Abstract
Contact with stinging spines venom from several Lepidoptera larvae may result in skin lesions. In Mexico, envenomation outbreaks caused by Megalopyge opercularis were reported between 2015 and 2016. The aim of this study was to identify the venomous caterpillars in Nuevo Leon, Mexico and evaluate several biological activities of their hemolymph (HEV) and spine setae (SSV) venoms. M. opercularis was identified by cytochrome oxidase subunit (COI) designed primers. HEV and SSV extracts cytotoxic activity was assessed on the L5178Y-R lymphoma cell line. For apoptotic cells number and apoptosis, cells were stained with acridine orange/ethidium bromide and validated by DNA fragmentation. Human peripheral blood mononuclear cells (hPBMC) cytokine response to the extracts was measured by the cytometric bead array assay. Extracts effect on pro-coagulation activity on human plasma was also evaluated. HEV and SSV extracts significantly inhibited (p < 0.01) up to 63% L5178Y-R tumor cell growth at 125–500 µg/mL, as compared with 43% of Vincristine. About 79% extracts-treated tumor cells death was caused by apoptosis. Extracts stimulated (p < 0.01) up to 60% proliferation of resident murine lymphocytes, upregulated IL-1β, IL-6, IL-8, and TNF-α production by hPBMC, and showed potent pro-coagulant effects. The pharmacological relevance of these venoms is discussed.
Similar content being viewed by others
Introduction
Lepidoptera order consists of moths and butterflies distributed throughout almost the entire planet. About 12 families have poisonous species associated with human skin inflammatory responses1. Most of them belong to the Saturniidae, Limacodidae, Megalopygidae, Arctiidae, Lasiocampidae, Notodentidae, and Lymantridae families2,3,4. One of the most important Megalopygidae species is represented by the Megalopyge genera5. Larvae of these genera have spines, which contain a high number of specialized cells with a sensory function and potential to respond to mechanical stimulation by secreting and injecting toxins into the skin through fur-like spines6. Envenomation caused by contact with Megalopyge opercularis spines causes severe pain, burning, and swelling, in addition to nausea and headache symptoms.
In mid-November of 2015 and October of 2016, increased skin injury alerts after venomous puss caterpillar (“oruga peluche”) stings were reported, mainly among infants, in the Mexican states of Jalisco, Nuevo León, and Tamaulipas (https://www.milenio.com/region/Reportan_presencia_oruga_peluchecasos_oruga_peluche_NL-alertan_oruga_peluche_0_630537166.html). In the USA, M. opercularis envenomation period ranged from July to November, but higher case numbers were reported from September to November7. Among others, the puss caterpillar, with a characteristic “hairy fur-like” appearance resembling a Persian cat, represents the insect’s larval stage, whereas M. opercularis adults are known as southern flannel moths7. This species is the most widely distributed of the genus, being reported from the southern states of the USA to South America8. Other Megalopyge species, including M. urens, M. lanata, and M. krugi from Central and South America, have the potential to cause severe stings9,10. Therefore, taxonomic identification of venomous caterpillar species at the outbreak location is ecologically and epidemiologically relevant11.
Because of its appearance, the puss caterpillar is very alluring to touch, particularly by children. Unfortunately, hidden in its furry hair, the larva has hollow spines ended in setae. After contact, a sensory and mechanical response activates spines insertion and toxin secretion into the skin12. Affected individuals acquire the venom from each broken setae, thus increasing the injury in the skin and surrounded area3,8.
The insect’s advantage of producing such poisoning compounds, in addition to avoid predation by other animals, may be related to improve its cellular longevity by inhibiting apoptosis11,13,14,15,16, thus showing application in biotechnology, mainly in the clinical area.
Evaluating M. opercularis venom symptoms in the Central Texas Poison Center during 1996, almost 99% of patients experienced local pain, 27% referred intense radiating pain and presented edema, 42% developed erythema, and 9% had welts, among other symptoms17. These varying symptoms among humans exposed to M. opercularis venom may be an indication of differences in their immune responses.
Compounds with venom-like properties have been isolated from the spine setae and hemolymph extracts of stinging caterpillars11. Such agents may consist of peptidic and non-peptidic components, however, their full nature and activities have not been fully investigated11. Recently, Sánchez et al. reported that M. lanata peptidic venom induced mild skin inflammatory lesions in mice and elicited a limited shortening clotting time triggered by calcium17.
In addition, spine structures produce and inoculate chemical urticating compounds. They are filled with a fluid that is secreted by cells located at the base of the tegument3. It has been suggested that spine cells are responsible for the venom secretion by the South America specie Lonomia obliqua Walker (Lepidoptera: Saturnidae), which has structures involved in venom production located in the caterpillar body’s proximity and in the hemolymph8.
In regard to megalopygids toxicity, Sánchez et al.18 showed that proteins found in the species Megalopyge lanata (Stoll) and Podalia orsilochus (Cramer) have serine peptides similar to those reported by the urticating setae of the neotropical moth Hylesia metabus (Cramer), as well as other enzymes reported by Lenomia obliqua. In fact, they discussed that such proteins have very low activity towards hyaluronic acid, which is related to venoms, since > 45 kDa hyaluronidases are associated with extracellular matrix components degradation. Although they did not identify the predominant infiltrating mononuclear cell type in skin lesions upon M. lanata venom exposure, after testing the spine setae extracts in vivo using a murine model, such injuries were similar to those reported by L. obliqua venom, since in vivo cell reaction against M. opercularis venom was mostly lymphocytic.
