Abstract
The endothelin system has an important role in bone modelling during orthodontic tooth movement (OTM); however, little is known about the involvement of endothelin B receptors (ETB) in this process. The aim of this study was to evaluate the role of ETB in bone modelling during OTM using ETB knockout rats (ETB-KO). Thirty-two male rats were divided into 4 groups (n = 8 per group): the ETB-KO appliance group, ETB-KO control group, wild type (ETB-WT) appliance group, and ETB-WT control group. The appliance consisted of a super-elastic closed-coil spring placed between the first and second left maxillary molar and the incisors. Tooth movement was measured on days 0 and 35, and maxillary alveolar bone volume, osteoblast, and osteoclast volume were determined histomorphometrically on day 35 of OTM. Next, we determined the serum endothelin 1 (ET-1) level and gene expression levels of the osteoclast activity marker cathepsin K and osteoblast activity markers osteocalcin and dentin matrix acidic phosphoprotein 1 (DMP1) on day 35. The ETB-KO appliance group showed significantly lower osteoblast activity, diminished alveolar bone volume and less OTM than the ETB-WT appliance group. Our results showed that ETB is involved in bone modelling in the late stage of OTM.
Similar content being viewed by others
Introduction
Endothelin 1 (ET-1) plays an important role in the regulation of bone metabolism in physiological as well as in pathophysiological processes1,2,3,4. It has a known role in the maintenance of bone homeostasis and the regulation of osteoblastic function. ET-1 stimulates the proliferation, differentiation and activity of osteoblasts5,6 and inhibits osteoblast apoptosis, promoting osteoblastic growth7. ET-1 acts through both endothelin A (ETA) and endothelin B (ETB) receptors. Specifically, it has been shown that the ET-1/ETA axis is an important regulator of osteoblast activity; targeted inactivation of ETA in mature osteoblasts induced lower tibial trabecular bone volume in vivo8. However, less is known about the role of ETB in osteogenesis. In one study, treatment with the dual ETA and ETB antagonist Macitentan showed decreased vertebral bone mass in mice, potentially from decreased osteoblast activity as well as from the increased osteoclast activity9.
Several studies also indicate the involvement of endothelins during orthodontic tooth movement (OTM)10,11,12. Orthodontic movement is a consequence of applying a force to the teeth. It is a mechanism that involves the biomechanical adaptation of the alveolar process and supporting periodontium. Alterations in the vascularity within the periodontal ligament (PDL), the connective tissue that connects a tooth to its surrounding alveolar bone, may trigger responses at the cellular level, such as alveolar bone modelling13,14. During OTM areas of pressure and tension are formed in the PDL. At pressure areas, which appear in the direction of the application of force, alveolar bone is resorbed, and at tension areas, which appear on the opposite side, new bone is formed (Fig. 1). Therefore the animal model of OTM can be used to study the accelerated bone modelling15,16. OTM consists of three different phases—the initial, lag and late phase. In the late phase linear teeth movement can be observed17.
Model of the OTM. Applying of orthodontic force to the tooth compresses the PDL. At the compression side of the tooth, which appears in the direction of the orthodontic force, bone resorption takes place, carried out mainly by osteoclasts. At the tension side, osteoblasts are responsible for the bone formation process.
ET-1 release in the PDL begins after 3 h of continuous loading of a rat molar18. However, in the alveolar bone ET-1 gene expression levels increased for the following 4 weeks after the start of the application of orthodontic force and predominated in the late phase of OTM, when the ETB expression rate was also upregulated11. A selective ETA antagonist significantly increased alveolar bone volume and decreased osteoclast volume and the amount of OTM, indicating decreased bone resorption in the late stage of OTM, and confirming the role of ETA in accelerated bone modelling11. On the other hand, a dual-selective endothelin antagonist (ETA/ETB) increased the amount of OTM in rats after 25 days of treatment12. Furthermore, the gene expression levels of both ETA and ETB were increased in the late phase of OTM11, when bone formation on the tension side and bone resorption on the pressure side of the loaded tooth were reported17, suggesting that both receptor subtypes could be involved in the process of the late stage of OTM.
