Abstract
European grassland birds are experiencing major population declines, mainly due to changes in farmland management. We analyzed the role of habitat availability, grazing management and linear infrastructures (roads and power lines) in explaining spatial and temporal variation in the population density of little bustards (Tetrax tetrax) in Portugal, during a decade in which the species population size halved. We used data from 51 areas (totaling ca. 1,50,000 ha) that were sampled in two different periods (2003–2006 and 2016). In 2003–2006, when the species occurred at high densities, habitat availability was the only factor affecting spatial variation in bustard density. In the 2016 survey, variation in density was explained by habitat availability and livestock management, with reduced bird numbers in areas with higher proportions of cattle. Population declines across the study period were steeper in areas that initially held higher densities of bustards and in areas with a higher proportion of cattle in the total stocking rate. Areas with higher densities of power lines also registered greater density declines, probably due to avoidance behavior and to increased mortality. Overall, our results show little bustards are currently lacking high quality grassland habitat, whose persistence depends on extensive grazing regimes and low linear infrastructure densities.
Similar content being viewed by others
Introduction
Natural and semi-natural grasslands across the world are known for their high biodiversity value1,2,3. Extensively managed High Nature Value (HNV) farmlands are recognized as key habitats for several taxa in Europe, namely butterflies4 and birds5,6, and the maintenance of such landscapes is considered a priority for European biodiversity7. However, they are often subjected to changes in management, such as conversion to other land uses, agriculture intensification or abandonment3. Intensification of management is usually associated to an increase in the use of agrochemicals or in livestock density8,9. Often, these changes are supported by targeted policies. For example, in Europe, changes in livestock management are promoted by the Common Agricultural Policy (CAP) through coupled payments (per livestock head), leading to an increase in livestock numbers, mainly cattle10,11.
Grasslands or pseudosteppes of the Iberian Peninsula are considered key farmlands habitats for European biodiversity, mainly due to their importance for threatened grassland bird species12,13,14,15. The traditional management of these systems created a heterogeneous landscape due to the extensive cultivation of dry cereal crops in rotation with pastures to support sheep raising, which produced a patchy system of cereal fields, stubbles, fallow lands, plowed lands and pastures that supported a widely rich and variable grassland bird community, including several species with unfavorable conservation status at the European and world level such as great bustard (Otis tarda), Montagu’s harrier (Circus pygargus) or little bustard (Tetrax tetrax)14,16. Despite the ecological importance of these HNV systems, so far EU policies have failed to maintain these landscapes. In fact, such systems are being rapidly replaced by systems specialized on cattle and consequently increasing the proportion of pastures in the landscape10,11. Even though these changes maintain grasslands, they potentially reduce the quality of the habitat for biodiversity by reducing landscape heterogeneity17, and promoting changes in sward structure and crop management, e.g. hay harvesting times18,19,20.
In addition to grassland management changes, infrastructures are also known to affect grassland birds. Infrastructures are major drivers of human-related effects in the Anthropocene, associated with habitat changes (e.g. habitat loss and fragmentation), behavioral effects (avoidance and displacement due to disturbance) and mortality of numerous animal taxa21,22. Roads and power lines are known to cause high levels of mortality of bird species23, and population effects have already been described in raptors24, bustards25 and owls26, among other bird groups. Regarding grassland birds, several studies report high mortality levels due to collision, mainly with power lines, and a displacement effect related with human structures, as power lines, roads and wind farms, has also been described27,28,29,30.
The little bustard is a Near Threatened ground-nesting grassland bird31 and considered a bioindicator species of grassland quality in southern Europe32. In Iberia, which holds ca. 95% of the population of the species in Western Europe (Iñigo and Barov, 2010) the species depends principally on grasslands to breed34,35,36. A network of Special Protection Areas (SPA) holding well conserved grasslands were designated for the protection of this and other steppe species. Despite that, little bustard numbers dropped dramatically during the twenty-first century; a mean population decline of 49% was recorded in Portugal between 2003–2006 and 201637 and a decline of 48% was recorded in Spain for the same period38. Habitat loss due to agricultural intensification, leading to reductions in fallow land area, were associated with declines and local extinctions of the little bustard in other parts of Europe33,39. However, evidence collected so far suggests that the declines in the Iberian Peninsula may not be fully explained by such drivers. In Portugal, for example, the decline in male densities was stronger within SPAs than in unprotected areas, even though the amount of grassland habitat did not change markedly within protected areas37. This indicates that a reduction in the amount of grassland habitat is not the only driver of the population decline, and that a deterioration of the quality of this habitat, due to changes in management, is also likely to be involved37,38. Collision with power lines is the main anthropogenic cause of mortality of the little bustard, affecting 3.4–3.8% of adult birds per year40. This is the highest mortality rate per collision with power lines ever recorded for a species. Morphological characteristics and bird sensorial perception are key species-specific features that explain such a high mortality rate41,42,43,44, but behavioural changes (as flocking behaviour or flight height) across seasons were also considered relevant45,46. In addition to mortality, little bustards also avoid the vicinity of anthropogenic infrastructures, mainly to roads and power lines47,48,49,50,51,52. Therefore, such structures are likely to influence little bustard spatial distribution and population density.
In this study, we used the results of two countrywide surveys of little-bustard (2003–2006 and 2016) in Portugal to analyze the drivers of spatial and temporal variation in little bustard densities at a landscape level. Bird data was gathered through a standardized field protocol applied across the Iberia Peninsula since 2003 that targets male birds, as females have a cryptic behavior and are hard to survey, due to their morphological characteristics and behavior. We specifically tested the potential roles of (1) the availability of the grassland habitat, i.e. habitat quantity; (2) livestock density as a proxy of habitat quality and (3) the amount of linear infrastructures, in explaining spatial and temporal changes in the density of the species in the two study periods.