In regard to antitumor properties of insect toxins and proteins, Apis mellifera L. (Apidae: Hymenoptera) bee venom (also known as api-toxin) contains phospholipases (also found in L. obliqua venom) and melittin peptide, which demonstrated cytotoxic activity against several tumor cell lines, where the melittin-induced anticancer effect was mediated by apoptosis19. Furthermore, Suttmann et al. reported that cecropins A and B inhibited bladder cancer cells growth, but were non-toxic against fibroblasts20. In contrast, Mazzoni et al. reported an anti-apoptotic effect in VERO and Sf-9 cells of a protein isolated from the close relative Megalopyge albicollis Walker hemolymph21.
The aim of the present study was to investigate in vitro antitumor, pro-inflammatory, and pro-coagulant activities of M. opercularis hemolymph (HEV) and spine setae (SSV) extracts, which may prompt pre-clinical studies for application in biomedicine.
Results
Venomous caterpillar identification
The analysis of the COI gene sequence (https://boldsystems.org/index.php/Taxbrowser_Taxonpage?taxon=+Megalopyge&searchMenu=taxonomy&query=+Megalopyge) confirmed that collected larvae belonged to M. opercularis species (GenBank access No. MF621253). This is the first molecular identification description of this species in Mexico, according to the Barcode of Life Data Systems (BOLDSYSTEMS v3 database, https://v3.boldsystems.org/index.php/Public_SearchTerms).
In the present study, fresh and washed oak leaves (Q. virginiana fusiformis) were used to feed collected larvae and maintain their natural wild type diet. Although all rearing larvae were eating, only six reached the pupal stage and from these, only 2 adults emerged, which may be related to sub-optimal environmental conditions present in the insect colony rearing room; in nature, they seek for colder, tree-shaded outdoor recreation areas during warmer fall and spring months, which synchronizes with peak larval instar seasons7.
Antitumor activity of M. opercularis extracts
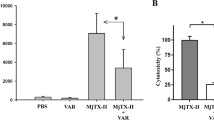
M. opercularis HEV and SSV extracts significantly (p < 0.01) inhibited 35%, 63%, and 60%, and 56%, 61%, and 51% L5178Y-R growth at 125, 250, and 500 µg/mL, respectively (Fig. 1a), whereas Vincristine (positive control) caused from 32 to 43% tumor growth inhibition at concentrations ranging from of 3.1 to 125 µg/mL15,37. For HEV, the IC95 was 210.15 µg/mL (± 97.76 to 377.47 µg/mL), whereas for SSV, the IC95 was 308.65 µg/mL (± 188.70 to 539.40 µg/mL). The lymphoma cytotoxicity peak was observed at 250 μg/mL (Fig. 1a).
Tumor cell cytotoxicity and apoptosis induced by HEV and SSV extracts. Exponentially growing L5178Y-R cells (5 × 104 cells/mL) were incubated for 48 h in the presence or absence of 7.8–500 µg/mL of HEV and SSV extracts, as described in the text. After incubation for 44 h, MTT was added to all wells, and cultures were incubated for additional 4 h. (a) Optical densities at 540 nm and percent cytotoxicity were then determined, as explained in the text. Data represent means ± SD of triplicate determinations from three independent experiments. Optical density value for untreated control was 0.269 ± 0.004. **p < 0.01, compared with untreated control. (b) L5178Y-R culture of 3 × 106 cells were exposed to HEV, SSV, or Actinomycin D (positive control), and number of apoptotic cells, were evaluated, as described in the text.
Apoptosis assay was examined after 24 h to achieve an intermediate point of cells toxicity, which was observed after 48 h. Figure 1b shows that 68% and 79% HEV- and SSV-treated tumor cells, respectively, resulted in death by apoptosis (DNA ladder is shown in Supplementary Fig. 1), as compared with 69% of Actinomycin D control-treated cells.
Effect of HEV and SSV on T cell proliferation
HEV and SSV extracts significantly (p < 0.01) stimulated 22–50% and 32–60% resident lymphocytes proliferation from 62.5 µg/mL respectively, as compared with positive control Concanavalin A (6.25 μg/mL) (Fig. 2).
Lymphoproliferation induced by HEV and SSV extracts. Thymus cell suspensions (100 µL of 1 × 107 cells/mL) were incubated in the presence or absence of HEV and SSV at various concentrations, and proliferation assessed as described in the text. Data represent means ± SD of triplicate determinations from three independent experiments. **p < 0.01, compared with untreated control. Optical density at 540 nm for untreated cells was 0.95 ± 0.01. Lymphocyte proliferation index of 1 indicates no alteration on proliferation.
hPBMC cytokine response to M. opercularis extracts
As shown in Fig. 3, HEV and SSV extracts increased IL-1β, IL-6, IL-8, and TNF-α cytokines production by hPBMC in a concentration-dependent fashion, as compared with untreated control (undetectable cytokine levels).
