Abstract
There has been insufficient investigation of the differences in long-term outcomes between surgical resection (SR) and radiofrequency ablation (RFA) among patients with hepatocellular carcinoma (HCC) and esophagogastric varices (EGV). We retrospectively enrolled 251 patients with treatment-naïve HCC and EGV who underwent SR or RFA as a first-line treatment. Prognostic factors were analyzed using a Cox proportional hazards model. A total of 68 patients underwent SR, and the remaining 183 patients received RFA. Patients who underwent SR were younger, had better liver functional reserves, and had larger tumors. After a median follow-up duration of 45.1 months, 151 patients died. The cumulative 5-year overall survival (OS) rate was significantly higher among patients who underwent SR than those treated with RFA (66.7% vs. 36.8%, p < 0.001). Multivariate analysis showed that age > 65 years, multiple tumors, RFA, albumin bilirubin grade > 1, and the occurrence of major peri-procedural morbidity were the independent risk factors that are predictive of poor OS. In conclusion, SR could be recommended as a first-line treatment modality for HCC patients with EGV if the patients are carefully selected and liver function is well preserved.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death among males and the sixth among females1. Worldwide, it is estimated that around 745,000 patients die of HCC annually1. Vaccination programs for hepatitis B virus (HBV) have been successfully implemented for newborns, and antiviral therapy is widely prescribed for chronic HBV or hepatitis C virus (HCV) infection. Thus, the incidence and mortality of HCC have seemed to decline in several traditionally high-risk countries in East Asia2,3. Nevertheless, the prevalence rates of HCC are still rising in North America and Europe, especially among the elderly3,4. In the United States, the age-adjusted annual incidence rate of HCC increased from 4.4 to 6.7 per 100,000 individuals between 2000 and 20124. Moreover, the prevalence of HCC for patients on a waitlist for liver transplant increased from 6.4% in 2002 to 22.0% in 20175.
With advances in surveillance programs for HCC, more and more patients are diagnosed of HCC at an early stage6. For patients with HCC in stage 0 or A in the Barcelona Clinic Liver Cancer (BCLC) system, the recommended curative treatment modalities are liver transplantation, surgical resection (SR), and local ablation therapy according to the current guidelines for HCC management7. Among local ablation therapies, percutaneous radiofrequency ablation (RFA) could result in more reliable tumor ablation effects, fewer treatment sessions, lower rates of local recurrence, and higher overall survival (OS) rates than percutaneous ethanol injection therapy8,9,10. Moreover, due to the shortage of organs for liver transplantation, SR and RFA are the most commonly applied therapies for patients with early-stage HCC in daily practice11.
Several studies have been conducted to compare the treatment efficacy and prognosis between SR and RFA for patients with early-stage HCC, but the results are inconsistent12,13,14,15,16. This might be due to differences in the etiologies, liver functional reserves, and tumor factors among the studies. These factors should be taken into consideration when choosing the optimal treatment modality for patients with early-stage HCC.
Most patients with HCC have an underlying advanced chronic liver disease, such as chronic HBV or HCV infection, alcoholism, or nonalcoholic steatohepatitis17,18. With the progression of liver injury and fibrosis, portal hypertension might develop over time. Esophagogastric varices (EGV) start to emerge when the hepatic venous pressure gradient (HVPG) level is greater than 10 mmHg, which is considered to indicate clinically significant portal hypertension (CSPH). This leads to the development of ascites, hepatic encephalopathy, and EGV bleeding with increases in HVPG levels. These conditions are recognized as hepatic decompensation19,20. Moreover, CSPH and EGV have been identified as poor prognostic factors for patients with cirrhosis or HCC18,21,22. However, the measurement of HVPG levels is costly and not applicable in most hospitals, so EGV has served as a surrogate for CSPH in clinical practice7,23.