Results
Major changes between surveys
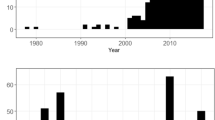
The mean little bustard density significantly declined from 2.68 ± 0.38 males/ha in 2003–2006 to 1.44 ± 0.28 males / ha in 2016 (Table S1, Fig. 1). Most of the sampled areas (n = 35 out of 51 study areas) showed a negative trend between the two surveys and the species disappeared in 12 of them.
Variation in little bustard density and environmental predictors (mean and standard errors) between the two surveys periods (2003–2006 and 2016). The grazing regime predictors (stocking rate and cattle proportion) are presented for 1999 and 2009, the only periods with livestock statistics available for the study area.
Habitat availability, i.e. the proportion of grassland area within the surveyed areas, significantly declined from 0.57 ± 0.03 to 0.45 ± 0.04 (Table S1, Fig. 1), with declines in 28 of the sampled areas. Most of these areas lost over 5% of grassland habitats (n = 19), 6 of which with losses between 40 and 89%.
There was a significant increase of the estimated stocking rate and the proportion of cattle in the total stocking rate experienced a significant increase (Table S1, Fig. 1), across the whole region. A significant increase of the linear infrastructures also occurred across the study period (Table S1, Fig. 1), road density went from 0.27 km/km2 ± 0.03 to 0.33 ± 0.03 km/km2, and power line density from 0.43 km/km2 ± 0.04 to 0.49 ± 0.04 km/km2.
Drivers of spatial variation in little bustard densities for each period
In the first survey (2003–2006), habitat availability was the only significant predictor of spatial variation in little bustard densities, with higher densities occurring in areas with higher proportions of grasslands. In the 2016 survey, habitat availability was also important, but the model also included a variable related to grazing management, i.e. the species was more abundant in areas with lower proportion of cattle in the total stocking rate (< 60%) (Table 1 and Fig. 2). This latter model had a higher explanatory power compared to the former one (71.2% vs. 24.6%). There was no significant autocorrelation in both model residuals (Figs. S1 and S2).
Generalized additive model partial effects for the two spatial models of the relationship between the little bustard density in each survey (2003–2006 and 2016) and the environmental predictors. Shaded areas represent 95% confidence intervals. The y-axis shows the contribution of the fitted centered smooth terms s (names of the predictor, estimated degrees of freedom) to the response variable (little bustard density in each survey). Ticks on the x-axis represent the location of observations along the predictor.
Drivers of little bustard temporal density variation
As for the population variation model (Table 1 and Fig. 3), larger declines in little bustard density occurred in areas with higher densities of bustards in the first survey, a larger proportion of cattle in the stocking rate and a higher density of power lines. Areas with a high proportion of available habitat (> 40%) were the ones with smaller losses. There was no significant autocorrelation in model residuals (Fig. S3).
Generalized additive model partial effects for the population variation model of the relationship between the delta in little bustard density across surveys (2016–2003–2006) and the environmental predictors. Shaded areas represent 95% confidence intervals. The y-axis shows the contribution of the fitted centered smooth terms s (names of the predictor, estimated degrees of freedom) to the response variable (delta in little bustard density between survey). Ticks on the x-axis represent the location of observations along the predictor.
Discussion
Changes in bustard densities and their drivers across time
The little bustard is considered a bioindicator species of HVN farmlands supporting a high diversity community of birds in Iberia32,39. Contrary to other ground-nesting birds, such as great bustard, Montagu’s harrier or stone curlew (Burhinus oedicnemus), the little bustard is subject to coordinated monitoring programs across the Iberian Peninsula, which makes it a good indicator of the overall conservation status of the agricultural landscapes within Iberia. In this study, we recorded a 46.3% variation of little bustard male density in our study areas, from 2003–2006 to 2016, matching the patterns previously reported for the whole region37. Although large scale monitoring programs of little bustard only collect data on male birds (as females have a cryptic and secretive behavior that makes it difficult to study them), studies conducted so far suggest that a sex-ratio biased towards males may be an issue in areas of intensive agriculture53,54, whilst in extensive and large grasslands areas the sex-ratio seems to be balanced55.
Changes in key population drivers likely explain the observed trends. First, there was a reduction of available habitat across time. The major losses were observed in areas allocated to recent irrigation projects, which converted grasslands to permanent crops such as olive groves or vineyards. In fact, the whole Alentejo region is facing major landscape changes during the twenty-first century, as large expanses of open agricultural lands have been transformed into permanent crops. As a result, the area devoted to annual crops decreased 66%, while permanent crops increased by 23% between 2000 and 2016 (Fig. S456). These changes were mostly located outside SPA, in accordance with the findings of recent studies37,57 that also reported habitat loss outside these protected areas, in contrast with a greater habitat persistence within SPA. Our results are also consistent with those found in Spain, where declines in little bustard density are correlated to habitat loss, and fallow land in particular39.