Effect of HEV and SSV on pro-inflammatory cytokine production. (a) IL-1β, (b) IL-6, (c) IL-8, and (d) TNF-α production by human PBMC exposed to HEV and SSV extracts at concentrations ranging from 3.91 to 125 µg/mL, was evaluated, as described in the text. Data represent means ± SD of triplicate determinations from three independent experiments. **p < 0.01, compared with untreated control.
Pro-coagulation effect of HEV and SSV on human plasma
M. opercularis HEV and SSV extracts plasma recalcification assay revealed that extracts lacked thrombin-like activity (plasma did not clot in Ca2+ absence). Both extracts displayed a pro-coagulant effect by shortening the clotting time (from minute 1) of human plasma, compared with untreated control (coagulation initiated at minute 7) (Fig. 4).
Discussion
The term erucism, also called lepidopterism, refers to an injury caused by contact with the venom bristles after adult or larval skin contact. It relates to a typical dermal medical condition caused from one out of 12 butterflies and moths venom species known to harm humans. From North America, 3484 cases of M. opercularis caterpillar stings were reported to the Texas Poison Center Network in Texas, USA, during 2000–2016, being the highest number of cases during July (12%) and October–November (59%). Thus showing that this caterpillar has two generations within a year. Patients mostly reported that the sting occurred at their own residence (91% of patients) and were treated outside of a healthcare (89% of patients), where clinical signs mainly involved the dermis (90% of patients), presenting irritation and local pain, wound, erythema, and edema. About 10% presented nausea, vomiting, abdominal pain, numbness, dizziness, headache, and fever. Treatments included washing by irrigation, applying tape to the sting site and pulling it off (to remove spines), and applying ice packs and baking soda, in addition to antihistamines, corticosteroids, or analgesics administration22.
After investigating how a disturbance affects M. opercularis abundance, using netting to cover live oak trees (Quercus virginiana) to exclude urban pest birds in Texas, results demonstrated its increase in more than 7,300% on netted versus non-netted trees. This result emphasizes the ecological alteration consequences among species interactions, the potential risk on human health, and the need of considering ecology disturbance under urban planning strategies23. One case was a 14-month-old boy who presented symptoms related to this species sting (M. opercularis venom causes a painful and pruritic cutaneous reaction). The patient recovered after washing the affected area and antihistamine administration, but was admitted again to the Care Center with lepidopterism after visiting a park. Special care should be implemented with atopic and asthmatic patients, since although rare, anaphylactic reactions to the venoms have been reported24.
In South America, after analyzing the progress of pain complaint and its outcome by erucism caused by larvae of several moths (Automeris spp., Hylesia spp., Lonomia spp., Megalopyge spp., and Podalia spp.), testing five different analgesic schemes among stringed patients admitted in the emergency department in Campinas (southeastern Brazil), Branco et al.25 reported that the genus related to most erucism cases was Podalia, and that the combined use of local anesthesia plus oral analgesics improved management of severe pain among 278 treated patients. Most reports of envenomation caused by the genus Megalopyge in French Guiana, South and North America are related to M. opercularis. Nevertheless, three sting cases involving caterpillars of the genus Megalopyge reported there were identified as a different species. Clinical symptoms from three patients reporting sharping intense pain (injection of local anesthetic was required), leading to mobility limitation for two of the patients, were not associated with a visible skin lesion. Moreover, patients’ recovery occurred in a few hours, without side effects. From photographs of the caterpillar involved in one case, M. albicollis was suspected to be the responsible species26. However, involved species in our study was identified as M. opercularis.
Due to the multiple reports of affected people by similar larvae within mid-November 2015 and October 2016, and because of the biotechnological interest of caterpillar venom biomolecules17, the present study was aimed to identify the endemic venomous caterpillar collected in our region and evaluate some HEV and SSV biological activities on murine and human cells. This represents the first evaluation of their pharmacological and biotechnological potential to date.
HEV and SSV extracts were evaluated for cytotoxicity activity against murine L5178Y-R lymphoma cells and normal resident lymphocytes proliferation, as well as pro-inflammatory cytokine production by hPBMC and human plasma pro-coagulation activity. Heinen & Veiga reported cytotoxic potential of several compounds produced by caterpillars16. They mainly reported the role of cecropins, a group of peptides first isolated from the hemolymph of the giant silk moth Hyalophora cecropia L. (Lepidoptera: Saturniidae), which possess antitumor and antimicrobial activities20. In addition, the “fire caterpillar” Lonomia obliqua Walker (Lepidoptera: Saturniidae) venom produces antimicrobial peptides and antitumor activity enzymes (mainly hyaluronidases and phospholipases)27. Since resistance against conventional chemotherapy increases, cationic anticancer peptides, as those produced by insects are feasible alternatives28.
M. opercularis extract has proteolytic activity29. After skin contact, its hollow spines release an uncharacterized venom7. Therefore, M. opercularis is being considering a phanerotoxic species, since it produces and releases its venom throughout spines or setae, whereas among cryptotoxic species, the produced venom remains in the hemolymph11. The spines and the hemolymph venoms have similar compounds due to the spines proximity with the hemolymph and the lack of barriers to separate them11,30,31. Lepidopteran spines extracts may contain a wide variety of active compounds, such as the storage protein (assembled from six ∼80 kDa polypeptide subunits) that represents the major component of hemolymph32.
