Abstract
The Yunnan province has one of the most serious outbreaks of the plague epidemic in China. Small mammals and fleas are risk factors for the occurrence of plague in commensal plague foci. Understanding the relationship between fleas and small mammals will help control fleas and prevent the onset of the plague. Four hundred and twenty-one small mammals, belonging to 9 species, were captured. Of these, 170 small mammals (40.4%) were found infested with fleas. A total of 992 parasitic fleas (including 5 species) were collected. The number of Leptopsylla segnis and Xenopsylla cheopis accounted for 91.03% (903/992). The final multiple hurdle negative binomial regression model showed that when compared with Rattus tanezumi, the probability of flea infestation with Mus musculus as well as other host species decreased by 58% and 99%, respectively, while the number of flea infestations of the other host species increased by 4.71 folds. The probability of flea prevalence in adult hosts increased by 74%, while the number of fleas decreased by 76%. The number of flea infestations in small male mammals increased by 62%. The number of fleas in small mammals weighing more than 59 g has been multiplied by about 4. R. tanezumi is the predominant species in households in the west Yunnan province, while L.segnis and X. cheopis were dominant parasitic fleas. There is a strong relationship between the abundance of fleas and the characteristics of small mammals (e.g. Species, age, sex, and body weight).
Similar content being viewed by others
Introduction
Plague is a zoonotic bacterial infectious disease transmitted by fleas with reservoirs in rodent populations, in which fleas play a potentially fundamental role as bridge vectors to transmit the bacteria (Yersinia pestis) to animals and humans1. The potential role of fleas as carriers and host-supported local reservoirs can help explain the persistent occurrence of plague2.
The plague has played a huge role in human history and is still prevalent in most parts of China. In China, animal plague is reported almost every year, and occasional human plagues did occur3,4. Transmission to humans sometimes occurs through contact with fleas that have fed on an infected small mammal or by skinning infected small mammals (e.g. Marmot)5,6,7. The province of Yunnan is one of the most serious plague epidemic foci in China8,9, and especially the most active in commensal rodent plague foci2. The province of Yunnan is characterized by rich varieties of natural conditions, which high in the north and low in the south of the terrain, spanning 8 latitude zones and possessing a rich geographical landscape. Also it possesses an obvious vertical climate and incorporates in cold, temperate and tropical, consisting of embedded microhabitats that create a series of ecological niches. The above all increased possibility survival and reproduction of small mammals and fleas and risk spread and preservation of Y. pestis in them10.
Adults fleas are obligatory hematophagous insects and rodents are the most common host of fleas, although they can appear in other types of mammals and birds11,12. At different stages of the life cycle, fleas are located in the hosts or in the host's nest. In most cases, the imago flea is usually parasitic on the host and until the end of its life cycle, differentiating with the larvae. When leaving a pupa, a new flea of imago finds a host because the reproduction of most fleas is not possible without the feeding of blood13. Therefore, it is likely to handle flea infestations on live hosts. The parasites get their food and other biological supplies from their hosts. Consequently, they can affect the host directly by reducing host resources, and indirectly by provoking behavioral responses14,15 or immunological responses1,16. Fleas with high density and richness not only favor the bacteria plague, but also spread widely among the hosts, and also suppress the immune response of the hosts, thus improving the susceptibility of the hosts17,18, which is vital for the maintenance of the plague19,20.
The abundance of hosts is an important factor that affects the distribution and abundance of parasites. In terms of the reservoir, some individuals, populations or host species are characterized by a higher level of parasite infestation than others21. It is well known that there is a great variation in the abundance of a parasite among the different host species. The host with the highest abundance of parasites is commonly defined as the main host, while those with lower abundance of parasites are defined as auxiliary hosts21,22.
In this study, we have attempted to describe the distribution of small mammals and fleas and to examine the effect of small mammals on parasites abundance in the western province of Yunnan. The information on the factors that affect the flea abundance in hosts is essential to provide evidence-based recommendations on flea control and to implement plague control and prevention programs in plague natural foci.