According to the current international guidelines for the management of HCC, SR is recommended for only patients with a single nodule of any size, good performance status, well-preserved liver function, and normal serum bilirubin levels, as well as a lack of tumor-related symptoms, CSPH, extra-hepatic spread, and major vascular invasion7. This is based on several studies that indicate that the presence of CPSH would increase the risk of postoperative liver failure and reduce the OS for HCC patients who undergo SR22,24,25.
Instead, local ablation therapy is recommended for HCC patients with CSPH or EGV7. Nevertheless, thanks to the recent advances in perioperative management for patients with CSPH, the risk of surgery-associated death has been reduced26. Several studies have debated the role the SR in HCC patients with CSPH, and SR could yield acceptable long-term survival in this clinical setting26,27,28,29. Hence, for patients who have HCC concomitant with EGV, the benefits of SR in comparison to RFA have not been sufficiently investigated30. Therefore, we compared the outcomes of SR and RFA in HCC patients with EGV to elucidate this issue.
Results
Baseline clinical characteristics
The baseline demographic data of the patients examined are shown in Table 1. Patients who underwent SR were younger than those who received RFA. Both groups were predominantly male, but the ratio of males-to-females was higher in the SR group. Chronic HBV infection was more prevalent in the SR group than the RFA group (58.8% vs. 38.3%, p = 0.005). Liver functional reserves were better in the SR group, which had lower scores in the model for end-stage liver disease (MELD), more patients with Child–Pugh Grade A, more patients with albumin bilirubin (ALBI) grade 1, higher serum albumin levels, lower bilirubin levels, lower prothrombin time international normalized ratios (PT INRs), and higher platelet counts. Furthermore, tumor sizes were larger in the SR group than the RFA group (median size: 3.2 cm vs. 2.2 cm, p < 0.001).
There were 251 patients who had EGV that was confirmed by an esophagogastroduodenoscopy (EGD). Regarding EGV status, 209 of these patients had esophageal varices (EV) alone at the time of HCC diagnosis, while 2 patients had gastric varices (GV) alone. The remaining 40 patients had both EV and GV. Moreover, 131 (52.2%) patients received prophylaxis therapy for EV bleeding, including 21 patients with non-selective beta-blockers (NSBB), 75 patients with esophageal variceal ligation (EVL) therapy and 35 patients with NSBB and EVL combination therapy.
Compared to the SR group, the RFA group had more cases of high-risk EV (68.3% vs. 55.9%, p = 0.092), and more patients received prophylaxis therapy for EV bleeding (57.9% vs. 36.8%, p = 0.005). However, among those who had high-risk varices, 23 patients (60.5%) patients in the SR group received prophylaxis therapy, while 92 patients (73.6%) received it in the RFA group (p = 0.179).
The safety of SR and RFA in HCC patients with EGV
No patients in our cohort died during the operations, both the SR group and the RFA group. As shown in Tables 1 and 2, there were 40 patients (15.9%) who developed peri-procedural morbidity and 15 patients (6.0%) who had major morbidity. The SR group had more peri-procedural morbidity than the RFA group (35.3% vs. 8.7%, p < 0.001), but the rates of major morbidity were comparable between both groups (8.8% vs. 4.9%, p = 0.390). The 90-day mortality rates were 2.9% and 1.1% in the SR group and RFA group, respectively (p = 0.371).
Among the 68 patients in the SR group, 63 patients underwent conventional open liver resection (OLR), including 15 patients with major resection (defined as resection of three or more segments). Among the 5 patients who underwent laparoscopic liver resection (LLR), 2 patients had two segmentectomies, and the other 3 patients had one segmentectomy. The patients who underwent major resection had more major morbidity than those who received minor hepatectomy (26.7% vs. 3.8%, p = 0.025).