The multipotent stem cells in the alveolar bone marrow, the PDL and the periosteum all participate in the regulation of bone remodelling and tooth movement. Mesenchymal stem cells (MSCs), when stimulated by a mechanical strain, differentiate into an osteo-chondrogenic lineage with increased expression of osteoblastic and chondrogenic markers19. Periodontal ligament stem cells (PDLSCs) are tissue-specific MSCs in PDL that play an important role during OTM20. It has been shown that both tension and compression can regulate the osteogenic differentiation of PDLSCs16,21. Interestingly, it has also been reported that ET-1 promotes the osteogenic differentiation of bone marrow-derived MSCs22 and PDLSCs23. Moreover, some studies mention an important role of both ETA and ETB receptors in the differentiation of different types of MSCs into different cellular phenotypes24,25.
ET-1 has been shown to have anti-apoptotic activity in numerous tissues. For example, it inhibits bone degradation by the induction of anti-apoptotic activity, and stimulates bone formation via endothelin ETA receptors in vitro and in vivo26. ET-1 is also known as an anti-apoptotic factor in osteoblasts7, and it promotes osteosarcoma cell invasion and survival against cisplatin-induced apoptosis through the ETA receptor4. The anti-apoptotic effects of ET-1 have been described in several other tissues: neurons27, cardiomyocytes28, and airway smooth muscle cells29.
The role of ETB has mostly been studied in cancer cell lines and in cancer tissue cultures30. The activation of ETB receptors by ET-1 has been shown to affect the processes involved in the inhibition of cancer, inducing cell death by apoptosis and promoting ET-1 clearance31,32. ETB expression has also been reported in many tumour types, including prostate cancer33,34 melanomas35, and oligodendrogliomas36. For example, in prostate cancer, the downregulation of ETB results in a higher local concentration of ET-1 which, through the stimulation of ETA receptors, facilitates cancer progression, including proliferation, escape from apoptosis and new bone formation34. ETB has been predominantly classified as a “clearance receptor”, and the role of circulatory ET-1 clearance by ETB has been confirmed in several studies32,37,38. In healthy men an injection of a selective ETB antagonist increased the plasma concentration of ET-1 and confirmed the crucial role of ETB in the clearance of endothelins in humans39.
Despite the fact that the role of ETA in bone modelling during OTM has been studied, the role of ETB in bone modelling in OTM is not well understood at present. The aim of this study was to determine the role of ETB in bone modelling in OTM using ETB–KO rats and to evaluate its effect on osteoclastogenesis and osteoblastogenesis in comparison to bone modelling in ETB–WT rats.
Material and methods
Laboratory animal model
The study was performed on ETB knockout (ETB-KO −/−) and wild type (ETB-WT +/+) rats. The ETB-KO rat line and its control line ETB-WT was established at the laboratory of Masashi Yanagisawa, PhD, at Howard Hughes Medical Institute (University of Texas Southwestern Medical Centre, Dallas, Texas, USA). The ETB-KO animals were incorporated with a dopamine beta-hydroxylase (DβH) transgene to enable the development of a normal enteric nervous system. The DβH transgene was also inserted into the control animals40. The rats were bred in a homozygous line at the Faculty of Medicine, University of Ljubljana (Slovenia). In this study we used 32 male ETB-KO rats (285 ± 27 g, 13–15 weeks old) and 32 male ETB-WT rats (286 ± 30 g, 13–15 weeks old). The animals were housed as well as procedures were identical to our previous studies11. The part of daily intake of rat chow (Teklad 2016 Global rodent diet, Harlan, The Netherlands) was soaked in water to facilitate food intake due to its mild impairment during orthodontic force application.
To ensure general anaesthesia, the anaesthetics were injected intraperitoneally: ketamine 50 mg/kg body weight (Bioketan, Vetoquinol Biowet, Gorzów Wielkopolski, Poland), medetomidine hydrochloride 67 mg/kg body weight (Domitor, Pfizer, Brooklyn, NY, USA), and thiopental 3 mg/kg body weight (Tiopental, Pliva, Zagreb, Croatia)12.
All the animal procedures and the study protocol were approved by the Ethics Committee for Animal Experiments of the Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection (34401-62/2008/20), and complied with the guiding principles in ''The Care and Use of Animals''.
Study protocol
Thirty-two rats were divided into the following four groups: (1) ETB-KO appliance group (n = 8); (2) ETB-KO control group (n = 8); (3) ETB-WT appliance group (n = 8); (4) ETB-WT control group (n = 8).