Results
Distribution and description of small mammals
A total of 12,000 traps were placed in 800 households in 40 villages from 10 counties in the western Yunnan province and 421 small mammals were captured. The overall density of small mammals was 3.51%. Small mammals were divided into 9 species, 6 genera, 2 families and 2 orders (Table 1). Rattus tanezumi is the dominant species and it represents 66.03% (278/421) of the entire sample. Among the 10 counties, the highest number and the smallest number of small mammals were captured in Mangshi (100) and Deqin (16) counties, respectively. The number of small mammals richness was 9. The highest number and lowest number of richness were in Yongren (5), Nanhua (1) and Deqin (1) counties, respectively. (Table 1).
In this study, the weight, body length and tail length of 407 small mammals were calculated (for other 14 small mammals there was no calculation due to incomplete data). The mean body weight was 62.93 g, standard deviation 42.67 g, median 59.31 g (range 7.39–186.89 g); the average body length was 22.79 cm, the standard deviation of 8.57 cm and the median 21.00 cm (range 7.50–39.30 cm); the average length of the tail was 12.30 cm, the standard deviation of 3.76 cm and the median 13.30 cm (extremes: 1.00 and 20.00 cm).
Distribution of parasitic fleas
Of 421 small mammals, 170 small mammals were infested with fleas and their prevalence was 40.38%. A total of 992 fleas, divided into 5 species, 5 genera, and 3 families, were collected and the flea index was 2.36 (992/421). The highest flea prevalence and the highest flea index occurred in Yunxian county (71.79%) and Yulong county (7.33), respectively. Out of 992 fleas, the number of Xenopsylla cheopis and Leptopsylla segnis was 903, which accounted for 91.03%. The proportion of X. cheopis exceeds 90% in Yunxian and Gengma counties (Table 2).
Among the 9 small mammal species, the flea prevalence of R. tanezumi was highest (53.60%, 149/278). The infestation of R. tanezumi by X. cheopis was particularly high (35.61%, 99/278). Crocidura attenuata and Anourosorex squamipes were not infested with fleas (Table 3).
The results of the prototype multiple HNB regression model
Table 4 shows the prototype multiple HNB regression model for the abundance of fleas. Of the six potential factors, compared to R. tanezumi, the probability of flea infestation of Mus musculus and other host species decreased, while the number of flea infestations of other host species increased. The number of fleas in adult hosts decreased; the number of fleas in small mammals weighing more than 59 g increased (Table 4).
The results of the final multiple HNB regression model
The final model showed that four factors—species, age, sex and body weight of small mammals—are closely associated with the abundance of flea (Table 5). Compared to R. tanezumi, the probability of flea infestation with M. musculus as well as other host species decreased by 58% and 99% respectively, while the number of flea infestations of the other host species increased by 4.71 folds. The probability of flea prevalence in adult hosts increased by 74%, while the number of fleas decreased by 76%. The number of flea infestations in small male mammals increased by 62%. The number of fleas in small mammals weighing more than 59 g increased by about 4 times (Table 5).
Discussion
In this study, 421 small mammals were captured and R. tanezumi was the predominant species. A total of 992 fleas were collected from small mammals. The number of L. segnis and L. cheopis was accounted for 91.03%. A close relationship between fleas’ abundance and small mammal traits (including species, age, body weight, sex) was evident in households in the western Yunnan province.