Factors associated with OS
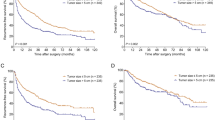
After a median follow-up duration of 45.1 (months interquartile range (IQR): 19.6–69.8 months), 151 patients died. In the SR group vs. the RFA group, the cumulative OS rates at 1, 2, 3, 5, and 10 years were 91.0% vs. 92.2%, 81.5% vs. 73.3%, 78.2% vs. 61.0, 66.7% vs. 36.8%, and 57.4% vs. 15.9%, respectively (Fig. 1; p < 0.001). As shown in Table 3, the multivariate analysis revealed that the independent risk factors for poorer OS were age > 65 years (hazard ratio (HR): 1.721; 95% confidence interval (CI): 1.213–2.441; p = 0.002), having multiple tumors (HR: 1.630; 95% CI: 1.129–2.354; p = 0.009), RFA (HR: 2.271; 95% CI: 1.427–3.616; p = 0.001), ALBI grade > 1 (HR: 1.583; 95% CI: 1.032–2.427; p = 0.035), and the development of major peri-procedure morbidity (HR: 3.201; 95% CI: 1.774–5.777; p < 0.001).
Risk factors associated with tumor recurrence
After therapy, 169 patients developed tumor recurrence, and the median recurrence time was 15.2 (IQR 7.3–31.5) months. In the SR group, 49 patients had tumor recurrence with a median development time of 20.8 (IQR 12.4–38.5) months. Among patients who underwent RFA, 120 of them developed tumor recurrence within a median time of 11.9 (IQR 5.4–27.2) months. Patients who underwent SR had a significantly higher rate of recurrence-free survival (RFS) than who received RFA (Fig. 2). As shown in the Table 4, the multivariate analysis showed that multiple tumors (HR 1.421, 95% CI 1.002–2.016, p = 0.049) and RFA (HR 1.583, 95% CI 1.128–2.221, p = 0.008) were associated with higher recurrence rates after therapy.
Discussion
This study shows that SR could provide acceptable long-term outcomes for patients with HCC and EGV. The 10-year cumulative OS rates were 57.4% and 15.9% for patients who underwent SR and RFA, respectively. The survival benefits of SR over RFA were confirmed by the multivariate analysis. Moreover, SR could provide a lower rate of recurrence and a higher RFS rate than RFA. This indicates that SR is not contraindicated for HCC patients with EGV. On the contrary, it could have a survival advantage over RFA if patients are carefully selected.
The presence of EGV, is a surrogate for CSPH and has been validated as an independent factor for poor prognosis among patients with HCC18,31,32. Several studies show that the incidence of developing liver decompensation after SR is high among HCC patients with CSPH, which would increase the risk of mortality24,25,33 (Table 5). Consequently, it has been suggested that SR be reserved for patients without CSPH in the current guidelines for the management of HCC, whereas RFA is recommended for HCC patients with CSPH23,34.
Nevertheless, recent technical innovations in surgical techniques, anesthesia, critical care, and spatial understanding of the intra-hepatic anatomy of the liver have led to an increasing number of liver resections, fewer post-operative hepatic failures, and lower treatment-related mortality26,35. As shown in Table 5, several studies from Eastern and Western countries have validated that CSPH alone is not a contraindication for SR33,36,37 These findings suggest that the indications for SR could be extended to HCC patients with CSPH or EGV if they have well-preserved liver function.
For patients with early-stage HCC, RFA is relatively safe and has lower costs, less serious adverse effects, and less destruction of non-neoplastic tissue than SR7. Moreover, it could provide an acceptable long-term OS for certain patients38,39,40. It has been reported that 5-year accumulative OS rates over 60% could be achieved with RFA among HCC patients with early-stage HCC12,38,39,40. In our previous study, the 5-year and 10-year OS rates after RFA were 63.1% and 48.7%, respectively40. Consequently, RFA is regarded as a curative treatment modality for HCC patients7.
However, the recurrence rate after RFA is still high. For example, the 10-year RFS rate after RFA was only 12.4% in our previous report40. This might be caused by the incomplete ablation of liver tumors due to insufficient ablation-needle technology, tissue cooling by the neighboring blood vessels (through a heat sink effect), large tumor masses, and the ablation of tumors in close proximity to heat-sensitive organs41.
