic networks involved in the regulation of biosynthesis as well as plant growth, development, and stress responses. Overall, the results confirm the accuracy of our sequencing approach and provide the foundation for future molecular research related to genetic gains in faba bean.
The instability of the yield of faba bean is mostly due to exposure to abiotic and/or biotic stresses. Because climate change will likely result in more extreme environmental conditions, enhancing the stress resistance of faba bean has become a high priority. However, stress responses are tightly regulated by complex genetic networks. More specifically, plants have developed sophisticated mechanisms for perceiving subtle changes in the environment and triggering signal transduction to regulate the activity of stress-responsive genes38. Transcriptome profiling, especially with long reads, can quickly provide insights into stress-related genetic cascades. Although covering this vast network is beyond the scope of this study, we identified a few important gene families based on Pfam domains and TFs. Many stress-related genes were annotated, including those encoding late embryogenesis abundant (LEA) proteins, dehydrins, and heat shock proteins. Earlier research proved that the overexpression of these genes could increase the tolerance to multiple stresses39. Most disease resistance proteins contain the NB-ARC domain. In this study, we identified many unigenes encoding this domain.
Unigenes were associated with major TFs, such as MYB, bHLH, and WRKY family members, directly related to stress responses or AB2/ERF, ARF, and C3H TFs, which are in turn related to specific hormone pathways involved in stress responses. These large TF classes are important for regulating gene expression and signal transduction. Overall, these stress resistance genes reflect the diversity of the faba bean transcriptome and may form the basis of future molecular research.
In South Korea, cold stress, including frost, is a major abiotic factor limiting faba bean production. Focusing on cold-responsive genes, we further classified the unigenes associated with the ICE-CBF-COR pathway, including unigenes related to WCOR413, DHN2, HAV22, CBF1-3, ICE1-2, and COR15a-b. Some of these genes encode TFs or positive regulators of TFs, whereas some are cold-regulated genes encoding cryoprotective proteins. For example, the ICE gene encodes an MYC-like bHLH transcriptional activator. In response to cold conditions, this activator binds to the CBF promoter and activates the expression of downstream cold- and dehydration-responsive genes41,42. The CBF gene encodes an AP2/ERF (APETALA2/Ethylene-responsive factor)-type TF that specifically binds to the C-repeat dehydration-responsive element to regulate the expression of cold-responsive genes (COR)43.
In contrast, COR15a-b, included in the nuclear genome, encodes a protein belonging to the LEA-4 group with a putative N-terminal signal sequence related to chloroplast import44. Freezing stress leads to overexpression of the mature COR15 protein in the chloroplast stroma. This protein functions as a cryoprotectant that stabilizes the membrane bilayer and minimizes electrolyte leakage from cells42. Earlier research suggested that COR15 is important for maintaining the activity of protoplast and chloroplast enzymes, such as lactate dehydrogenase, under freezing conditions45. Similarly, DHN, a hydrophilic protein with many charged amino acids, belongs to the LEA protein family. Ubiquitous among plant species, DHN proteins accumulate during late embryogenesis, or their production is induced in vegetative tissues following exposure to freezing stress46. These proteins protect cell metabolism during cellular dehydration, scavenge hydroxyl radicals, and protect lipid membranes from peroxidation47.
We also identified one of the less characterized members of the COR gene family, WCOR41348. Studies proved that the expression of COR413 genes is strongly induced by cold stress in cereals and Arabidopsis48,49. To validate our observations, we selected six unigenes encoding COR and dehydrin family members. To examine the expression patterns of these genes, we analyzed two faba bean accessions that vary regarding their tolerance to the cold conditions of South Korean winters50. Although the expression patterns of COR-related unigenes were generally the same at 4 °C, the expression levels were slightly higher in the cold-tolerant accession, suggesting that these genes contribute to low-temperature stress responses. Their potential roles influencing cold stress tolerance require further investigation.
We revealed a direct correlation between high expression levels and a decrease in temperature regarding the dehydrin-related unigenes. Moreover, we detected an over-accumulation of dehydrin proteins as the temperature decreased, suggesting that they are involved in a late response to freezing or frost stress. The cold tolerance of faba bean is controlled by various genes or quantitative trait loci (QTL)11. Previous investigations of faba bean involving SNP and AFLP markers detected QTL associated with frost tolerance in natural accessions and a multi-parent advanced generation inter-cross population51,52,53. In the current study, we identified faba bean isoform sequences and validated the expression of a few cold-responsive genes under cold stress conditions. Thus, to analyze cold tolerance-related genes more thoroughly, additional genetic approaches are needed, including QTL mapping, GWAS, and DEG analyses. We are currently developing faba bean mutant genetic resources produced via gamma irradiation. The isoform data may be relevant to other quality-related loci in faba bean.