The results of our investigation showed that R. tanezumi is the predominant species in households in the western Yunnan province. X. cheopis and L. segnis are dominant fleas, and R. tanezumi is their main host. These results are consistent with the previous study that R. tanezumi is the dominant rodent species in commensal plague foci, and X. cheopis and L. segnis are dominant parasitic fleas23. In the commensal plague foci of the western Yunnan province, R. tanezumi is the main host species, while M. musculus is the auxiliary host species. Yin et al.24 have suggested that the abundance of fleas is related to host species. Our data indicated that R. tanezumi was more likely to be infested with fleas than M. musculus and other species. The main host may or may not be the species in which the parasite evolved for the first time, but it’s currently home to most individuals in the parasite population22. It is therefore believed that R. tanezumi is the best species in which the majority of parasites live and the probability of flea infestation on M. musculus as well as other species has decreased compared to R. tanezumi. The reason for the increase in the number of fleas in other species could be due to the fact that the number of flea infestations on other species was sufficient, although the number of other host species is lower in other species in our study, like Apodemus chevrieri. In addition, the fundamental reasons for the diversity in parasitic abundance between the main host and any auxiliary host are often related to the different successes of exploitation and reproduction of a parasite25. Previous studies have shown that the phylogenetic correlation between the main host and the auxiliary host can determine parasite abundance on its auxiliary hosts, as it should reflect the similarities between host species in terms of physiological, ecological characteristics and immunological26. On the other hand, it was found that for flea infestation in small mammals, the abundance of a flea on its auxiliary hosts decreased with increasing phylogenetic distance of these hosts relative to the main host26. The mechanisms underlying this model are not yet clear but are supposed to be consistent with the differential performance of a flea on auxiliary hosts, which in turn is correlated with a phylogenetic distance from the auxiliary host to the main host27.
Recent studies have shown that parasites appear to make favorable choices and decisions for hosts in which their reproductive benefits are maximized28. In the meantime, the choice of the host is important, which may contribute to the variation in the abundance of the fleas collected when the potential risk of flea-mediated diseases are assessed. In addition, the variation in the level of infection in host individuals is well known, indicating that some individuals may represent better patches for parasites than other individuals21. Namely, reservoirs providing better patches for parasites are usually defined as primary hosts.
Differences in flea infestation due to the age of the host may be influenced by differences in parasite aggregation. We examined the effect of age of small mammals on parasitic fleas and found that the probability of flea prevalence in adult hosts increased but the number of fleas in adult hosts decreased. This phenomenon has generally been linked to host immune responses and parasite-host associations. Firstly, it is known that immune defenses often deteriorate with increasing age29, so that in smaller mammals the decline of antiparasitic defense has been reported with increasing age30. These hosts have different defenses that can affect the number of blood a flea can acquire31. Fleas were found to take more blood from juveniles and older animals than from sub-adult and adult animals32. The degree of deterioration of immune function with age may, however, be affected by environmental conditions (e.g. environmental stress)33 and may also differ between males and females (due to faster aging of men)34. Secondly, differential parasite abundance in hosts belonging to different age cohorts has been reported for various host and parasite taxa35,36,37. However, the influence of host age on the distribution pattern of parasite abundance differs among different host-parasite associations. In some host-parasite associations, the abundance of parasites increases with the age of the host35, while in other associations it increases or decreases in the youngest and oldest hosts compared to hosts of median age (called "adult hosts")36. In fact, acquired resistance against parasites may be lower in young or old hosts than in median-age hosts. Young hosts may simply not have the time to develop parasite resistance, while older hosts may lose their ability to resist parasites due to immunosenescence29,38,39. As a result, hosts in the youngest and the oldest cohorts would have better habitat for parasites, so the relationship between parasite abundance and host age would be convex. If the negative effect of heavy parasite loads causes mortality mainly in young instead of old hosts, the abundance of parasites will increase with the age of the host. In all cases, fleas performed better in younger and older hosts than in middle-aged hosts.
In addition, the effect of the age of the host was strongly influenced by the effect of the sex of the host. In particular, from the point of view of resource acquisition (i.e. The size of the blood meal), there has been an improvement in the quality of cohorts of young and old hosts in females, but not in males, while, in the context of resource processing (digestion of blood), some trends in age-dependent host quality has been observed in male hosts but not in female hosts31. In other words, the results of the Liberman et al.32 suggest that the age of the host does not predict unequivocally whether it is more or less beneficial for a flea.