As for habitat quality, the remaining grasslands likely degraded over time due to different reasons: (1) from 2000 to 2016 the number of beef cattle increased by 48% in the Alentejo region, while sheep declined by 27% (Fig. S456), leading to the observed overall increase of the stocking rate along with the increased proportion of cattle, with expected negative impacts on vegetation structure and nest trampling and desctruction58,59. (2) A consequence of the increased numbers of cattle is that the area devoted to hay production has been increasing in the region to ensure the increased fodder demands19, a land use that did not occur in the traditional system based on rotational cereal crop. This increase of hay production has likely consequences for the bustard population as the mowing of hay fields is carried out over one month earlier, compared to the traditional system, and coinciding with the beginning of egg hatching leading to nest destruction and increasing adult mortality, primarily females19. (3) Changes in livestock management also represent the loss of the traditional rotation system based on cereal production and its resulting heterogeneous landscape, which is replaced by permanent pastures of lower quality for ground nesting birds (Fig. S456). The intensification of land use and livestock management were promoted by the CAP reform of 200310,11. It should be noted, however, that the available data relating to livestock management used in this study was not detailed enough to fully characterize the livestock density and management in the study area, hence future studies should focus on providing further details on the effect of grazing and variable stocking rate levels on ground nesting birds. (4) Finally, there was an overall increase of anthropogenic infrastructures (namely roads and power lines), which are known to negatively impact little bustards. Collisions with power lines are a major source of mortality for the species in the Iberia Peninsula40 and power lines and roads are also a known source of displacement effects for the species47,48,49,50,51,52.
Drivers of spatial variation in little bustard densities
In 2003–2006, higher population densities of bustards were found in areas with higher habitat availability and this was the only significant driver of spatial variation in bustard densities. This shows that habitat availability had a very strong influence on population size, as it promoted a disproportionate increase in bustard densities, providing evidence that habitat availability was also the single best indicator of habitat quality. This effect can also be explained by conspecific attraction that is known to occur in grassland species60,61, including in the little bustard62. In 2016, habitat availability was still relevant, but livestock management also became significant explaining spatial variation in male densities. In fact, following the overall increase of livestock density, the bustards occurred in higher densities in areas with lower (< 60%) proportions of cattle (compared to sheep) (Fig. 2). Previous studies found that intermediate levels of cattle grazing density were preferred by little bustards54,63 and the highest densities of the species in Alentejo (in 2007–2008) were recorded in areas grazed by cattle with seasonal rotational pastures36,55. However, in our study, the increased proportion of cattle, also associated with higher stocking rates (Pearson r = 0.40, p = 0.004), seems to have led to the avoidance of some areas by the bustards in 2016. The reduced little bustard density in areas with higher proportions of cattle suggests that grassland management associated with cattle cause a decrease in habitat quality, when compared with similar stocking rate values for sheep.
Drivers of temporal changes in population densities
In spite of the clear trend for population density declines over time, the regional variation in population densities during the ca. 10-year period were quite variable across areas (ranging from losses of 6.9 males/ha to gains of 2.9 males/ha), thereby suggesting that the prevalence of driving forces behind such changes varied across areas.
Major declines in density (absolute value) were observed in areas where the bustards occurred in higher densities during the first survey. These high densities are likely a good indicator of overall habitat quality for the species36, coinciding with areas where the magnitude of the impacts of habitat loss and degradation, as well as the overall population decline, are more clearly expressed. The loss of male densities in remaining areas was limited by their already low densities.
Habitat quantity was also relevant to attenuate the decline of the species between 2003–2006 to 2016, as study areas with the largest expanses of grasslands (> 40% of the study area surface) were the ones with smaller density losses (some even with gains). Large and continuous grasslands are known to host high density of little bustards in Portugal35,36, and our results suggest that such landscapes have more resilient populations, which are able to buffer the general population decline. Little bustards preference for extensive and open landscapes is probably due to their exploded lek mating system, as larger fields (> 50 ha) allow greater aggregations of males, which are preferred and are visited more frequently by females, with lower disturbance levels, higher proportion of nesting females and lower rates of predation as a result of reduced edge effects36.
Areas dominated by cattle were the ones with higher reductions of little bustard density between surveys. This suggests that higher cattle densities and respective changes in grassland management are causing changes in the quality of grassland habitats (see previous sections).
Areas with a higher density of power lines suffered greater density losses between surveys. Power lines are known to affect the spatial distribution of the little bustard, although it is not clear if the causal factor is an increased perceived predation risk (as power line poles are frequently used as perches by little bustard predators, such as large raptors) or neophobia52,64. Additionally, power lines represent a major cause of anthropogenic mortality for the species40, with high fatalities due to collisions with power line cables known to occur at the onset of the breeding season45. These mechanisms may explain the reduction of male density in areas with more power lines.
The fact that the road network was not relevant in explaining spatial variation in the distribution of little bustards was unexpected, as roads are responsible for habitat fragmentation and a source of disturbance65, previously reported to affect the spatial distribution of the little bustard during the breeding season36,47,48,49,51. The lack of a statistically significant effect of road density on little bustard may be explained by the fact that both structures tend to occur spatially clustered, with power lines and roads running parallel, and we had a moderate positive correlation between these predictors in our dataset (Pearson r = 0.39, p = 0.004).
Implications for conservation
Our work shows that both habitat quantity and quality are key for the little bustard. Conservation actions should focus principally on (1) avoiding further habitat losses, mainly due to the plantation of permanent crops (i.e. olive groves, vineyards or almond orchards); (2) avoid the expansion of linear infrastructures and mitigate the impacts of either existing and future structures, e.g. with the use of wire marking devices; and (3) reduce the cattle levels. Although we acknowledge that cattle livestock plays an important role to maintain the grassland habitat in Iberia, and some grassland bird species may even benefit from such livestock systems, current cattle stocking rates in Alentejo are not compatible with the conservation of the little bustard. This also highlights that management guidelines should take into account the historical background of each system, respecting the singularities and biodiversity value of each location, as general policies will not adjust for every farmland system66. To reduce the dominance of cattle in Iberian grasslands we suggest: (a) the total decoupling of cattle in the next CAP reform, so that farmers are paid per unit area with a fixed maximum cattle density, rather than a payment per cattle head; and (b) using the same mechanism to promote sheep grazing at moderate densities, so that these are not totally replaced by cattle. Reduced cattle densities will imply lower hay needs, with direct benefits for little bustards. The proposed recommendations aimed to manage the little bustard will also be beneficial to the overall grassland bird community, as high cattle densities likely affect other ground-nesting birds in our study area, including high conservation concern species as the great bustards or the Montagu’s harrier, mainly because of trampling and haying activities during the nesting period.