In summary, characterizing the full-length faba bean transcriptome should facilitate dissection of the molecular and genetic basis of agronomic traits. Using the data generated in this study, we identified and analyzed transcripts involved in various biological and metabolic processes in faba bean. Combining information regarding the putative stress-related genes and networks with appropriate biotechnological approaches will enable researchers to improve faba bean varieties genetically. The results of this study may be useful for future functional and comparative genomic studies. Moreover, our findings demonstrate the advantages of the SMRT Iso-seq method for identifying genes in nonmodel plants.
Materials and methods
Plant materials and RNA extraction
Faba bean accession PI 469181 (highly adapted to the environmental conditions in South Korea) was grown in the experimental field at the Korea Atomic Energy Research Institute (Jeongup, Korea) in 2018. The faba bean accessions were provided by the USDA-National Plant Germplasm System (NPGS) (http://npgsweb.ars-grin.gov/) at Pullman, WA, USA. All experiments were carried out in accordance with national regulations in Korea. Young apical stems and leaves were collected, immediately frozen in liquid nitrogen, and ground into a fine powder. Total RNA was isolated from the leaves using the RiboPure Kit (Applied Biosystems, Foster City, CA, USA). The RNA concentration was determined using the ND1000 spectrophotometer (NanoDrop Technologies, DE, USA), whereas the RNA quality was assessed using the 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA). Samples comprising 1–10 μg of RNA with an RNA integrity number (RIN) > 8.0 were used for sequencing.
Library preparation and SMRT sequencing
A total of 6 μg of RNA was used to synthesize cDNA using the Clontech SMARTer PCR cDNA Synthesis Kit (Takara Bio USA Inc., CA, USA). Large double-stranded cDNA was produced in optimized PCR cycles, after which the BluePippin™ Size Selection System (Sage Science Inc., Beverly, MA, USA) was used for size fractionation and selection (1–10 kb). A template library was prepared using the SMRTbell library kit (Pacific Biosciences Inc., CA, USA) and the PacBio RSII platform, in accordance with the manufacturer’s protocol at the National Instrumentation Center for Educational Management (Seoul National University, Seoul, South Korea). Libraries were extracted in two batches (< 3 kb and > 3 kb) using the BluePippin™ Size Selection System. Two SMRTbell libraries were constructed with the Pacific Biosciences DNA Template Prep Kit (version 2.0) for SMRT sequencing on the Pacific Biosciences Sequel System.
Quality filtering and long-read processing
The PacBio long raw reads were processed using SMRTlink (version 4.0) software. The pipeline included generating CCS, identifying full-length reads (classification), clustering isoforms, and polishing to generate high-quality isoforms. The obtained subread BAM file reads were processed into error-corrected CCS. After identifying the 5′ and 3′ adapters and the poly-A tail, CCS were classified into full-length and non-full-length reads. The CCS with all 5′ and 3′ reads were designated as non-concatemer reads with 5′ and 3′ primers, whereas those with all three elements and no additional copies of the adapter sequence within the DNA fragment were designated as non-concatemer reads with 5′ and 3′ primers and a poly-A tail or full-length non-concatemer (FLNC) reads. Consensus isoforms were identified from the FLNC reads using the ICE (Iterative Clustering for Error Correction) algorithm and were further polished based on non-full-length reads using Quiver to obtain high-quality isoforms with post-correction accuracy exceeding 99%54. Redundant sequences were collapsed using the COding GENome reconstruction tool (Cogent version 3.9)36 and unique isoforms were defined using the Cupcake ToFU package (https://github.com/Magdoll/cDNA_Cupcake)55.
Functional annotation and classification
Functional annotations were completed using BLASTX (E-value cut-off of 10−6) to screen the following databases: NR (NCBI non-redundant proteins), NT (nucleotide database), UniProt, EggNOG (orthology prediction), GO (gene ontology), Pfam (conserved protein families and domains), and TAIR (Arabidopsis information resources). On the basis of the annotations, TFs) in PlantTFDB 4.0 were identified56.