Similarly, the sex of the host was also a determining factor influencing the abundance of fleas. Previous studies have shown the effect of host sex in flea reproduction40,41. In this study, we studied the consequences of host sex on flea abundance. Finally, we observed an increase in the number of flea infestations in small male mammals compared to females. The effect of host sex on parasite performance is essential to understanding the mechanisms of male-biased parasitism.
Under normal conditions, two non-mutually exclusive mechanisms are invoked to explain the variation in the number of fleas observed. Male hosts are characterized by higher mobility and poorer immunological defense than female hosts. In general, fleas feed faster, take relatively more blood and digest more quickly when feeding on male rather than female, although the pattern of blood digestion related to the sex of the host depends on external conditions (relative humidity). In addition, fleas produced more eggs exploiting male hosts than female hosts40. This would lead to a better ability of parasites exploiting male hosts rather than female hosts because of the immunosuppressive effects of androgens, which would lead to fleas favoring male41. It is suggested that, in many cases, male hosts represent better patches for parasites than females. Thus, it can be seen that more flea prefers to stay in male hosts than in females. Consequently, a greater infestation of a male host than a female host in terms of abundance, prevalence and species richness of parasites, this has been reported for a wide variety of parasite and host taxa42, although higher levels of parasite infection have been reported in some mammalian female43.
The body weight of the hosts could be considered as an additional indicator of the number of fleas assembled. The results of this study showed that the number of fleas increased in small mammals weighing more than 59 g. Hawlena44 noted that the number of fleas, mainly X. cheopis and Xenopsylla Astia (Siphonaptera: Pulicidae), caught in the wild (Rodentia: Muridae) increased with the weight of the host (and thus its age) at an optimum before decreasing. In practice, the body weight of the host increases with age. As mentioned above, the probability of flea infestation on the host increases with the age of the host. Therefore, the number of fleas would increase with the body weight of the host.
Associations between the aggregation of fleas and the abundance of their hosts vary according to different factors. This study confirmed that the variation in the number of fleas was due to different factors in the host.
It is concluded that the host is an important factor to consider when comparing fleas variation in natural rodent populations. It is necessary that the factors mentioned above be taken into account to control the abundance of fleas. Fitness related measures should be directly involved, such as reducing the number of small mammals and fleas, preventing and controlling flea-borne diseases.
Conclusions
The results of the study show the distribution of small mammals and parasitic fleas in households of the western Yunnan province, China. In addition, a new discovery revealed by the hurdle binomial regression model is that there is a close relationship between the abundance of fleas and the characteristics of small mammals (e.g. species, age, sex, and body weight). Our study provides direct evidence to explore the relationship between the fleas and host by the characteristic of instinct and to reveal host structure playing an important role in the allocation of flea abundance in the ecosystem of the plague. The knowledge of flea factors will help control and predict the number of fleas in commensal rodent plague foci and further control the onset of the plague. Although it is relatively limited that the current model predicts the factors affecting the abundance of fleas. In addition to the characteristics of the host, the number of fleas may be affected by other potential factors (e.g. environment, climate, economy, human disturbance). Thus, the above factors should be measured and included in the regression model, it would be useful to establish benefit model to predict the abundance of parasitic fleas.
Methods
Description of the study area
The western Yunnan province is located in the central region of the Hengduan mountains of the terrestrial part of the Tibetan plateau, which belongs to the low latitude mountain valley. It is influenced by the enormous difference in latitude and altitude gradient, which is characterized by complex terrain and varied climate45. Therefore, it is one of the regions that possess the most abundant animal and plant species in China. There are many minority nationalities of human populations, including Yi, Dai, Naxi, Hui, Bai et al. inhabiting this area. The main economic source is planting and economic development is relatively poor. These conditions contribute to the breeding of small mammals and the spread of the plague.