The decline of the little bustard in Alentejo is probably not only a result of pressures occurring during the breeding season. In fact, multiple threats, such as loss of post-breeding and wintering areas, high anthropogenic mortality and global warming40,67,68, may be acting synergistically. Little bustards are short-distance migrants69,70 that move towards more productive agriculture areas in northern, coastal or higher-altitude locations in Iberia during the dry summer season, in search of green vegetation69,71. Hence, the patterns we are witnessing at breeding areas may also be a consequence of the still unknown drivers occurring elsewhere. Regular monitoring programs would be important for a comprehensive understanding of the overall bustard decline and to understand the effect of land use shifts and other drivers of the current population trend. These programs should be implemented at different stages of the little bustard annual cycle, namely in the post-breeding and wintering areas (much less known that the breeding areas), and including a focus on the threats to the species during these seasons.
Overall, a successful recovery of little bustard populations will depend, not only on a better management of breeding habitat, but also on wider conservation actions during the remaining annual cycle that promote the reduction of anthropogenic mortality levels and maintain favorable non-breeding habitat.
Methods
Little bustard data
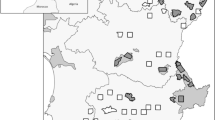
Little bustard male densities were surveyed in two different time periods, 2003–2006 and 201637, across 51 areas (totaling ca. 1,50,000 ha; mean = 2,889 ha; range = 1,657–9,997 ha) located in the Alentejo region, southern Portugal (Fig. 4). The region is home to the large majority of the national breeding population of the species72. Bird density was estimated following a standardized protocol targeting male birds, as females have a cryptic behavior and are hard to detect34,73,74. A network of point counts defined along non-paved roads, 600 m from each other and from paved roads or inhabited houses, was used to survey birds in each area, covering an average density of approximately 1.0 points/km2 per survey (range 0.47–3.00). In all 51 areas, 1,526 and 1,441 survey points were sampled in 2003–2006 and in 2016, respectively (differences due to changes in dirty road availability, as the access to some properties were closed by the land owners). Still, 99% of all sampling points were replicated in the exact same location of the previous survey. At each point location, little bustard males were counted within a 250 m radius during 5 min within the first three hours after dawn or three hours before dusk, during April and May. For further details on little bustard surveys see Moreira et al.35 and Silva et al.37.
Location of the 51 survey areas in Alentejo, Portugal, and open habitats in the region (open farmlands and permanent pastures, based on the official land cover maps of mainland Portugal for 2007 and 201575, publicly available on-line at https://mapas.dgterritorio.pt/geoportal/catalogo.html). Map created using the Free and Open Source QGIS 2.14.2088 by A.T.M.
Potential drivers of bustard population density
The analysis was focused on a regional and landscape scale, hence, for each survey period, and for each of the 51 areas, information on four major types of potential drivers of spatial and temporal changes in little bustard densities were gathered (Table 2):
Habitat availability
We quantified the amount of permanent pastures, non-irrigated annual crops and fallow land, the major land use types considered suitable for the species35,36,46, in each area. Land use data was collected from the official land cover maps of mainland Portugal for 2007 and 201575, publicly available on-line at https://mapas.dgterritorio.pt/geoportal/catalogo.html. We used land use classes 2.1.1 and 2.3.1 as our cartographic base for non-irrigated annual crops (including fallow land) and permanent pastures, respectively. This information was later refined to match the surveys years based on (1) visual inspection of Google Earth and Bing images and (2) field validation. The proportion of the total surface of each area covered by suitable habitat was estimated (Table 2).
Grazing management
Agricultural statistics were used to characterize livestock densities in our survey areas, focused on the two main grazers of the region: cattle and sheep. Two variables were estimated: (1) stocking rate, i.e. the number of livestock units (LU) per area of pastures and fallow land (stocking rates were calculated according to the following ratio: cattle = 1 LU; adult sheep = 0.15 LU76), and (2) the proportion of cattle in the total (cattle + sheep) stocking rate (Table 2). Both variables were derived from the results of the national agrarian census (RGA—Recenseamento Geral Agrícola) of 1999 and 2009, complemented with information obtained from Instituto Nacional de Estatística regarding the amount of pastures and fallow land76,77. We used the smallest administrative region in the country (i.e. Freguesia) as our unit, and applied a weighted mean based on the area occupied by each Freguesia in our individual survey areas to obtain an estimate for each area. Due to the temporal lag between the little bustards’ surveys and the available data on livestock we used the mean value between 1999 and 2009 data as a proxy of average grazing intensity in our sampled areas. Some obtained values of estimated livestock densities were considered artificially high (in 3 of the sampled areas, with estimated values often one order of magnitude higher), as they do not represent the real density of livestock in the field and are probably related to animals in stables or declared by farmers but grazing in farms outside the region. So, we set densities to a maximum of 3 LU/ha, corresponding to the highest values estimated by direct counts in the field.