We observed that HEV and SSV extracts were cytotoxic against L5178Y-R murine lymphoma cells by the mechanism of apoptosis (Fig. 1), but stimulated proliferation of normal lymphocytes (Fig. 2). In this regard, Heinen et al.15 showed cytotoxic effect after treating Chinese hamster fibroblasts (V-79) with Lonomia obliqua caterpillars crude venom extracts for 24–48 h, at concentrations ranging from 5 to 450 µg/mL, as measured by the MTT colorimetric reduction assay. This work supported concentrations, times, and viability staining procedure used in the present study.
In addition, HEV and SSV extracts showed potential to stimulate IL-1β, IL-6, IL-8, and TNF-α by hPBMC (Fig. 3). Cytokines are known to regulate host immune responses to infection, cancer, and tissue damage. Although IL-1β, IL-6, IL-8, and TNF-α are pro-inflammatory cytokines serving as immune response activators, when they are overexpressed as in an acute response, their effects are exacerbated causing fever, inflammatory processes, tissue destruction, and even death by anaphylactic shock33.
On the other hand, thrombosis is considered a major cause of mortality nowadays34. The coagulation process is regulated by inhibitors that control clot formation and thrombosis, which may be altered by increased pro-coagulant activity or production of inhibitors35. Considering that the hemorrhagic syndrome resulting from L. obliqua sting, venom may contain both fibrinolytic and procoagulant activities. In this regard, Veiga et al.13 found that in addition to the procoagulant activity related to prothrombin activation, L. obliqua venom contains at least one fibrinogen/fibrinolytic activity, thus authors suggested the potential use of L. obliqua venom as anti-thrombotic agent. Since coagulation alterations have been reported by Lepidoptera’ toxins3, in the present study the pro-coagulation effect of HEV and SSV extracts on human plasma was evaluated, showing strong coagulation properties (Fig. 4). Similarly, two species of Lonomia caterpillars, L. obliqua and L. achelous Cramer have been reported to produce toxins that may lead to potentially fatal coagulation activity3.
In conclusion, M. opercularis HEV and SSV extracts showed antitumor activity against L5178Y-R murine lymphoma cells, involving apoptosis as the cell death mechanism, but stimulated murine proliferation of normal thymus lymphocytes. However, additional experiments using other tumor cell lines are needed to elucidate their full antitumor potential. In addition, HEV and SSV extracts induced production of the pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α by human PBMC. Furthermore, the extracts showed a potent pro-coagulant effect on human plasma. Overall, results demonstrated that M. opercularis HEV and SSV extracts possess anti-tumor, pro-inflammatory, and pro-coagulant activities. This study represents an initial exploration on the bioactivity of M. opercularis HEV and SSV venoms, whose results demonstrated their potential for biomedicine application.
Methods
Ethical statement
All methods involving human samples were performed in accordance with Institutional guidelines and regulations. Volunteers donating blood samples for experiments in this study provided a signed informed consent and remained anonymous. The donor sample consent informs and the assay involving human samples were reviewed and approved by the Institutional Ethics Committee at Autonomous University of Nuevo Leon (UANL). Experiments related to the use of animals were reviewed and approved by the Institutional Committee for Research Ethics and Animal Welfare of “The College of Biological Sciences” (CEIBA) at UANL with application number CEIBA-2017-005, following Mexican regulation NOM-062-ZOO-1999 entitled Technical Specifications for the Production, Care and Use of Laboratory Animals, normative that aligns with the guidelines and basic principles in the NIH Guide for the Care and Use of Laboratory Animals. In addition, standard ethical guidelines for ascites tumor induction in mice and rats36 were followed for experiments involving tumor cells obtained from tumor-bearing mice.
Reagents, culture media, and tumor cell line
Penicillin–Streptomycin solution, and RPMI 1640 and AIM-V media were obtained from Life Technologies (Grand Island, NY). Fetal bovine serum (FBS), Actinomycin D, dimethyl sulfoxide (DMSO), and 3-[4,5-dimethyl thiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). Taq & Go Master Mix 5X, pGEM-T Easy plasmid, and all molecular biology reagents were obtained from Promega (Madison, WI). Oligonucleotides were synthesized by Integrated DNA Technologies (UNIPARTS S.A., Monterrey, N.L., Mexico).
The tumor cell line L5178Y-R (mouse DBA/2 lymphoma) was obtained from The American Type Culture Collection (Rockville, MD), and maintained in culture flasks with RPMI 1640 medium supplemented with 10% FBS, 1% L-glutamine, and 0.5% Penicillin–Streptomycin solution (referred as complete RPMI 1640 medium) at 37 ºC, in a humidified atmosphere of 5% CO2 in air. Cellular density was kept between 105 and 106 cells/mL.