In contrast, SR could have a higher chance of complete excision of not only tumor tissue but also the hepatic parenchyma around the tumor, which might have microvascular invasion and micro-metastases13,42. Therefore, it could result in better local tumor control than RFA. However, the risk of liver decompensation and mortality after the operation are concerns when performing SR for HCC patients with a poorer liver functional reserve. Several studies compared the outcomes between SR and RFA for patients with early-stage HCC12,13,14,15,16,43. Most of these studies demonstrated that SR could reduce the risk of recurrence and might provide superior OS to RFA, although some studies observed that the OS rates were comparable between SR and RFA12.
Nevertheless, the differences in prognosis between SR and RFA for HCC patients with CSPH have not yet been investigated sufficiently. Qiu et al. demonstrated that SR is safe and could provide a better OS and RFS than ablation therapy for patients with HBV-related HCC and CSPH30. However, the patients enrolled in that study were limited to those with HBV-related HCC, and the ablation therapies included both RFA (79 patients) and microwave ablation (57 patients).
In the current study, we enrolled HCC patients from all etiologies and compared the prognoses between patients who underwent SR and RFA. In the SR group, no patient expired during the surgeries, and only one patient died within one month due to post-operative liver failure. The patients who underwent SR had a higher rate of post-procedure morbidity than those in the RFA group, but the rates of major morbidity and 90-day mortality were not statistically different between the two groups. Notably, our cohort revealed that SR could offer better long-term prognoses than RFA among HCC patients with EGV in terms of OS and recurrence. This was further validated by the multivariate analysis. The results could provide robust evidence for performing SR as a front-line treatment modality for patients with HCC and EGV if they have well-preserved liver function.
The patients in our cohort who underwent RFA as the primary treatment modality were older and had a poorer liver functional reserve than those who underwent SR. Older patients might choose RFA because of the greater chance of comorbidities than younger patients. RFA features less invasiveness, a lower complication rate, and lower costs, as well as higher repeatability in the event of recurrence44. This finding is similar to those of a nationwide cohort from Japan45. However, the survival benefit of SR over RFA was persistent after adjusting for the confounding factors for prognosis in the multivariate analysis.
There were some limitations to our study. First, although EGD was recommended to screen for EGV among patients with a new diagnosis of HCC, the completion rate was only 48.6% in this cohort (Fig. 3). Selection bias might be present because of the retrospective nature of the study. Second, measuring HVPG levels is the gold standard for assessing the degree of portal hypertension. However, it is invasive, expensive and not feasible in most medical centers. We did not perform HVPG measurement in out cohort. Furthermore, the spleen diameter was not recorded uniformly due to the retrospective study design. Therefore, we used the presence of EGV diagnosed by EGD as a surrogate for CSPH, which is more practical in the daily practice. Third, decisions made for treatment were shared between the physicians and patients. This patient-tailored approach is based on the multidisciplinary evaluation of each case and includes any alternative treatment options. This might have caused the significant demographic difference between the two groups of patients. Fourth, LLR is a recent technical innovation in SR for the treatment of HCC. It could provide shorter hospitalization, less blood loss, less wound pain, and a lower rate of postoperative liver failure and ascites formation than conventional OLR for cirrhotic patients with HCC46,47,48. Regarding the long-term outcomes, HCC patients who underwent LLR had similar OS and RFS rates to those who received OLR46,47,48,49. However, we could not compare the treatment efficacy and outcomes between LLR and OLR because the majority of patients in our cohort underwent OLR and only 5 patients received LLR. Further prospective studies are warranted to elucidate this issue. Lastly, this study enrolled HCC patients over a relatively long span of time, so the diagnoses, assessment of HCC patients, and SR and RFA techniques might not have been the same between different time periods.
Despite these limitations, this study provides robust evidence to reassure physicians that SR could serve as a first-line treatment option for HCC, in spite of evidence of CSPH and EGV. However, the patients should be selected carefully. Consequently, aggressive treatments beyond the current guidelines could be considered when clinically applicable to achieve the maximum survival benefit.