Cold stress treatments
We identified a set of gene families involved in abiotic and biotic stress responses based on the protein domains. We subsequently identified six gene groups, WCOR413 (wheat COR-413), DHN2 (dehydrin), HVA22, CBF1 (C-repeat binding factor), ICE1-2 (inducer of CBF expression), and COR15a-b (cold regulated 15), involved in the ICE-CBF-COR pathway associated with cold stress responses in diverse crops. We randomly selected six unigenes from these groups and studied their expression profiles to validate the transcriptome data. To confirm the cold tolerance/susceptibility phenotype of the two accessions and validate the identification of cold-responsive genes, we conducted cold stress experiments as previously described51, with some modifications (e.g., exposure times and the lowest temperature). Briefly, the cold-tolerant line (PI 469181) and the cold-susceptible line (PI 271634) were grown in pots, which were placed in a growth chamber (Vision Science, Korea) set at 18 °C for 2 weeks. The temperature in the growth chamber was gradually lowered at a rate of 1 °C/h and then held at 4 °C for 3 days (12-h light/12-h dark). To investigate the gene expression levels at extremely low temperatures, the temperature was lowered further (1 °C/h) to − 7 °C. After 12 h of exposure to − 7 °C, the plants were allowed to recover at 18 °C.
Quantitative real-time (qRT)-PCR
Total RNA was isolated from cold-treated/untreated leaf tissue using TRIzol reagent and treated with DNase I (Ambion, USA). For both accessions, RNA samples were prepared in triplicate. The extracted RNA (1 μg) served as a template to synthesize the first-strand cDNA using the SuperScript III First-Strand Synthesis SuperMix (Invitrogen, USA). Gene-specific primers were designed for the selected unigene sequences using Primer3 software (http://primer3plus.com/primer3web/primer3web_input.htm). A qRT-PCR assay was performed using the iTaq Universal SYBR Green SuperMix (Bio-Rad, USA) and the Bio-Rad CFX96 real-time PCR detection system. The PCR program was as follows: 94 °C for 10 min; and then 45 cycles of 94 °C for 10 s, 60 °C for 15 s, and 72 °C for 30 s. The analysis was completed using three independent biological replicates. Gene expression levels were normalized against the expression of the cyclophilin (CYP2) gene57 and calculated according to the 2−ΔΔCt comparative threshold method58.
Data availability
The PacBio sequencing raw reads can be accessed from the NCBI Sequence Read Archive (SRA) under accession number SRX10148269.
References
Mudryj, A. N., Yu, N. & Aukema, H. M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 39, 1197–1204 (2014).
Roy, F., Boye, J. I. & Simpson, B. K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 43, 432–442 (2010).
Barton, L., Thamo, T., Engelbrecht, D. & Biswas, W. K. Does growing grain legumes or applying lime cost effectively lower greenhouse gas emissions from wheat production in a semi-arid climate?. J. Clean. Prod. 83, 194–203 (2014).
FAOSTAT. The global economy of pulses. Rome, Italy. Available online at: http://faostat.fao.org (2019).
Duc, G. Faba bean (Vicia faba L.). Field Crops Res. 53, 99–109 (1997).
Chang, P. R. Q. & McCurdy, A. R. Lipoxygenase activity in fourteen legumes. Can. Inst. Food Sci. Technol. J. 18, 94–96 (1985).
Katerji, N., Mastrorilli, M., Lahmer, F. Z., Maalouf, F. & Oweis, T. Faba bean productivity in saline–drought conditions. Eur. J. Agron. 35, 2–12 (2011).
Pociecha, E., Kościelniak, J. & Filek, W. Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor). Acta Physiol. Plant. 30, 529–535 (2008).
Zhou, R. et al. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 214, 1–13 (2018).
O’Sullivan, D. M. & Angra, D. Advances in faba bean genetics and genomics. Front. Genet. 7, 150 (2016).
Arbaoui, M., Link, W., Satovic, Z. & Torres, A.-M. Quantitative trait loci of frost tolerance and physiologically related trait in faba bean (Vicia faba L.). Euphytica 164, 93–104 (2008).
Inci, N. E. & Toker, C. Screening and selection of faba beans (Vicia faba L.) for cold tolerance and comparison to wild relatives. Genet. Resour. Crop Evol. 58, 1169–1175 (2011).