Linear infrastructures
We gathered data on the distribution of paved roads and power lines in both surveys and calculated their density (km/km2) per study area (Table 2). We used the data from Open-StreetMap contributors, namely the classes: motorway, trunk, primary, and secondary78, to identify the main paved roads. For power lines, we mapped both the transmission (> 110 kV) and the distribution (< 110 kV) networks, based on data provided by the electric companies in Portugal (REN and EDP). Both data were validated for each surveys period based on Google Earth, Bing images and field checks. We considered that roads bordering our study areas had a reduced effect when compared to the ones traversing them, as only one side of these structures is expected to directly affect the bustard populations within area boundaries. Therefore, the length of the roads at the boundaries of the survey areas was down weighted when calculating its density, by dividing the length in half.
Data analysis
First, we fitted univariate Generalized Linear Mixed Models (GLMM) to test if little bustard density and potential drivers (habitat, stocking rate, cattle proportion, roads and power lines) varied across surveys, using the surveys year as a fixed factor and the sampled area as a random effect. These models were fitted in R79 with packages lme480 and lmerTest81.
To access the main drivers influencing the density of the little bustard across the study region, in each survey, we first performed two models that assessed the factors influencing spatial variation in bird densities separately for each survey date (hereafter referred to as spatial models). These models included the density in each survey area as the response variable, and the predictors included: breeding habitat availability, stocking rate, cattle proportion, roads and power lines (Table 2). In each spatial model values for predictors corresponded to the sampled year, except for the two grazing predictors, where we used the mean value of the available data as described above. A third model, the population variation model, assessed the factors underlying spatial patterns of changes in the little bustard density. In this model the variation in bird density across time (survey 2016–survey 2003/2006) in each area was the response variable. As predictors, we included the mean value across surveys of breeding habitat availability, stocking rate, cattle proportion, roads, and power lines (Table 2), which aimed to access the main global pressures in each survey area, as values of these predictors across time were highly positively correlated (Spearman's rho = 0.74. 0.81, 0.85, 0.85 and 0.94, respectively, p < 0.001; Fig. S5). Additionally, we included the little bustard density in the first surveys as a predictor, as the magnitude of the absolute variation in density is obviously constrained by the initial value in the area.
We used Spearman correlation coefficient and variance inflation factors to check for collinearity between the explanatory variables (Zuur et al.83). Variance inflation factor values (all < 2.0) and pairwise correlations between explanatory variables (all |r|< 0.55) were low for our dataset, so all variables were used in the analysis.
Generalized Additive Models (GAM) were used to fit the three models, thereby accounting for potential non-linear responses82,83. The spatial models were fitted using a Gaussian distribution and a logarithmic link function, ensuring that fitted values were positive. To model the variation on the density values across surveys we used a Gaussian distribution with an identity link function. For the three models the optimal smoothing parameter was estimated by restricted maximum likelihood estimation (REML), and a basis dimension (k = 3) was defined to allow some complexity in the functions, while avoiding over-fitting the data. The models were fitted in R79 with the package mgcv84.
The modelling procedure involved the fitting of the full model, followed by backward elimination of non-significant (p > 0.05) variables to find the optimal model. The final model adequacy was evaluated by plotting residuals versus fitted values and explanatory variables, and the model fit was evaluated by the proportion of the null deviance explained (Zuur et al.83). Spline correlogram plots with 95% pointwise confidence intervals calculated with 1,000 bootstrap resamples were used to check for spatial autocorrelation in model residuals85. We assumed that variable selection and parameter estimation were unbiased if there was no significant autocorrelation in model residuals86. Correlograms were estimated in R with the ncf package87.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author.
References
Tilman, D. & Downing, J. A. Biodiversity and stability in grasslands. Nature 367, 363 (1994).
Watkinson, A. R. & Ormerod, S. J. Grasslands, grazing and biodiversity: editors’ introduction. J. Appl. Ecol. 38, 233–237 (2001).
Dengler, J., Janišová, M., Török, P. & Wellstein, C. Biodiversity of Palaearctic grasslands: a synthesis. Agric. Ecosyst. Environ. 182, 1–14 (2014).
Dover, J. W., Spencer, S., Collins, S., Hadjigeorgiou, I. & Rescia, A. Grassland butterflies and low intensity farming in Europe. J. Insect Conserv. 15, 129–137 (2011).
Morelli, F. High nature value farmland increases taxonomic diversity, functional richness and evolutionary uniqueness of bird communities. Ecol. Indic. 90, 540–546 (2018).
Morelli, F., Benedetti, Y. & Tryjanowski, P. Introduction. In Birds as Useful Indicators of High Nature Value Farmlands (eds Morelli, F. & Tryjanowski, P.) 1–26 (Springer, Berlin, 2017). https://doi.org/10.1007/978-3-319-50284-7_1.
Sutcliffe, L. M. E. et al. Harnessing the biodiversity value of Central and Eastern European farmland. Divers. Distrib. 21, 722–730 (2015).
Donald, P. F., Green, R. E. & Heath, M. F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. Ser. B Biol. Sci. 268, 25–29 (2001).
Donald, P. F., Pisano, G., Rayment, M. D. & Pain, D. J. The Common Agricultural Policy, EU enlargement and the conservation of Europe’s farmland birds. Agric. Ecosyst. Environ. 89, 167–182 (2002).
Fragoso, R., Marques, C., Lucas, M. R., Martins, M. B. & Jorge, R. The economic effects of common agricultural policy on Mediterranean montado/dehesa ecosystem. J. Policy Model. 33, 311–327 (2011).
Ribeiro, P. F. et al. Modelling farming system dynamics in high nature value farmland under policy change. Agric. Ecosyst. Environ. 183, 138–144 (2014).
Suárez, F., Naveso, M. A. & De Juana, E. Farming in the drylands of Spain: birds of the pseudosteppes. In Farming and Birds in Europe. The common Agricultural Policy and its Implications for Bird Conservation (eds Pain, D. & Pienkowsky, M.) 297–330 (Academic Press, New York, 1997).