Conclusion
SR could be recommended as the first-line treatment modality for HCC patients with EGV if the patients are carefully selected and liver function is well preserved.
Methods
Patients
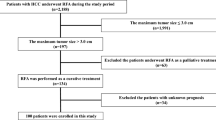
This study retrospectively enrolled 251 treatment-naïve HCC patients who underwent SR or RFA as the first treatment modality for HCC. All of the patients had EGV diagnosed by EGD at the time of HCC diagnosis at Taipei Veterans General Hospital (Fig. 3). EV was classified as follows: F1: small and straight varices; F2: moderately sized, tortuous varices; and F3: large, tumorous varices50. High-risk EV was defined by the F2 and F3 classifications or by F1 accompanied by red coloring51.
The diagnosis of HCC was established based on the criteria from the American Association for the Study of Liver Disease consensus52. Multidisciplinary expert meetings and an HCC registration system in Taipei Veterans General Hospital have been conducted since October 2007. The diagnosis and treatment strategy are discussed at weekly multidisciplinary meetings for all of the HCC patients at this hsopital53. The decision about the treatment modality is shared between the patient and the physician after discussing the risks, benefits, complications, and efficacies of the available treatments, as well as the recommendations from the multidisciplinary expert meetings.
In our center, the indications for SR are as follows: (1) Child–Pugh grade of liver function of A or B, with an indocyanine-green 15-min retention rate (ICG-15R) of ≤ 30%; (2) tumor involving no more than two Healey’s segments without involvement of the main portal vein trunk; and (3) the absence of extra-hepatic tumor dissemination17. RFA was performed in cases of (1) a single tumor with size < 5 cm or 2‒3 tumors < 3 cm; (2) no extra-hepatic metastasis or major vascular invasion; (3) Child–Pugh grade A or B; (4) platelet count > 50,000 /mm3; (5) the absence of ascites; and (6) no other comorbid diseases that might complicate RFA40. Liver transplantation was indicated for patients with end-stage liver disease, HCC, or acute liver failure according to the criteria of United Network for Organ Sharing (UNOS)54. For patients with HCC, the tumor size criterion for liver transplantation was based on the criteria of the University of California San Francisco (UCSF)55.
The indications for SR and RFA were not the same, so we selected HCC patients using the Milan criteria for this study. Consequently, the inclusion criteria were as follows: (1) solitary tumor with size < 5 cm, in which anatomic resection could be achieved after through evaluation or 2‒3 tumors < 3 cm; (2) no extra-hepatic metastasis or major vascular invasion; (3) grade A or B Child–Pugh classification of liver function; (4) platelet count > 50,000 /mm3; (5) absence of ascites; and (6) no other comorbid diseases that might complicate SR or RFA (e.g., infection, arrhythmia, acute myocardial infarction, uncontrolled congestive heart failure, chronic obstructive pulmonary disease with acute exacerbation, recent stroke, etc.).
There were 2958 consecutive patients who were enrolled in the HCC registration system database from 2008 to 2017. As shown in Fig. 3, 1465 of these patients received an EGD, and 625 patients had EGV at the time of HCC diagnosis. Patients were excluded for having tumor size > 5 cm, tumor number > 3, ascites or major vascular invasion. After the exclusion, 51 patients were enrolled in the SR group, and 163 patients were enrolled in the RFA group. Moreover, we also retrospectively recruited 17 patients in the SR group and 20 patients in the RFA group who fulfilled the inclusion criteria between 2003 and 2017 before the establishment of the HCC registration system. Consequently, a total of 68 patients who underwent SR and 183 patients who received RFA were enrolled in the final analysis.
After SR or RFA, the peri-procedural morbidities were recorded and graded by the Clavien-Dindo classification56. Grade III-V complications were defined as major morbidities. Postoperative liver failure was defined according to the International Study Group of Liver Surgery (ISGLS)57. All of the patients were followed up regularly every 3 months after SR or RFA until their last visit to our hospital or death.