Animals and tumor intraperitoneal implantation
Six- to eight-week old BALB/c female mice were purchased from Harlan Mexico S.A. de C.V. (Mexico, D.F.). Regarding housing conditions, up to five animals per cage were kept in a pathogen- and stress-reduced environment at 24 °C, under a light–dark cycle (light phase, 06:00–18:00 h) in a One Cage 2100 System (Lab Products, Inc., Seaford, DE) and given water and food ad libitum36. Three mice were used for L5178Y-R lymphoma induction, which was performed by intraperitoneal (i.p.) administration of 0.2 mL of L5178Y-R tumor cells suspension (5 × 106 cells/mouse). After 13 d inoculation, mice were euthanized by cervical dislocation and peritoneal cavity ascites was collected. The ascites suspension was placed in a 50 mL tube containing 10 mL PBS for in vitro cytotoxicity assays37.
Insect source and rearing conditions
Venomous caterpillars were collected from the escarpment live oak Quercus virginiana var. fusiformis Mill. (Fagaceae) trees growing in the Cumbres National Park of Sierra Madre Oriental, in Monterrey, Nuevo Leon, located northeastern México at 25° 42′ 28.8″ N and 100° 22′ 11.4″ W. Insects collection was performed with a collaboration of Biological Science College (UANL) and the Environmental Education Program of the Wild-Life Cumbres National Park of Nuevo Leon State (Parques y Vida Silvestre, https://www.nl.gob.mx/servicios/programa-de-educacion-ambiental). Collected larvae and escarpment live oak leaves were placed inside of a 2-L glass jar with a 2 cm × 2 cm open square metallic cap, covered with wire mesh screen for air exchange. Collected material was transported to the laboratory for larval rearing. Jars with larvae and leaves were incubated at 25 ± 2 °C, 65% ± 5% relative humidity, and 16:8 h light:darkness cycles, inside of a rearing insect room. Larvae were fed on fresh escarpment live oak leaves, previously rinsed in tap water for 30 s. Incubated larvae were tested after reaching the fourth instar. Extra reared larvae were kept feeding until reaching the pupa stage, followed by adults’ emergence, in order to generate and maintain new insect colonies for further experiments.
Caterpillar venom molecular identification
DNA from three fourth instar caterpillar larvae was extracted, using the Wizard Genomic DNA Purification Kit (Promega) and following the isolating genomic DNA from tissue culture cells and animal tissue protocol. DNA extract was used as a template for PCR amplification of specific primers for the cytochrome oxidase subunit (COI) F1 5′AAC WYT ATA YTT TAT TTT TGG 3′ R and 5′TGT TGR TAW ARR ATW GGR TC 3′, designed from Genbank Megalopyge genus sequences.
PCR was performed using GoTaq Green Master Mix (Promega) in a 50 µL volume, with 100 ng of DNA as template and 1 µM of forward and reverse primers. Thermal cycling conditions included an initial denaturation step at 94 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 40 s, annealing at 60 °C for 40 s, and elongation at 72 °C for 2 min.
Amplified PCR products were ligated into pGEM-T Easy (Promega) in competent E. coli TOP-10 cells. Detected plasmids were purified using the Wizard Plus SV Minipreps DNA Purification System. Sanger sequencing was performed with standard vector M13F and M13R primers by the Instituto de Biotecnología at Universidad Nacional Autónoma de México. The sequence obtained was analyzed on platform Boldsystem.
HEV and SSV spine setae samples
HEV was obtained by performing a puncture on the third false leg from each larva head. Released fluid (~ 200 µL) was collected and centrifuged at 9,600 rpm for 2 min. The resulting supernatant protein content was quantified on a NanoDrop Lite kit and adjusted to 1 mg/mL. This was used as a stock for further dilution and dosage preparations38. In addition, SSV was obtained from four reared fourth instar venomous caterpillars, extracted according to da Silva et al.39. Spine setae were cut from the caterpillars’ integument, homogenized, sonicated in sterile PBS, and processed as described for HEV.
HEV and SSV cytotoxicity against murine L5178Y-R lymphoma cells
To determine the direct in vitro effect of HEV and SSV on tumor cell growth, L5178Y-R cell suspensions (from i.p. lymphoma grown in female BALB/c mice as explained above) were adjusted to 5 × 104 cells/mL in complete RPMI 1640 medium. We evaluated the antitumor effect of a broad range of concentrations of HEV and SSV, following the cytotoxicity assay previously described15. One hundred microliters of the cell suspensions were then added to flat-bottomed 96-well plates (Becton Dickinson, Lincoln Park, NJ), containing triplicate cultures (100 µL) of complete RPMI 1640 medium (unstimulated control), HEV or SSV (7.8–500 µg/mL)37, using 3.1–125 µg/mL Vincristine (Sigma-Aldrich), as positive control. After incubation for 44 h at 37 °C in 5% CO2, MTT (0.5 mg/mL, final concentration) was added, and cultures were incubated for additional 4 h. Cell cultures were then incubated for 16 h with 100 µL DMSO to dissolve formazan crystals, and optical densities (ODs) were read in a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT) at 540 nm37. Percentage of cytotoxicity was calculated as follows:
The Statistical Package for the Social Sciences version 17.040, was used to calculate the inhibitory concentration at 95% (IC95), selecting the Probit analysis.