Hoogeveen, Y., Petersen, J. E., Balazs, K. & Higuero, I. High Nature Value Farmland: Characteristics, Trends and Policy Challenges. EEA Report. No 1/2004. European Environment Agency, Copenhagen, Denmark (2004).
Moreira, F., Pinto, M. J., Henriques, I. & Marques, T. The importance of low-intensive farming systems for fauna, flora and habitats protected under the european "birds" and "habitats" directives: is agriculture essential for preserving biodiversity in the mediterranean region? In Trends in Biodiversity Research (ed. Burk, A. R.) 117–145 (Nova Science Publishers, Huappauge, 2005).
Lomba, A. et al. Mapping and monitoring high nature value farmlands: challenges in European landscapes. J. Environ. Manag. 143, 140–150 (2014).
Delgado, A. & Moreira, F. Bird assemblages of an Iberian cereal steppe. Agric. Ecosyst. Environ. 78, 65–76 (2000).
Ribeiro, P. F. et al. An applied farming systems approach to infer conservation-relevant agricultural practices for agri-environment policy design. Land Use Policy 58, 165–172 (2016).
Stoate, C. et al. Ecological impacts of early 21st century agricultural change in Europe—a review. J. Environ. Manag. 91, 22–46 (2009).
Faria, N., Morales, M. B. & Rabaça, J. E. Exploring nest destruction and bird mortality in mown Mediterranean dry grasslands: an increasing threat to grassland bird conservation. Eur. J. Wildl. Res. 62, 663–671 (2016).
Santana, J. et al. Using beta diversity to inform agricultural policies and conservation actions on Mediterranean farmland. J. Appl. Ecol. 54, 1825–1835 (2017).
Trombulak, S. C. & Frissell, C. A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30 (2000).
Bernardino, J. et al. Bird collisions with power lines: state of the art and priority areas for research. Biol. Conserv. 222, 1–13 (2018).
Loss, S. R., Will, T. & Marra, P. P. Direct mortality of birds from anthropogenic causes. Annu. Rev. Ecol. Evol. Syst. 46, 99–120 (2015).
Hernández-Matías, A., Real, J., Parés, F. & Pradel, R. Electrocution threatens the viability of populations of the endangered Bonelli’s eagle (Aquila fasciata) in Southern Europe. Biol. Conserv. 191, 110–116 (2015).
Shaw, J. M., Reid, T. A., Schutgens, M., Jenkins, A. R. & Ryan, P. G. High power line collision mortality of threatened bustards at a regional scale in the Karoo, South Africa. Ibis (Lond. 1859) https://doi.org/10.1111/ibi.12553 (2017).
Borda-de-Água, L., Grilo, C. & Pereira, H. M. Modeling the impact of road mortality on barn owl (Tyto alba) populations using age-structured models. Ecol. Model. 276, 29–37 (2014).
Reijnen, R., Foppen, R. & Meeuwsen, H. The effects of traffic on the density of breeding birds in Dutch agricultural grasslands. Biol. Conserv. 75, 255–260 (1996).
Mcnew, L. B., Hunt, L. M., Gregory, A. J., Wisely, S. M. & Sandercock, B. K. Effects of wind energy development on nesting ecology of greater prairie-chickens in fragmented grasslands. Conserv. Biol. 28, 1089–1099 (2014).
Wolfe, D. H., Patten, M. A., Shochat, E., Pruett, C. L. & Sherrod, S. K. Causes and patterns of mortality in lesser prairie-chickens Tympanuchus pallidicinctus and implications for management. Wildl. Biol. 13, 95–104 (2007).
Shaw, J. M., Jenkins, A. R., Smallie, J. J. & Ryan, P. G. Modelling power-line collision risk for the Blue Crane Anthropoides paradiseus in South Africa. Ibis (Lond. 1859) 152, 590–599 (2010).
Birdlife International. The IUCN Red List of Threatened Species 2018 (2018). www.iucnredlist.org. Accessed 2nd August 2019.
Faria, N. Implications of Dry Grassland Management in the Ecology and Conservation of Grassland Birds in South Portugal (Universidad Autónoma de Madrid, Madrid, 2015).
Iñigo, A. & Barov, B. Action plan for the Little Bustard Tetrax tetrax in the European Union. Report. SEO| BirdLife and BirdLife International for the European Commission (2010).
Morales, M. B., García, J. T. & Arroyo, B. Can landscape composition changes predict spatial and annual variation of little bustard male abundance?. Anim. Conserv. 8, 167–174 (2005).
Moreira, F. et al. Mosaic-level inference of the impact of land cover changes in agricultural landscapes on biodiversity: a case-study with a threatened grassland bird. PLoS ONE 7, e38876 (2012).
Silva, J. P., Palmeirim, J. M. & Moreira, F. Higher breeding densities of the threatened little bustard Tetrax tetrax occur in larger grassland fields: implications for conservation. Biol. Conserv. 143, 2553–2558 (2010).
Silva, J. P. et al. EU protected area network did not prevent a country wide population decline in a threatened grassland bird. PeerJ 6, e4284 (2018).
García de la Morena, Bota, G., Mañosa, S. & Morales, M. B. El sisón común en España. II Censo Nacional (2016). Report (2018).
Traba, J. & Morales, M. B. The decline of farmland birds in Spain is strongly associated to the loss of fallowland. Sci. Rep. 9, 9473 (2019).
Marcelino, J. et al. Tracking data of the Little Bustard Tetrax tetrax in Iberia shows high anthropogenic mortality. Bird Conserv. Int. https://doi.org/10.1017/S095927091700051X (2017).