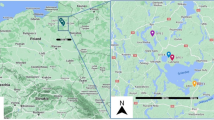
The study was carried out in 800 households (Twenty households per village were selected by simple random sampling method) in 40 villages from 10 counties, in the western part of Yunnan province (Fig. 1) (The Fig. 1 was made using QGIS 2.8.1, https://www.qgis.org/en/site/). In the study area, the 10 counties have a total area of approximately 41,130 square kilometers and a human population of approximately 3.4 million.
Study subjects
The study received ethical approval from the National Natural Science Foundation of China (No. 81860565) and Yunnan Institute of Endemic Disease Control and Prevention (No.YIEDC2011-01). All experiments were performed in accordance with relevant guidelines and regulations.
Small mammals capture and identification
Five traps were placed in each household for three continuous nights to capture small mammals. The bait was fried ham sausage. Each trap was checked in the morning of the next day. If a small mammal was captured, a new trap with fresh bait was replaced in the same place. The species of small mammals, sex and age were identified according to their morphological characteristics. The age of small mammals was identified by the development of the reproductive organs, teeth, and the body weight46. Their weight, body length and tail length were measured.
Fleas collections and identification
The fleas were harvested by combing from the tail end forward using a toothbrush in a white enamel tray after anesthetizing small mammals with ether. The fleas were stored in labeled vials containing 75% ethanol. Fleas from each small mammal were kept in separate vials and kept at room temperature. After the fleas were taken from vials and rinsed with 0.9% normal saline, the species was identified under a light microscope based on the external morphology of the fleas47.
Data analysis
The distribution of small mammals and fleas was summarized using descriptive statistics. Then the flea prevalence and the abundance of fleas of each small mammal were generated48. Species, sex, age, weight, body length and tail length of each small mammal were considered potential factors for the abundance of fleas. For continuous variables, the median was used to classify these variables into binary variables. The relationship between potential factors and a result was explored using the hurdle negative binomial (HNB) regression model using R software 3.0249,50,51. This regression model was applied to take into account of the current dataset that shows a count of excess zeros and an overdispersion distribution. It is a two-component model: one is the logistic model adjusting counts against zero, the other is the hurdle binomial model adjusting positive counts. The factors with P < 0.20 in univariate analysis were regarded as the potential factors related to fleas and entered the prototype multiple HNB regression model. The final model to predict the factors associated with the flea abundance was refined using a backward method (α = 0.10 as a criterion of statistical significance). The proportion of prevalence odds ratio (OR) and abundance ratio (AR) for a parasitic infestation of fleas were calculated based on pieces of literature24,52.
Ethical statement
The study has been approved by the National Natural Science Foundation of China (No. 81860565) and Yunnan Institute of Endemic Disease Control and Prevention (No. YIEDC2011-01).
References
Gallizzi, K., Alloitteau, O., Harrang, E. & Richner, H. Fleas, parental care, and transgenerational effects on tick load in the great tit. Behav. Ecol. 19, 1225–1234. https://doi.org/10.1093/beheco/arn083 (2008).
Gage, K. L. Factors affecting the spread and maintenance of plague. Adv. Exp. Med. Biol. 954, 79–94. https://doi.org/10.1007/978-1-4614-3561-7_11 (2012).
Yin, J. X. et al. Human plague outbreak in two villages, Yunnan Province, China, 2005. Southeast Asian J. Trop. Med. Public Health 38, 1115–1119 (2007).
Cai, W. F., Zhang, F. X. & Wang, G. L. Structure and community diversity of small mammals in plague natural focus of Yulong County and Gucheng District. Chin. J. Control Endem. Dis. 30, 333–335 (2015).
Stenseth, N. C. et al. Plague: past, present, and future. PLoS Med. 5, e3. https://doi.org/10.1371/journal.pmed.0050003 (2008).