This study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. Approval was obtained from the Institutional Review Board (IRB) of Taipei Veterans General Hospital (VGHIRB No. 2018-07-029BC). Informed consent was obtained before the patients underwent SR or RFA.
Biochemical and serological markers
Serum biochemistry was measured using a Roche/Hitachi Modular Analytics System (Roche Diagnostics GmbH, Mannheim, Germany). Serum alpha-fetoprotein (AFP) levels were tested using a radioimmunoassay kit (Serono Diagnostic SA, Coinsins, Switzerland). The ALBI score was calculated using the following equation: (– 0.085 × albumin in g/L) + (0.66 × log10 bilirubin in μmol/L)58. The ALBI grades were defined as grade 1 (score ≤ – 2.60), grade 2 (score > − 2.60 and ≤ – 1.39), or grade 3 (score > – 1.39).
Statistical analyses
The primary endpoint of the study was OS, which was calculated from the date of HCC diagnosis until death, the last visit, or loss to follow-up. Pearson’s chi-squared analysis or Fisher’s exact test was used to compare categorical variables. The medians with IQRs were used to express continuous variables and compared using the Mann–Whitney U test.
Cumulative rates of OS and RFS were estimated by the Kaplan–Meier method, and the results were compared using a Cox proportional hazards model. Variables that had statistical significance (p < 0.05) or were proximate to it (p < 0.1) in the univariate analysis were included in a multivariate analysis, which was conducted using a forward stepwise Cox regression model. The ALBI scores were derived from serum albumin and bilirubin levels, so we used the ALBI grade but not the serum albumin and bilirubin levels in the multivariate analysis.
A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. https://doi.org/10.3322/caac.21262 (2015).
Kulik, L. & El-Serag, H. B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156, 477–491e471. https://doi.org/10.1053/j.gastro.2018.08.065 (2019).
Choo, S. P., Tan, W. L., Goh, B. K. P., Tai, W. M. & Zhu, A. X. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 122, 3430–3446. https://doi.org/10.1002/cncr.30237 (2016).
White, D. L., Thrift, A. P., Kanwal, F., Davila, J. & El-Serag, H. B. Incidence of hepatocellular carcinoma in All 50 United States, from 2000 to 2012. Gastroenterology 152, 812–820e815. https://doi.org/10.1053/j.gastro.2016.11.020 (2017).
Younossi, Z. et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 17, 748–755e743. https://doi.org/10.1016/j.cgh.2018.05.057 (2019).
Kudo, M. Management of hepatocellular carcinoma in japan as a world-leading model. Liver Cancer 7, 134–147. https://doi.org/10.1159/000484619 (2018).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462. https://doi.org/10.1056/NEJMra1713263 (2019).
Lin, S., Lin, C., Lin, C., Hsu, C. & Chen, Y. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 54, 1151–1156 (2005).
Lencioni, R. & Crocetti, L. Local-regional treatment of hepatocellular carcinoma. Radiology 262, 43–58. https://doi.org/10.1148/radiol.11110144 (2012).
Germani, G. et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J. Hepatol. 52, 380–388 (2010).
Kao, W. Y. et al. Prognosis of early-stage hepatocellular carcinoma: the clinical implications of substages of Barcelona clinic liver cancer system based on a cohort of 1265 patients. Medicine (Baltimore) 94, e1929. https://doi.org/10.1097/MD.0000000000001929 (2015).
Pompili, M. et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J. Hepatol. 59, 89–97. https://doi.org/10.1016/j.jhep.2013.03.009 (2013).
Hung, H. H. et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin. Gastroenterol. Hepatol. 9, 79–86. https://doi.org/10.1016/j.cgh.2010.08.018 (2011).
Hasegawa, K. et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J. Hepatol. 58, 724–729. https://doi.org/10.1016/j.jhep.2012.11.009 (2013).