Bevanger, K. Biological and conservation aspects of bird mortality caused by electricity power lines: a review. Biol. Conserv. 86, 67–76 (1998).
Janss, G. F. E. Avian mortality from power lines: a morphologic approach of a species-specific mortality. Biol. Conserv. 95, 353–359 (2000).
Martin, G. R. Understanding bird collisions with man-made objects: a sensory ecology approach. Ibis. 153, 239–254 (2011).
Martin, G. R. & Shaw, J. M. Bird collisions with power lines: failing to see the way ahead?. Biol. Conserv. 143, 2695–2702 (2010).
Marques, A. T., Martins, R. C., Silva, J. P., Palmeirim, J. M. & Moreira, F. Power line routing and configuration as major drivers of collision risk in two bustard species. Oryx https://doi.org/10.1017/S0030605319000292 (2020).
Silva, J. P. et al. A spatially explicit approach to assess the collision risk between birds and overhead power lines: a case study with the little bustard. Biol. Conserv. 170, 256–263 (2014).
García, J., Suárez-Seoane, S., Miguélez, D., Osborne, P. E. & Zumalacárregui, C. Spatial analysis of habitat quality in a fragmented population of little bustard (Tetrax tetrax): implications for conservation. Biol. Conserv. 137, 45–56 (2007).
Osborne, P. E. & Suárez-Seoane, S. Identifying core areas in a species’ range using temporal suitability analysis: an example using little bustards Tetrax Tetrax L. in Spain. Biodivers. Conserv. 16, 3505–3518 (2007).
Santangeli, A. & Dolman, P. M. Density and habitat preferences of male little bustard across contrasting agro-pastoral landscapes in Sardinia (Italy). Eur. J. Wildl. Res. 57, 805–815 (2011).
Santos, M. et al. Impacts of land use and infrastructural changes on threatened Little Bustard Tetrax tetrax breeding populations: quantitative assessments using a recently developed spatially explicit dynamic modelling framework. Bird Conserv. Int. 26, 418–435 (2016).
Suárez-Seoane, S., Osborne, P. E. & Alonso, J. C. Large-scale habitat selection by agricultural steppe birds in Spain: identifying species-habitat responses using generalized additive models. J. Appl. Ecol. 39, 755–771 (2002).
Silva, J. P. et al. Estimating the influence of overhead transmission power lines and landscape context on the density of little bustard Tetrax tetrax breeding populations. Ecol. Model. 221, 1954–1963 (2010).
Morales, M. B., Traba, J., Carriles, E., Delgado, M. P. & de la Morena, E. L. G. Sexual differences in microhabitat selection of breeding little bustards Tetrax tetrax: ecological segregation based on vegetation structure. Acta Oecologica 34, 345–353 (2008).
Faria, N., Rabaça, J. E. & Morales, M. B. The importance of grazing regime in the provision of breeding habitat for grassland birds: the case of the endangered little bustard (Tetrax tetrax). J. Nat. Conserv. 20, 211–218 (2012).
Silva, J. P., Estanque, B., Moreira, F. & Palmeirim, J. M. Population density and use of grasslands by female Little Bustards during lek attendance, nesting and brood-rearing. J. Ornithol. 155, 53–63 (2014).
INE. Statistical data: Database. (2019). https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_base_dados. Accessed 9th May 2019.
Gameiro, J., Silva, J. P., Franco, A. M. A. & Palmeirim, J. M. Effectiveness of the European Natura 2000 network at protecting Western Europe’s agro-steppes. Biol. Conserv. 248, 108681 (2020).
Beja, P. et al. Predators and livestock reduce bird nest survival in intensive Mediterranean farmland. Eur. J. Wildl. Res. 60, 249–258 (2014).
van der Wal, R. & Palmer, S. C. Is breeding of farmland wading birds depressed by a combination of predator abundance and grazing?. Biol. Lett. 4, 256–258 (2008).
Lane, S. J., Alonso, J. C. & Martín, C. A. Habitat preferences of great bustard Otis tarda flocks in the arable steppes of central Spain: are potentially suitable areas unoccupied?. J. Appl. Ecol. 38, 193–203 (2001).
Ahlering, M. A., Johnson, D. H. & Faaborg, J. Conspecific attraction in a grassland bird, the Baird’s Sparrow. J. Field Ornithol. 77, 365–371 (2006).
Tarjuelo, R. et al. Not only habitat but also sex: factors affecting spatial distribution of Little Bustard Tetrax tetrax families. Acta Ornithol. 48, 119–128 (2013).
Reino, L. et al. Effects of changed grazing regimes and habitat fragmentation on Mediterranean grassland birds. Agric. Ecosyst. Environ. 138, 27–34 (2010).
Walters, K., Kosciuch, K. & Jones, J. Can the effect of tall structures on birds be isolated from other aspects of development?. Wildl. Soc. Bull. 38, 250–256 (2014).
Fahrig, L. & Rytwinski, T. Effects of roads on animal abundance: an empirical review and synthesis. Ecol. Soc. 14, 21 (2009).
Tryjanowski, P. et al. Conservation of farmland birds faces different challenges in Western and Central-Eastern Europe. Acta Ornithol. 46, 1–12 (2011).
Gudka, M., Santos, C. D., Dolman, P. M., Abad-Gómez, J. M. & Silva, J. P. Feeling the heat: elevated temperature affects male display activity of a lekking grassland bird. PLoS ONE 14, e0221999 (2019).
Silva, J. P., Catry, I., Palmeirim, J. M. & Moreira, F. Freezing heat: thermally imposed constraints on the daily activity patterns of a free-ranging grassland bird. Ecosphere 6, art119 (2015).