The orthodontic appliance consisted of a superelastic closed coil spring (25 cN; wire diameter, 0.15 mm; GAC Dentsply International, York, PA, USA) which was placed between the first and second left maxillary molars and the incisors by a stainless-steel ligature in the ETB-KO and ETB-WT appliance groups11. The coil spring was attached to a steel thread placed around the first and second molar on one side and through a drilled hole into the upper incisors on the other side (Fig. 2). The hole was drilled laterally in the incisors through the area where the vivid tooth structures were unaffected12. The orthodontic appliance was placed in each animal under general anaesthesia at the beginning of the study, and replaced to the correct position every 7 days, ensuring its proper activation and the exertion of constant force on the teeth41.
Tooth movement measurements
The distance between the most mesial point of the maxillary first molar and the most distal point of the ipsilateral incisor at the gingival level was measured on the experimental sides in all four groups. The measurements were obtained using a digital calliper (Digitronic Calliper, 144–15 D (Wilson & Wolpert, Utrecht, The Netherlands)) while the animals were under general anaesthesia at days 0 and 35. All the measurements were carried out twice by two investigators independently within a period of few minutes and the reliability of the measurements was assessed by using the intraclass correlation coefficient (ICC) as described in previous studies11,42. Tooth movement was calculated by subtracting the distance between the teeth on day 35 from the distance between the teeth on day 0.
Preparation of histology samples and bone histomorphometry
On day 35, all the animals were sacrificed by intraperitoneal injection of anaesthetics and carbon dioxide. Tissue samples of the maxilla containing all 3 molars were taken. Samples were prepared in vertical section perpendicular to the occlusal plane of the molars. Paraffin blocks were then prepared and stained with haematoxylin and eosin (Fig. 3) as previously published in Plut et al.42.
(A) Schematic representation of the examined areas in part of the maxilla with all three molars (M1, M2, M3). Alveolar bone volume was determined around the first and second maxillary molars (area surrounded by dashed line). Osteoblast and osteoclast volumes were determined along the mesial and distal roots of the second maxillary molar (green areas surrounded by dashed line). The arrow shows direction of tooth movement. (B) Examples of osteoclast (left picture) and osteoblast (right picture) under 40-fold magnification. Al.b—alveolar bone; Ob—osteoblast; Oc—osteoclast; PDL—periodontal ligament; T—tooth.
Bone histomorphometry was used to determine alveolar bone, osteoclast and osteoblast volume density in all four groups. Histomorphometry was performed by using a point-counting method. For this purpose, the stereologic cycloid grid system incorporated into the ocular of a light microscope (BX-60, Olympus, Tokyo, Japan) was used. As described by Sprogar et al.11, the alveolar bone area was expressed as the percentage of alveolar bone area versus the tissue area consisting of tooth, PDL, connective tissue and bone marrow spaces. In addition, the osteoblast and osteoclast areas were defined as the alveolar bone area covered with osteoblasts or osteoclasts versus alveolar bone area. The cells were counted in the alveolar bone alongside the mesial and distal roots of the second molar42. However, because 20 sections from each specimen were examined, alveolar bone area, osteoblast area and osteoclast area were extrapolated to alveolar bone volume, osteoblast volume and osteoclast volume, as we already described in previous studies11,42.
Endothelin 1 (ET-1) determination
Endothelin 1 levels were measured in rat serum in all 4 groups using the commercially available kit Endothelin 1 ELISA Kit (ab133030, Abcam, Cambridge, MA, USA), according to the manufacturer’s instructions. Briefly, standards and samples were added to the designated wells of a microplate, precoated with an endothelin-1 specific antibody. The plate was incubated at room temperature for 1 h, washed and HRP-conjugated. The endothelin 1 detection antibody was added to each well. After incubation, the unbound detection antibodies were removed and TMB was added to visualise the HRP enzymatic reaction. After incubation, a stop solution was added and the absorbance was read at 450 nm, with correction at 570 nm, using Tecan Safire (Tecan Group Ltd., Switzerland).
RNA isolation and semiquantitative RT-PCR
Osteocalcin and DMP1 gene expression levels were used to determine osteoblast activity, and the cathepsin K gene expression level was used to determine osteoclast activity in all 4 groups43,44. The maxillary bones with all 3 molars and their PDLs were excised and immediately frozen in liquid nitrogen. RNA isolation and semiquantitative RT-PCR was performed as described by Plut et al.42. Oligonucleotides for cathepsin K, osteocalcin and DMP1 were chosen from predesigned assays (TaqMan Gene Expression Assays, Applied Biosystems). Thermal cycling, construction of standard curve and cDNA amplification and quantification were performed as we reported in a previous study43. In order to exclude variations from different inputs of total mRNA to the reaction, data on cathepsin K, osteocalcin and DMP1 were normalised to an internal housekeeping gene, GAPDH, for which data was obtained by using TaqMan GAPDH predesigned assays (TaqMan Gene Expression Assays, Applied Biosystems). All the reactions for standard samples and for samples from all 4 groups were performed in duplicate. The data were averaged from the values obtained in each reaction43. For all the transcripts tested, a time-course-dependent gene expression consensus profile was observed after normalisation to the expression of the housekeeping gene GAPDH.