Apoptosis assay
Cellular death type resulting from HEV- or SSV-mediated L5178Y-R cytotoxicity was determined according to Reyna-Martínez et al.41. For this, 3 × 106 cells were exposed to HEV or SSV IC50 using flat-bottomed, 24-well plates (Becton Dickinson), and incubated for 24 h under the same conditions as for the cytotoxicity assay. Treated cells were aliquoted into microtubes, washed by centrifugation at 9,600 rpm (Sorvall ST16R Centrifuge; ThermoScientific, Pittsburgh, PA), and suspended in 500 μL of complete RPMI 1640 medium. Cells were then stained adding 1 μL of 100 μg/mL acridine orange and 1 μL of 100 μg/mL ethidium bromide, and incubated for 5 min. Next, cultured cells were washed three times by centrifugation 9600 rpm with 1 mL PBS and suspended in 100 μL of PBS 1×, after which 10 μL of cell suspension samples were observed in a fluorescence microscope adapted with a rhodamine filter (540–570 nm), using Actinomycin D (800 ng/mL) as positive control.
Uniform green stained cells were quantified as viable cells and spotty green or granular core cells were quantified as in early apoptosis. Orange dots or cells with large granules similar to those observed in early-apoptosis cells were quantified as in late apoptosis, whereas uniform orange hue cells were quantified as in necrosis42.
Staining cells results were validated by the DNA degradation method41, where DNA like-ladder fragmentation indicates apoptotic activity, whereas DNA smear represents cell death by necrosis. DNA extracted from 1 × 106 cells per treatment were tested using the AxyPrep Multisource Genomic DNA Miniprep kit (Axygen) in 1% agarose gel electrophoresis at 100 V for one hour. The gel was then stained with 5 ng/mL ethidium bromide and analyzed on a GelDoc XR photo-documenter (Bio Rad, Berkeley, CA).
Lymphocyte proliferation assay
The effect of venom caterpillar HEV and SSV extracts on murine lymphocyte proliferation was determined by the MTT reduction colorimetric technique37. Two mice were euthanized and thymuses were immediately removed after mice death, a single cell-suspension was prepared by disrupting the organs in RPMI 1640 medium, as previously reported37. Cell suspensions were then washed three times in this medium, suspended, and adjusted to 1 × 107 cells/mL in complete RPMI 1640 medium. One hundred microliters of thymus cell suspensions were added to flat-bottomed 96-well plates (Becton Dickinson) containing triplicate cultures (100 µL) of complete RPMI 1640 medium (unstimulated control), HEV and SSV at 7.8, 15.6, 31.25, 62.5, 125, 250, and 500 µg/mL15,37, and the positive control Concanavalin A (6.25 μg/mL) for 48 h at 37 °C in 95% air-5% CO2 atmosphere. After 44 h of incubation, MTT (0.5 mg/mL, final concentration) was added, and cultures were incubated for additional 4 h. Cell cultures were then incubated for 16 h with 100 µL of DMSO and ODs, resulting from dissolved formazan crystals, were then read in a microplate reader (DTX 880 Multimode detector, Becton Dickinson, Austria) at 570 nm37. To calculate the lymphoproliferation index, the obtained values between the samples were compared. For this, values recorded by extracts treated cells were divided with the value given by Concanavalin A (tested as mouse T-cell mitogen) as follows: OD570 in treated cells/OD570 in Concanavalin A treated cells. Therefore, all values were compared with the control, where the lowest concentrations have a value of 1, since there was no difference compared with the control.
Human peripheral blood mononuclear cells (hPBMC) cytokine response to M. opercularis extracts
Cytokine production by hPBMC was measured after HEV and SSV extracts exposure. For this, hPBMC were isolated with Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and adjusted to 1 × 106 cells/mL in complete RPMI 1640 medium. One hundred microliters of the cell suspension were placed in a 96-well plate in the presence or absence (untreated control) of 100 μL of HEV or SSV M. opercularis extracts at 3.91, 7.81, 15.62, 31.25, 62.5, and 125 µg/mL15 in complete RPMI 1640 medium. Plates were then incubated at 37 °C for 48 h and centrifuged at 400 rpm for 5 min.
Cell‐free supernatants were then subjected to IL-1β, IL-6, IL-8, and TNF-α levels determination by cytometric bead arrays (CBA) (BD Biosciences, San Jose, CA) on a BD Accuri C6 Flow Cytometer Sampler (BD Biosciences, Ann Arbor, MI), following manufacturer's instructions, and data analyzed with the FCAP Array v3.0 (SoftFlow Inc.). Results were adjusted by subtracting the basal levels of cytokines from untreated hPBMC (negative control) and data analyzed by Prism 6 software (GraphPad Software Inc., La. Jolla, CA)43.
Coagulation assay
The effect of HEV and SSV activity on plasma coagulation was assessed, using the re-calcification time assay44, adapted for a microplate reader. For this, 1 mg/mL HEV and SSV reactive samples were prepared in 20 mM Tris–HCl buffer pH 7.4 and sterilized by filtration with a 0.22 μm micropore filter. Reactive samples consisted of 50 μL of citrated human plasma, 50 μL of HEV or SSV samples at 250 μg/mL (based on the concentration that produced maximal cytotoxicity in lymphoma cells), and 100 μL Tris–HCl buffer to a final volume of 200 μL. They were then incubated for 5 min at 37 °C, after which 10 μL of 150 mM CaCl2 were added for coagulative process re-activation, following the reaction during 23 min at 37 °C, and ODs were read in a microplate reader (Bio-Tek Instruments, Inc.) at 565 nm.