Alonso, H. et al. Male post-breeding movements and stopover habitat selection of an endangered short-distance migrant, the Little Bustard Tetrax tetrax. Ibis (Lond. 1859) 162, 279–292 (2020).
García de la Morena, E. L. et al. Migration patterns of Iberian little bustards Tetrax tetrax. Ardeola 62, 95–112 (2015).
Silva, J. P., Faria, N. & Catry, T. Summer habitat selection and abundance of the threatened little bustard in Iberian agricultural landscapes. Biol. Conserv. 139, 186–194 (2007).
Equipa Atlas. Atlas das aves nidificantes em Portugal (1999–2005). (Instituto da Conservaçăo da Natureza e da Biodiversidade, Sociedade Portuguesa para o Estudo das Aves, Parque Natural da Madeira e Secretaria Regional do Ambiente e do Mar, 2008).
De Juana, E. & Martínez, C. Distribution and conservation status of Little bustard Tetrax tetrax in the Iberian Peninsula. Ardeola 43, 157–167 (1996).
Delgado, A. & Moreira, F. Between-year variations in Little Bustard Tetrax tetrax population densities are influenced by agricultural intensification and rainfall. Ibis (Lond. 1859) 152, 633–642 (2010).
DGT. Especificações técnicas da Carta de Uso e Ocupação do Solo de Portugal Continental para 1995, 2007, 2010 e 2015. 103 (2018).
INE. Recenseamento Agrícola 1999—Análise de resultados (2001).
INE. Recenseamento Agrícola 2009—Análise dos principais resultados (2011).
Haklay, M. & Weber, P. Openstreetmap: user-generated street maps. IEEE Pervasive Comput. 7, 12–18 (2008).
R Core Team. R: a language and environment for statistical computing (2016).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017).
Wood, S. N. Generalized Additive Models: An Introduction with R 2nd edn. (Chapman and Hall/CRC, Boca Raton, 2017).
Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Springer, New York, 2009). https://doi.org/10.1007/978-0-387-87458-6.
Wood, S. N. mgcv: mixed GAM computation vehicle with automatic smoothness estimation. R package version 1.8-24. 302 (2018).
Bjørnstad, O. N. & Falck, W. Nonparametric spatial covariance functions: estimation and testing. Environ. Ecol. Stat. 8, 53–70 (2001).
Rhodes, J. R., McAlpine, C. A., Zuur, A. F., Smith, G. M. & Ieno, E. N. Mixed Effects Models and Extensions in Ecology with R 469–492 (Springer, Berlin, 2009). https://doi.org/10.1007/978-0-387-87458-6_21.
Bjørnstad, O. N. ncf: spatial nonparametric covariance functions. R package version 1.1–7. (2016).
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.osgeo.org (2017).
Acknowledgements
We are grateful to Paulo Flores Ribeiro for the help with the livestock data and to all colleagues that carried out the field work during both surveys: Agostinho Tomás, Ana Martins, Carlos Franco, Célia Medeiros, Conceição Conde, Bruno Martins, Carlos Carrapato, Carlos Cruz, Carlos Pacheco, Cristina Cardoso, David Carvalho, Eduardo Santos, Eunice Pereira, Fernando Abegão, Fernando Queirós, Fernando Romba, Hany Alonso, Henrique Velez, Hugo Lousa, Hugo Sampaio, Ivan Kljun, Iván Prego, Joana Alves, João Carlos Claro, Joaquim Pífano, José Paulo Martins, Luís Venâncio, Marcello D'Amico, Márcia Pinto, Miguel Lecoq, Nuno Faria, Paulo Marques, Pedro Alverca, Pedro Capa, Pedro Salgueiro, Raquel Ventura, Ricardo C. Martins, Ricardo Silva, Rui Morgado, Rui Pedroso, Teresa Catry and Teresa Silva. The first national little bustard survey was coordinated by the Portuguese Nature Conservation Institute (ICNF—Instituto da Conservacão da Natureza e da Biodiversidade) and funded by a European Union LIFE project (LIFE02NAT/P/8476). The 2016 survey was coordinated by the REN Biodiversity Chair/CIBIO with the collaboration of ICNF, Liga para a Proteccão da Natureza, Quercus—Associação Nacional de Conservação da Natureza, Sociedade Portuguesa para o Estudo das Aves, and LABOR—Laboratório de Ornitologia/University of Évora. Fundação para a Ciência e a Tecnologia (FCT) funded ATM work through a doctoral grant (SFRH/BD/100147/2014) and JPS with a contract DL57/2019/CP 1440/CT 0021. FM was funded by REN Biodiversity Chair and FCT (IF/01053/2015).
Author information
Authors and Affiliations
Contributions
A.T.M.: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft. F.M.: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft. R.A.: Investigation, Resources. A.D.: Investigation, Resources. C.G.: Investigation, Resources. H.S.: Investigation, Resources. P.R.: Investigation, Resources. N.S.: Investigation, Resources. J.P.: Conceptualization, Supervision, Writing—Review and Editing. J.P.S.: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Supervision, Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marques, A.T., Moreira, F., Alcazar, R. et al. Changes in grassland management and linear infrastructures associated to the decline of an endangered bird population. Sci Rep 10, 15150 (2020). https://doi.org/10.1038/s41598-020-72154-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72154-9
This article is cited by
-
Temperature and microclimate refugia use influence migratory timings of a threatened grassland bird
Movement Ecology (2023)
-
A nationwide collapse of a priority grassland bird related to livestock conversion and intensification
Scientific Reports (2023)
-
Differential responses to habitat structure and degradation by two Grassbirds (Fam. Locustellidae) syntopic in floodplain grassland
Wetlands Ecology and Management (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.