Landry, E. J. & Hu, J. Increasing pre-acclimation temperature reduces the freezing tolerance of winter-type faba bean (Vicia faba L.). J. Agron. Crop Sci. 205, 46–53 (2019).
Link, W., Balko, C. & Stoddard, F. L. Winter hardiness in faba bean: Physiology and breeding. Field Crop Res. 115, 287–296 (2010).
Maalouf, F. et al. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 138, 465–473 (2018).
Duc, G. et al. Faba bean. In Grain Legumes. Handbook of Plant Breeding, vol 10 (ed. De Ron, A.) 141–178. https://doi.org/10.1007/978-1-4939-2797-5_5 (Springer, New York, 2015).
Gnanasambandam, A. et al. Impact of molecular technologies on faba bean (Vicia faba L.) breeding strategies. Agronomy 2, 132–166 (2012).
Gao, B. et al. Comprehensive transcriptome analysis of faba bean in response to vernalization. Planta 251, 22 (2020).
Neumann, P., Koblizkova, A., Navratilova, A. & Macas, J. Significant expansion of Vicia pannonica genome size mediated by amplification of a single type of giant retroelement. Genetics 173, 1047–1056 (2006).
Pearce, S. R. et al. The Ty1-copia group retrotransposons in Vicia species: Copy number, sequence heterogeneity and chromosomal localisation. Mol. Gen. Genet. 250, 305–315 (1996).
Arun-Chinnappa, K. S. & De McCurdy, D. W. novo assembly of a genome-wide transcriptome map of Vicia faba (L.) for transfer cell research. Front. Plant Sci. 6, 217 (2015).
Yang, F. et al. Transcriptome profile analysis of two Vicia faba cultivars with contrasting salinity tolerance during seed germination. Sci. Rep. 10, 7250 (2020).
Ocana, S. et al. Large-scale transcriptome analysis in faba bean (Vicia faba L.) under Ascochyta fabae infection. PLoS ONE 10, e0135143 (2015).
Ward, J. A., Ponnala, L. & Weber, C. A. Strategies for transcriptome analysis in non-model plants. Am. J. Bot. 99, 267–276 (2012).
Anvar, S. Y. et al. Full-length mRNA sequencing uncovers a widespread coupling between transcription initiation and mRNA processing. Genome Biol. 19, 46 (2018).
Pan, Q., Shai, O., Lee, L. J., Frey, B. J. & Blencowe, B. J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 (2008).
Nakano, K. et al. Advantages of genome sequencing by long-read sequencer using SMRT technology in medical area. Hum. Cell 30, 149–161 (2017).
Rhoads, A. & Au, K. F. PacBio sequencing and its applications. Genomics Proteomics Bioinform. 13, 278–289 (2015).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Chinnusamy, V., Zhu, J. & Zhu, J. K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451 (2007).
Wang, C. et al. Development and application of the Faba_bean_130K targeted next-generation sequencing SNP genotyping platform based on transcriptome sequencing. Theor. Appl. Genet. 134, 3195–3207 (2021).
Carrillo-Perdomo, E. et al. Development of new genetic resources for faba bean (Vicia faba L.) breeding through the discovery of gene-based SNP markers and the construction of a high-density consensus map. Sci. Rep. 10, 6790 (2020).
Yuan, X. et al. Single-molecule real-time and illumina-based RNA sequencing data identified vernalization-responsive candidate genes in faba bean (Vicia faba L.). Front. Genet. 12, 656137 (2021).
Kim, J. A. et al. Genome-wide transcriptome profiling of the medicinal plant Zanthoxylum planispinum using a single-molecule direct RNA sequencing approach. Genomics 111, 973–979 (2019).
Lyu, J. I. et al. Characterization of gene isoforms related to cellulose and lignin biosynthesis in Kenaf (Hibiscus cannabinus L.) mutant. Plants (Basel) 9, 631 (2020).
Tseng, E. cDNA Cupcake. Available online: https://github.com/Magdoll/cDNA_Cupcake (2019).
Chooi, W. Y. Variation in nuclear DNA content in the genus vicia. Genetics 68, 195–211 (1971).
Lamers, J., van der Meer, T. & Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 182, 1624–1635 (2020).