Huang, J. et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 252, 903–912. https://doi.org/10.1097/SLA.0b013e3181efc65600000658-201012000-00003[pii] (2010).
Feng, K. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 57, 794–802. https://doi.org/10.1016/j.jhep.2012.05.007 (2012).
Su, C. W. et al. Impact of steatosis on prognosis of patients with early-stage hepatocellular carcinoma after hepatic resection. Ann. Surg. Oncol. 22, 2253–2261. https://doi.org/10.1245/s10434-014-4221-5 (2015).
Hsieh, W. Y. et al. The impact of esophagogastric varices on the prognosis of patients with hepatocellular carcinoma. Sci. Rep. 7, 42577. https://doi.org/10.1038/srep42577 (2017).
D’amico, G. et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment. Pharm. Ther. 39, 1180–1193 (2014).
Garcia-Tsao, G. & Bosch, J. Varices and variceal hemorrhage in cirrhosis: a new view of an old problem. Clin. Gastroenterol. Hepatol. 13, 2109–2117. https://doi.org/10.1016/j.cgh.2015.07.012 (2015).
Giannini, E. G. et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 4, 1378–1384. https://doi.org/10.1016/j.cgh.2006.08.011 (2006).
Bruix, J. et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 111, 1018–1022 (1996).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
Llovet, J. M., Fuster, J. & Bruix, J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 30, 1434–1440 (1999).
Berzigotti, A., Reig, M., Abraldes, J. G., Bosch, J. & Bruix, J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 61, 526–536. https://doi.org/10.1002/hep.27431 (2015).
Ishizawa, T. et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 134, 1908–1916 (2008).
Vitale, A. et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona clinic liver cancer stages: a multicentre study. J. Hepatol. 62, 617–624 (2015).
Torzilli, G. et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann. Surg. 257, 929–937 (2013).
Chang, C. Y. et al. Esophageal varices are not predictive of patient prognosis after surgical resection of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 30, 1368–1377. https://doi.org/10.1097/MEG.0000000000001193 (2018).
Qiu, J. et al. Resection versus ablation in hepatitis B virus-related hepatocellular carcinoma patients with portal hypertension: a propensity score matching study. Surgery 158, 1235–1243. https://doi.org/10.1016/j.surg.2015.04.002 (2015).
De Franchis, R. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J. Hepatol. 63, 743–752 (2015).
Su, C. W. et al. Association between esophagogastric varices in hepatocellular carcinoma and poor prognosis after transarterial chemoembolization: a propensity score matching analysis. J. Formos. Med. Assoc. 119, 610–620. https://doi.org/10.1016/j.jfma.2019.09.003 (2020).
Cucchetti, A. et al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J. Hepatol. 64, 79–86. https://doi.org/10.1016/j.jhep.2015.08.025 (2016).
Fang, K.-C. et al. The impact of clinically significant portal hypertension on the prognosis of patients with hepatocellular carcinoma after radiofrequency ablation: a propensity score matching analysis. Eur. Radiol. 27, 2600–2609 (2017).
Schreckenbach, T., Liese, J., Bechstein, W. O. & Moench, C. Posthepatectomy liver failure. Dig. Surg. 29, 79–85. https://doi.org/10.1159/000335741 (2012).
Harada, N. et al. Surgical resection for hepatocellular carcinoma with concomitant esophageal varices. World J Surg 39, 2510–2518. https://doi.org/10.1007/s00268-015-3110-9 (2015).
Roayaie, S. et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 62, 440–451. https://doi.org/10.1002/hep.27745 (2015).
Shiina, S. et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 107, 569–577. https://doi.org/10.1038/ajg.2011.425 (2012).
Kim, Y. S. et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J. Hepatol. 58, 89–97. https://doi.org/10.1016/j.jhep.2012.09.020 (2013).
Kao, W. Y. et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology 285, 670–680. https://doi.org/10.1148/radiol.2017162382 (2017).