Statistical analyses
The data were expressed as means ± standard error of the mean (SEM) and calculated for each parameter for all the animal groups. The evaluated parameters were tooth movement, alveolar bone volume density, osteoclast and osteoblast volume densities, serum ET-1 level, and gene expression levels of cathepsin K, DMP1 and osteocalcin. Comparisons within and between the groups were performed using analysis of variance (ANOVA), followed by the Tukey multiple comparison test. A P value less than 0.05 was considered statistically significant.
Interexaminer reliability for the measurements of the distance between the teeth was tested using the intraclass correlation coefficient (ICC).
In the results, not all the groups contained the initial number of rats (n = 8 per group). During the experiment some of the rats had to be excluded. The number of samples in each group is explicitly stated in the Figures.
Results
Orthodontic tooth movement and physiologic distal drift measurements after 35 days
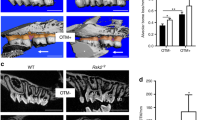
The overall mean value of the ICC for all the measurements of the distances was 0.938. In the ETB-KO appliance group the amount of OTM (1.67 mm ± 0.10 mm) was significantly less pronounced than in the ETB-WT appliance group (2.28 mm ± 0.17 mm) on day 35 (P = 0.0255). The physiologic distal drift did not significantly differ between the ETB-KO and ETB-WT groups (Fig. 4).
Amount of total tooth movement after 35 days. Significant differences were observed between the ETB-KO and ETB-WT appliance groups (P = 0.0255) and between appliance and control groups (P < 0.0001). The physiological distal drift was non-significantly decreased in the ETB-KO control group in comparison to the ETB-WT control group. The data are presented as mean ± SEM and analysed by the one-way ANOVA and Tukey post hoc tests.
Histomorphometric analyses
The histomorphometric analysis showed that after 35 days of OTM the alveolar bone volume was significantly lower in the ETB-KO appliance group (35.26% ± 1.47%) in comparison to ETB-KO control group (44.45% ± 1.63%) (P = 0.0004). Furthermore, the alveolar bone volume was significantly less in the ETB-KO appliance group than in the ETB-WT appliance group (45.26% ± 1.74%) (P = 0.0001) (Fig. 5a). No significant differences in the osteoblast volume were observed between the groups. Osteoblast volume was non-significantly increased in the ETB-KO control group in comparison to the other three groups (Fig. 5b). The osteoclast volume was significantly increased in the ETB-KO (1.08% ± 0.13%) and ETB-WT appliance (1.30% ± 0.10%) groups compared to their controls (0.47% ± 0.06% and 0.69% ± 0.06%, respectively) (P < 0.0001). The osteoclast volumes in the ETB-KO appliance and the ETB-KO control groups were less than in the ETB-WT groups, but the differences were not significant (Fig. 5c).
Histomorphometric analyses of the maxillary bone specimens after 35 days. The alveolar bone volume in the ETB-KO appliance group was significantly less than in the ETB-WT appliance group (P = 0.0001) (a); no significant (NS) differences in the osteoblast volume were observed between the groups (b); the osteoclast volume was significantly increased in the ETB-KO and ETB-WT appliance group compared to the control groups (P < 0.0001) (c). The data are presented as mean ± SEM and analysed by the one-way ANOVA and Tukey post hoc tests. (−)—control, ( +)—appliance.
Serum endothelin 1 levels
The serum endothelin level (ET-1) on day 35 was significantly higher in the ETB-KO control rats (3.04 pg/ml ± 0.43 pg/ml) compared to the WT control rats (1.46 pg/ml ± 0.18 pg/ml) (P = 0.0002) and between the ETB-KO appliance rats (2.78 pg/ml ± 0.13 pg/ml) and the ETB-WT appliance rats (1.70 pg/ml ± 0.12 pg/ml) (P = 0.0023) (Fig. 6).