Liang, X. C. & Wang, D. S. Analysis of epidemic situation of Marmota himalayana plague natural focus in Gansu Provence. Bull. Dis. Control Prev. 26, 38–48 (2011).
Bai, L. Q., Wang, J. J. & Si, X. Y. Overview of the survelliance of rodent populations from 2001 to 2011 in Inner Mongolia. Inner Mongolia Med. 46, 826–829 (2014).
Yin, J. X. et al. Predictors for presence and abundance of small mammals in households of villages endemic for commensal rodent plague in Yunnan Province, China. BMC Ecol. 8, 18. https://doi.org/10.1186/1472-6785-8-18 (2008).
Li, J. Y., Zhao, W. H., Dong, X. Q., Liang, Y. & Hong, M. Analysis on the current status of plague epidemics for Rattus flavipectus plague natural foci in Yunnan province. Chin. J. Endemiol. 25, 654–657 (2006).
Yang, C. G., Zhao, W. H. & Dong, X. Q. An analysis for epidemiological character and intensity of commensal rodent plague in Yunnan. Chin. J. Vector Biol. Control 18, 226–229 (2007).
Otranto, D. et al. Season-long control of flea and tick infestations in a population of cats in the Aeolian archipelago using a collar containing 10% imidacloprid and 45% flumethrin. Vet. Parasitol. 248, 80–83. https://doi.org/10.1016/j.vetpar.2017.10.023 (2017).
Lawrence, A. L. et al. Integrated morphological and molecular identification of cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) vectoring Rickettsia felis in central Europe. Vet. Parasitol. 210, 215–223. https://doi.org/10.1016/j.vetpar.2015.03.029 (2015).
Krasnov, B. R. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology (Cambridge University Press, Cambridge, UK, 2008).
Giorgi, M. S., Arlettaz, R., Christe, P. & Vogel, P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis). Proc. Biol. Sci. 268, 2071–2075. https://doi.org/10.1098/rspb.2001.1686 (2001).
Sánchez, S. & Gómez, M. S. Xenopsylla spp. (Siphonaptera: Pulicidae) in murid rodents from the Canary Islands: an update. Parasite 19, 423–426. https://doi.org/10.1051/parasite/2012194423 (2012).
Rouault, E. et al. Imaging visceral leishmaniasis in real time with golden hamster model: monitoring the parasite burden and hamster transcripts to further characterize the immunological responses of the host. Parasitol. Int. 66, 933–939. https://doi.org/10.1016/j.parint.2016.10.020 (2017).
Schmid-Hempe, P. & Ebert, D. On the evolutionary of specific immune deference. Trends Ecol. Evol. 18, 27–32 (2003).
Khokhlova, I. S., Spinu, M., Krasnov, B. R. & Degen, A. A. Immune response to fleas in a wild desert rodent: effect of parasite species, parasite burden, sex of host and host parasitological experience. J. Exp. Biol. 207, 2725–2733. https://doi.org/10.1242/jeb.01090 (2004).
Lorange, E. A., Race, B. L., Sebbane, F. & Hinnebusch, B. J. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J. Infect Dis. 191, 1907–1912. https://doi.org/10.1086/429931 (2005).
Eisen, R. J. et al. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc. Natl. Acad. Sci. USA 103, 15380–15385. https://doi.org/10.1073/pnas.0606831103 (2006).
Poulin, R. Evolutionary Ecology of Parasites: From Individuals to Communities (Princeton Univ. Press, Princeton, 2007).
Poulin, R. Relative infection levels and taxonomic distances among the host species used by a parasite: insights into parasite specialization. Parasitology 130, 109–115 (2005).
Wu, A. G., Li, T. Y., Feng, J. M. & Dong, X. Q. Study on the epidemiological significance related to community-structural difference of the rat plague host and vectors in Western Yunnan, China. Zhonghua liuxingbingxue zazhi 29, 346–350 (2008).