Ciarmiello, L. F., Woodrow, P., Fuggi, A., Pontecorvo, G. & Carillo, P. Plant genes for abiotic stress, abiotic stress in plants—Mechanisms and adaptations. Available from https://www.intechopen.com/books/abiotic-stress-in-plants-mechanisms-and-adaptations/plant-genes-for-abiotic-stress. https://doi.org/10.5772/22465 (2011).
Javed, T. et al. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants (Basel) 9, 491 (2020).
Liu, Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10, 1391–1406 (1998).
Thalhammer, A., Bryant, G., Sulpice, R. & Hincha, D. K. Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 166, 190–201 (2014).
Stockinger, E. J., Gilmour, S. J. & Thomashow, M. F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. U. S. A. 94, 1035–1040 (1997).
Lin, C. & Thomashow, M. F. DNA sequence analysis of a complementary DNA for cold-regulated arabidopsis gene cor15 and characterization of the COR 15 polypeptide. Plant Physiol. 99, 519–525 (1992).
Nakayama, K. et al. Arabidopsis Cor15am is a chloroplast stromal protein that has cryoprotective activity and forms oligomers. Plant Physiol. 144, 513–523 (2007).
Paul, A. & Kumar, S. Dehydrin2 is a stress-inducible, whereas Dehydrin1 is constitutively expressed but up-regulated gene under varied cues in tea [Camellia sinensis (L.) O. Kuntze]. Mol. Biol. Rep. 40, 3859–3863 (2013).
Samarina, L. S. et al. Physiological, biochemical and genetic responses of Caucasian tea (Camellia sinensis (L.) Kuntze) genotypes under cold and frost stress. PeerJ 8, e9787 (2020).
Breton, G., Danyluk, J., Charron, J. B. & Sarhan, F. Expression profiling and bioinformatic analyses of a novel stress-regulated multispanning transmembrane protein family from cereals and Arabidopsis. Plant Physiol. 132, 64–74 (2003).
Okawa, K., Nakayama, K., Kakizaki, T., Yamashita, T. & Inaba, T. Identification and characterization of Cor413im proteins as novel components of the chloroplast inner envelope. Plant Cell Environ. 31, 1470–1483 (2008).
Lee, M.-K. et al. Selection of the elite lines with high adaptability to autumn sowing of South Korea in faba bean. Plant Breed. Biotechnol. 6, 57–64 (2018).
Ali, M. B. M. et al. Association analyses to genetically improve drought and freezing tolerance of faba bean (Vicia faba L.). Crop Sci. 56, 1036–1048 (2016).
Sallam, A., Arbaoui, M., El-Esawi, M., Abshire, N. & Martsch, R. Identification and verification of QTL associated with frost tolerance using linkage mapping and GWAS in winter faba bean. Front. Plant Sci. 7, 1098 (2016).
Sallam, A. & Martsch, R. Association mapping for frost tolerance using multi-parent advanced generation inter-cross (MAGIC) population in faba bean (Vicia faba L.). Genetica 143, 501–514 (2015).
Chin, C. S. et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10, 563–569 (2013).
Bayega, A., Fahiminiya, S., Oikonomopoulos, S. & Ragoussis, J. Current and future methods for mRNA analysis: A drive toward single molecule sequencing. Methods Mol. Biol. 1783, 209–241 (2018).
Jin, J. et al. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 45, D1040–D1045 (2017).
Gutierrez, N., Giménez, M. J., Palomino, C. & Avila, C. M. Assessment of candidate reference genes for expression studies in Vicia faba L. by real-time quantitative PCR. Mol. Breed. 28, 13–24 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by the research program of KAERI, Republic of Korea and the Radiation Technology R&D Program (NRF‐2017M2A2A6A05018538) through the National Research Foundation of Korea funded by the Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
S.J.K., K.C.P., I.Y.C. conceived the study. J.M.K., N.N.H., J.S.S. performed phenotyping and preparation of SMRT Iso-seq. J.B.K. provided germplasm resources and project administration. I.Y.C., K.C.P. analyzed the SMRT Iso-seq data. J.I.L., R.R., S.J.K. wrote the manuscript. All authors reviewed and agreed to this submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lyu, J.I., Ramekar, R., Kim, J.M. et al. Unraveling the complexity of faba bean (Vicia faba L.) transcriptome to reveal cold-stress-responsive genes using long-read isoform sequencing technology. Sci Rep 11, 21094 (2021). https://doi.org/10.1038/s41598-021-00506-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00506-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.