Statistical analysis
Results were expressed as means ± SD of triplicate determinations from three independent experiments. Statistical significance (p ≤ 0.05) was assessed by one-way analysis of variance and by the Student’s t test.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information file).
References
Gullan, P. J., & Cranston, P. The Insects: an Outline of Entomology, 3rd ed. Wiley-Blackwell, 499 p. Malden, MA. (2005).
Hossler, E. W. Caterpillars and moths. Dermatol. Ther. 22, 353–366 (2009).
Hossler, E. W. Caterpillars and moths: Part I. Dermatologic manifestations of encounters with Lepidoptera. J. Am. Acad. Dermatol. 62, 1–12 (2010).
Hossler, E. W. Caterpillars and moths: Part II. Dermatologic manifestations of encounters with Lepidoptera. J. Am. Acad. Dermatol. 62, 13–28 (2010).
Haddad, Jr. V., Cardoso, J. L. C., Lupi, O., & Tyring, S. K. Tropical dermatology: Venomous arthropods and human skin: Part I. Insecta. J. Am. Acad. Dermatol. 67, 331 e1-331.e14 (2012).
Battisti, A., Holm, G., Fagrell, B. & Larsson, S. Urticating hairs in arthropods: their nature and medical significance. Ann. Rev. Entomol. 56, 203–220 (2011).
Eagleman, D. M. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin. Toxicol. 46, 201–205 (2008).
Villas-Boas, I.M., Alvarez-Flores, M.P., Chudzinski-Tavassi, A.M., &Tambourgi, D.V. Envenomation by caterpillars. In: Gopalakrishnakone P., Faiz S., Gnanathasan C., Habib A., Fernando R., Yang CC (eds) Clinical toxicology, pp 1–17. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6288-6_57-1(2016).
Ardao, M. I., Perdomo, C. S. & Pellaton, M. G. Venom of the Megalopyge urens (Berg) caterpillar. Nature 209, 1139–1140 (1966).
Henwood, B. P. & MacDonald, D. M. Caterpillar dermatitis. Clin. Exp. Dermatol. 8, 77–93 (1983).
Diaz, J. H. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. Am. J. Trop. Med. Hyg. 72, 347–357 (2005).
De Roodt, A., Salomon, O. & Orduna, T. A. Accidentes por lepidópteros con especial referencia a Lonomia sp. Medicina. 60, 964–972 (2000).
Veiga, A. B. G., Blochtein, B. & Guimarães, J. A. Structures involved in production, secretion and injection of the venom produced by caterpillar Lonomia obliqua (Lepidoptera: Saturniidae). Toxicon 39, 1343–1351 (2001).
Greco, K. N., Mendonça, R. M., Moraes, R. H., Mancini, D. A. & Mendonça, R. Z. Antiviral activity of the hemolymph of Lonomia obliqua (Lepidoptera: Saturniidae). Antiviral Res. 84, 84–90 (2009).
Heinen, T. E. et al. Effects of Lonomia obliqua caterpillar venom upon the proliferation and viability of cell lines. Cytotechnology 66, 63–74 (2014).
Heinen, T. E. & Veiga, A. B. G. Arthropod venoms and cancer. Toxicon 57, 497–511 (2011).
Stipetic, M. E., Rosen, P. B., & Borys, D. J. A retrospective analysis of 96 “asp” (Megalopyge opercularis) envenomations in Central Texas during 1996. J. Toxicol.: Clin. Toxicol. 37, 457–462 (1999).
Sánchez, M. N., Sciani, J. M., Quintana, M. A., Martínez, M. M., Tavares, F. L., Gritti, M.A., Fan. H. W., Teiblerc, G. P., & Peichoto, M. E. Understanding toxicological implications of accidents with caterpillars Megalopyge lanata and Podalia orsilochus (Lepidoptera: Megalopygidae). Comp. Biochem. Physiol. C. 216, 110–119 (2019).
Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 31, 173–194 (2012).
Suttmann, H. et al. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 8, 5. https://doi.org/10.1186/1471-2490-8-5 (2008).
Mazzoni, M. K. F., de Carvalho, N. D., Rofatto, H. K., Moraes, R. E. P. & Mendonça, R. Z. Study of antiapoptotic effect of a protein isolated from Megalopyge albicollis (Lepidoptera: Megalopygidae) hemolymph. BMC Proc. 8, P148. https://doi.org/10.1186/1753-6561-8-S4-P148 (2014).
Forrester, M. B. Megalopyge opercularis caterpillar stings reported to Texas Poison Centers. Wilderness Environ. Med. 29, 215–220 (2018).
Hood, G. R., Comerford, M., Weaver, A. K., Morton, P. M. & Egan, S. P. Human-mediated disturbance in multitrophic interactions results in outbreak levels of North America’s most venomous caterpillar. Biol. Lett. 15(9), 20190470 (2019).