Yin, J. X. et al. Predictors for abundance of host flea and floor flea in households of villages with endemic commensal rodent plague, Yunnan Province, China. PLoS Negl. Trop. Dis. 5, e997. https://doi.org/10.1371/journal.pntd.0000997 (2011).
Krasnov, B. R. et al. Host specificity and foraging efficiency in blood-sucking parasite: feeding patterns of the flea Parapulex chephrenis on two species of desert rodents. Parasitol. Res. 90, 393–399. https://doi.org/10.1007/s00436-003-0873-y (2003).
Krasnov, B. R., Shenbrot, G. I., Khokhlova, I. S. & Poulin, R. Relationships between parasite abundance and the taxonomic distance among a parasite’s host species: an example with fleas parasitic on small mammals. Int. J. Parasitol. 34, 1289–1297. https://doi.org/10.1016/j.ijpara.2004.08.003 (2004).
Khokhlova, I. S., Fielden, L. J., Degen, A. A. & Krasnov, B. R. Digesting blood of an auxiliary host in fleas: effect of phylogenetic distance from a principal host. J. Exp. Biol. 215, 1259–1265. https://doi.org/10.1242/jeb.066878 (2012).
Krasnov, B. R., Khokhlova, I. S. & Shenbrot, G. I. Density-dependent host selection in ectoparasites: an application of isodar theory to fleas parasitizing rodents. Oecologia 134, 365–372. https://doi.org/10.1007/s00442-002-1122-2 (2003).
Gruver, A. L., Hudson, L. L. & Sempowski, G. D. Immunosenescence of ageing. J. Pathol. 211, 144–156. https://doi.org/10.1002/path.2104 (2007).
Body, G. et al. Population density and phenotypic attributes influence the level of nematode parasitism in roe deer. Oecologia 167, 635–646. https://doi.org/10.1007/s00442-011-2018-9 (2011).
Liberman, V., Khokhlova, I. S., Degen, A. A. & Krasnov, B. R. Reproductive consequences of host age in a desert flea. Parasitology 140, 461–470. https://doi.org/10.1017/s0031182012001904 (2013).
Liberman, V., Khokhlova, I. S., Degen, A. A. & Krasnov, B. R. The effect of host age on feeding performance of fleas. Parasitology 138, 1154–1163. https://doi.org/10.1017/s0031182011000758 (2011).
Hayward, A. D., Wilson, A. J., Pilkington, J. G., Pemberton, J. M. & Kruuk, L. E. Ageing in a variable habitat: environmental stress affects senescence in parasite resistance in St Kilda Soay sheep. Proc. Biol. Sci. 276, 3477–3485. https://doi.org/10.1098/rspb.2009.0906 (2009).
Clutton-Brock, T. H. & Isvaran, K. Sex differences in ageing in natural populations of vertebrates. Proc. Biol. Sci. 274, 3097–3104. https://doi.org/10.1098/rspb.2007.1138 (2007).
Fichet-Calvet, E., Wang, J., Jomaa, I., Ben Ismail, R. & Ashford, R. W. Patterns of the tapeworm Raillietina trapezoides infection in the fat sand rat Psammomys obesus in Tunisia: season, climatic conditions, host age and crowding effects. Parasitology 126, 481–492 (2003).
Krasnov, B. R., Stanko, M. & Morand, S. Age-dependent flea (Siphonaptera) parasitism in rodents: a host’s life history matters. J. Parasitol. 92, 242–248. https://doi.org/10.1645/ge-637r1.1 (2006).
Alarcos, A. J. & Timi, J. T. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol. Res. 110, 2155–2166. https://doi.org/10.1007/s00436-011-2741-5 (2012).
Johansen, C. E., Lydersen, C., Aspholm, P. E., Haug, T. & Kovacs, K. M. Helminth parasites in ringed seals (Pusa hispida) from Svalbard, Norway with special emphasis on nematodes: variation with age, sex, diet, and location of host. J. Parasitol. 96, 946–953. https://doi.org/10.1645/ge-1685.1 (2010).