Lee, S. et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J. Hepatol. 69, 70–78 (2018).
Su, C. W. et al. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: a propensity score matching analysis. Arch. Surg. 147, 137–144. https://doi.org/10.1001/archsurg.2011.288 (2012).
Cucchetti, A. et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 59, 300–307. https://doi.org/10.1016/j.jhep.2013.04.009 (2013).
Santambrogio, R. et al. Surgical resection versus laparoscopic radiofrequency ablation in patients with hepatocellular carcinoma and Child-Pugh class a liver cirrhosis. Ann. Surg. Oncol. 16, 3289–3298 (2009).
Arii, S. et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology 32, 1224–1229 (2000).
Memeo, R. et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J. Surg. 38, 2919–2926. https://doi.org/10.1007/s00268-014-2659-z (2014).
Han, H. S. et al. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J. Hepatol. 63, 643–650. https://doi.org/10.1016/j.jhep.2015.04.005 (2015).
Wu, X. et al. Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: a propensity score matching study. Surg. Endosc. 33, 206–215. https://doi.org/10.1007/s00464-018-6296-8 (2019).
Vega, E. A. et al. Preoperative prognosticators of safe laparoscopic hepatocellular carcinoma resection in advanced cirrhosis: a propensity score matching population-based analysis of 1799 western patients. J. Gastrointest. Surg. 23, 1157–1165. https://doi.org/10.1007/s11605-019-04139-7 (2019).
Beppu, K. et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest. Endosc. 27, 213–218 (1981).
Chen, P. H. et al. Combination of albumin-bilirubin grade and platelets to predict a compensated patient with hepatocellular carcinoma who does not require endoscopic screening for esophageal varices. Gastrointest. Endosc. 88, 230-239e232. https://doi.org/10.1016/j.gie.2017.12.023 (2018).
Bruix, J. & Sherman, M. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022. https://doi.org/10.1002/hep.24199 (2011).
Fang, K. C. et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona clinic liver cancer stage A or B: the role of albumin-bilirubin grade. Liver Cancer 7, 335–358. https://doi.org/10.1159/000487407 (2018).
Schilsky, M. L. & Moini, M. Advances in liver transplantation allocation systems. World J. Gastroenterol. 22, 2922–2930. https://doi.org/10.3748/wjg.v22.i10.2922 (2016).
Yao, F. Y. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33, 1394–1403. https://doi.org/10.1053/jhep.2001.24563 (2001).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae (2004).
Rahbari, N. N. et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149, 713–724. https://doi.org/10.1016/j.surg.2010.10.001 (2011).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. 33, 550–558. https://doi.org/10.1200/JCO.2014.57.9151 (2015).
Acknowledgement
Part of this study was presented as a poster presentation at the 2019 Annual Conference of the International Liver Cancer Association, Chicago, USA, September 20–22, 2019. We thank Dr. Elise Chia-Hui Tan for her assistance with the statistical analysis.
Author information
Authors and Affiliations
Contributions
C.Y.W.: study concept and design; analysis and interpretation of data; drafting of the manuscript; G.Y.C.: study concept and design; performed surgical resection; analysis and interpretation of data; P.H.C.: study concept and design; analysis and interpretation of data; C.A.L.: study concept and design; performed RFA; Y.H.H.: study concept and design, valuable discussion and support; T.H.: study concept and design, valuable discussion and support; M.C.H.: study concept and design, valuable discussion and support; H.C.L.: study concept and design, valuable discussion and support; Y.H.S.: study concept and design, analysis and interpretation of data; J.C.W.: overall study concept and design; C.W.S.: overall study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content and final drafting of the manuscript. All authors reviewed and approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, CY., Chau, GY., Chen, PH. et al. A comparison of prognoses between surgical resection and radiofrequency ablation therapy for patients with hepatocellular carcinoma and esophagogastric varices. Sci Rep 10, 17259 (2020). https://doi.org/10.1038/s41598-020-74424-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74424-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.