Konstat-Korzenny, E., Yudovich, A. & Morgenstern-Kaplan, D. Lepidopterism: case report and review of the literature. Cureus. 12(1), e6567 (2020).
Branco, M. M. P., Borrasca-Fernandes, C. F., Prado, C. C., Galvão, T. F., Silva, M. T., Mello De Capitani, E., Hyslop, S., & Bucaretchi, F. Management of severe pain after dermal contact with caterpillars (erucism): a prospective case series. Clin. Toxicol. 57, 338–342 (2019).
Torrents, R., Simon, N., Schmitt, C., De Haro, L. & Agha, M. Envenomation by caterpillars of the genus Megalopyge in French Guiana with an atypical clinical presentation, based on three observations. Clin. Toxicol. (Phila) 53, 844–845 (2015).
da CB Gouveia, A. I., da Silveira, R. B., Nader, H. B., Dietrich, C. P., Gremski, W., & Veiga, S. S. Identification and partial characterisation of hyaluronidases in Lonomia obliqua venom. Toxicon. 45, 403–410 (2005).
Dinarello, C. A. Proinflammatory cytokines. Chest 118, 503–508 (2000).
Avilán, L., Guerrero, B., Álvarez, E., & Rodríguez-Acosta, A. Description of envenomation by the “gusano-pollo” caterpillar (Megalopyge opercularis) in Venezuela. Inv Clín.-Rev. Med. IMSS. 51 127–132 (2010).
Pinto, F. M., Silva, R. L. M. & Guimara, J. A. Proteases from Lonomia obliqua venomous secretions: comparison of procoagulant, fibrin (ogen) olytic and amidolytic activities. Toxicon 47, 113–121. https://doi.org/10.1016/j.toxicon.2005.10.004 (2006).
Papo, N. & Shai, Y. Host defense peptides as new weapons in cancer treatment. CMLS. 62, 784. https://doi.org/10.1007/s00018-005-4560-2 (2005).
Haunerland, N. H. Insect storage proteins: gene families and receptors. Insect Biochem. Mol. Biol. 26, 755–765 (1996).
Vagdatli, E., Papapreponis, P. & Gounari, E. Investigation of potential pro-coagulation activity markers in healthy individuals. Hippokratia 19, 377 (2015).
Previtali, E., Bucciarelli, P., Passamonti, S. M. & Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transf. 9, 120–138 (2011).
Veiga, A. B. G., Pinto, A. F. & Guimarães, J. A. Fibrinogenolytic and procoagulant activities in the hemorrhagic syndrome caused by Lonomia obliqua caterpillars. Thromb. Res. 111, 95–101 (2003).
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer. 102, 1555–1577 (2010).
Gomez-Flores, R. et al. Antitumor properties of Gymnosperma glutinosum leaf extracts. Cancer Invest. 27(2), 149–155 (2009).
Valadez-Lira, J. A. et al. Physiological alterations of immune parameters on Trichoplusia ni (Lepidoptera: Noctuidae) larvae exposed to extremely low-frequency electromagnetic fields. Environ. Entomol. 46, 376–382 (2017).
da Silva, W. D. et al. Development of an antivenom against toxins of Lonomia obliqua caterpillars. Toxicon 34, 1045–1049 (1996).
SPSS. Version 17.0. SPSS Inc., an IBM Company. Chicago, IL (2008).
Reyna-Martinez, R. et al. Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State México. PeerJ. 6, e4358 (2018).
Coligan, J. E., Kruisbeek, A. M., Margulies, D. H., Shevach, E. M. & Strober, W. Current protocols in immunology. In Related isolation procedures and functional assays (ed. Coico, R.) 3172 (Wiley, New York, 1995).
Tighe, P., Negm, O., Todd, I. & Fairclough, L. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods 61, 23–29 (2013).
Hougie, C. Fundamentals of blood coagulation and clinical medicine 25–48 (McGraw-Hill, New York, 1963).
Acknowledgements
This research was supported by the Consejo Nacional de Ciencia y Tecnología (Conacyt-México), scholarship to Alonso A. Orozco Flores (260650), and Sistema Nacional de Investigadores (SNI) to José A. Valadez-Lira (100687), Ricardo Gomez-Flores (9942), Diana Caballero-Hernández (97102), Reyes Tamez-Guerra (11925), Cristina Rodríguez-Padilla (11924) and Patricia Tamez-Guerra (16614), and by the Laboratorio de Inmunología y Virología (LIV-DEMI-FCB-UANL).
Author information
Authors and Affiliations
Contributions
A.A.O.F., J.A.V.L. and P.T.G. contributed to experimental design, its implementation and analysis, and manuscript writing. K.E.C.C., J.J.P.T., and R.G.F., contributed to the experimental design, data analysis and manuscript writing. D.C.H., R.T.G., and C.R.P. contributed to data analysis and interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orozco-Flores, A.A., Valadez-Lira, J.A., Covarrubias-Cárdenas, K.E. et al. In vitro antitumor, pro-inflammatory, and pro-coagulant activities of Megalopyge opercularis J.E. Smith hemolymph and spine venom. Sci Rep 10, 18395 (2020). https://doi.org/10.1038/s41598-020-75231-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75231-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.