Praet, N. et al. Age-related infection and transmission patterns of human cysticercosis. Int. J. Parasitol. 40, 85–90. https://doi.org/10.1016/j.ijpara.2009.07.007 (2010).
Khokhlova, I. S., Serobyan, V., Degen, A. A. & Krasnov, B. R. Host gender and offspring quality in a flea parasitic on a rodent. J. Exp. Biol. 213, 3299–3304. https://doi.org/10.1242/jeb.046565 (2010).
Khokhlova, I. S., Serobyan, V., Krasnov, B. R. & Degen, A. A. Is the feeding and reproductive performance of the flea, Xenopsylla ramesis, affected by the gender of its rodent host, Meriones crassus?. J. Exp. Biol. 212, 1429–1435. https://doi.org/10.1242/jeb.029389 (2009).
Patterson, J. E., Neuhaus, P., Kutz, S. J. & Ruckstuhl, K. E. Patterns of ectoparasitism in North American red squirrels (Tamiasciurus hudsonicus): sex-biases, seasonality, age, and effects on male body condition. Int. J. Parasitol. Parasites Wildl. 4, 301–306. https://doi.org/10.1016/j.ijppaw.2015.05.002 (2015).
Krasnov, B. R., Morand, S., Hawlena, H., Khokhlova, I. S. & Shenbrot, G. I. Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146, 209–217. https://doi.org/10.1007/s00442-005-0189-y (2005).
Hawlena, H., Khokhlova, I. S., Abramsky, Z. & Krasnov, B. R. Age, intensity of infestation by flea parasites and body mass loss in a rodent host. Parasitology 133, 187–193. https://doi.org/10.1017/s0031182006000308 (2006).
Feng, J. M. & Xu, C. D. Geographical distribution patterns of zonal plant community species diversity in Western Yunnan, China. Chin. J. Ecol. 28, 595–600 (2009).
Zheng, Z. M., Jiang, Z. K. & Chen, A. G. The Glires (Shanghai Jiaotong University Press, Shanghai, 2008).
Xie, B. Q. & Zeng, J. F. The Siphonaptera of Yunnan (Yunnan Science and Technology Press, Kunming, 2000).
Bush, A. O., Lafferty, K. D., Lotz, J. M. & Shostak, A. W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83, 575–583 (1997).
Zeileis, A., Kleiber, C. & Jackman, S. Regression models for count data in R. J. Stat. Softw. 27, 1–25 (2008).
Baughman, A. L. Mixture model framework facilitates understanding of zero-inflated and hurdle models for count data. J. Biopharm. Stat. 17, 943–946. https://doi.org/10.1080/10543400701514098 (2007).
Yin, J. X. & Dong, X. Q. Application of hurdle model in identifying predictors for flea abundance on rats. Endem. Dis. Bull. 25, 1–4 (2010).
Yin, J. X., Zhong, Y. H., Du, C. H., Dong, X. Q. & Yang, S. H. Predictors for abundance of Rattus tanezumi in households of commensal rodent plague foci. Zhonghua liuxingbingxue zazhi 34, 157–159 (2013).
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 81860565) and The Innovation Team of Natural Focal Diseases Epidemiology in Dali University (No.ZKPY2019201).
Author information
Authors and Affiliations
Contributions
J.X.Y. conceived and designed the study. J.X.Y., X.O.C., X.F.W., D.D.X., M.D.W., Y.Z. and Z.X.L collected data and analyzed the results. Q.F.Z and Z.F.W. prepared the figures. J.X.Y. and X.O.C. wrote the manuscript draft. L.Y.Y revised the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, JX., Cheng, XO., Luo, YY. et al. The relationship between fleas and small mammals in households of the Western Yunnan Province, China. Sci Rep 10, 16705 (2020). https://doi.org/10.1038/s41598-020-73690-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73690-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.