Blood serum endothelin level (ET-1) during the experimental period (35 days). The serum endothelin level (ET-1) was significantly higher in the ETB-KO control rats than in the WT control rats (P = 0.0002), and in the ETB-KO appliance group compared to the WT appliance group (P = 0.0023). The data are presented as mean ± SEM and analysed by the one-way ANOVA and Tukey post hoc tests. (−)—control; ( +)—appliance.
RT-PCR analysis
The gene expression level of osteocalcin on day 35 was significantly downregulated in the ETB-KO appliance group compared to the ETB-WT appliance group (P = 0.0157). A significant difference in the gene expression level of osteocalcin was also determined between the ETB-KO appliance group and the ETB-KO control group (P = 0.0288) (Fig. 7a). Similarly, the gene expression of DMP1 was significantly downregulated in the ETB-KO appliance group in comparison to the WT appliance group (P = 0.0040) (Fig. 7b). The gene expression level of cathepsin K was downregulated in both appliance groups compared to the control groups. Significant differences were determined between the ETB-KO control group compared to the ETB-KO appliance group (P = 0.0314) (Fig. 7c).
Relative gene expression levels of osteocalcin, DMP1 and cathepsin K in the alveolar bone and periodontal ligament after 35 days of OTM. Osteocalcin (a) and DMP1 (b) gene expression levels were significantly downregulated in the ETB-KO appliance group compared to the ETB-WT appliance group (P = 0.0157 and P = 0.0040, respectively). A significant difference was observed in the gene expression level of cathepsin K between the ETB-KO groups (P = 0.0314), but not between the ETB-WT groups (c). All gene expressions were normalised to the reference gene GAPDH. The data are presented as mean ± SEM and analysed by the one-way ANOVA and Tukey post hoc tests. (−)—control, ( +)—appliance.
Discussion
The results of this study showed that ETB is involved in bone modelling during the late stage of OTM in the rat animal model. The amount of OTM after 35 days of the experiment was significantly less in the ETB-KO appliance group than in the ETB-WT group (P = 0.0255). There was a significant difference in the alveolar bone volume in the ETB-KO appliance group compared to the ETB-WT appliance group (P = 0.0004), probably due to diminished osteoblast activity in the ETB-KO appliance group.
Some of the ETB antagonists showed significant inhibition of ET-1 effects in vitro45,46; however, none of the available antagonists have an established pharmacological and toxicological profile. Furthermore, they have to be administered intravenously and daily intravenous application over a longer period of time represents a great deal of stress for the animals. To study the effects of ET-1 in a reduced ETB response, the most suitable model is to use ETB knock-out animals. The ETB-KO strain of rats used in the present study is described as a natural mutation in the progeny of a Wistar rat. Mutations in the ETB gene have been linked to Hirschsprung’s disease in humans, a congenital disease characterised by aganglionic megacolon, an absence of enteric ganglia, and a lack of innervations to the lower gastrointestinal tract. The disease is associated with polymorphism and several missense mutations in the ETB gene which lead to decreased expression, changes in cell signalling, and loss of endothelin ET receptor function47,48. The animals used in this study were incorporated with a transgene (DβH) to enable the development of a normal enteric nervous system. The resulting transgenic rats are healthy but present with a total absence of ETB in all non-adrenergic tissues40. The animal model of OTM used in this study had already been confirmed as appropriate for studying the role of endothelin system in bone modelling10,11,12. The advantage of the model was the minimally invasive placement of the coil spring between the molars and incisors to maximally avoid injuries to the vital structures in the incisors and surrounding structures which may otherwise cause an inflammatory response and interfere with the results of the OTM. The force used in the experiment was constant and continuous41,49. The duration of the experiment is important in studying the processes in bone modelling during OTM. Bone formation on the tension side and bone resorption on the pressure side has been reported in the late stage of OTM; a time period usually around 2–4 weeks after the force has been applied17.
Both appliance groups showed lower alveolar bone volume compared to their control groups. Furthermore, the alveolar bone volume in the ETB-KO appliance group was significantly less than that of the ETB-WT appliance group. Differences in alveolar bone volume during physiological and pathological processes depend on the relationship between bone formation and bone resorption. Similarly to our study, histomorphometric analyses in previous studies have shown that alveolar bone volume was less in appliance groups than in their control groups11,42,43. We studied bone formation by determining osteoblast volume and osteoblast activity using osteocalcin and DMP1. There were no significant differences in osteoblast volumes between the four groups, but there was a significant decrease in osteoblast activity as determined by the gene expression levels for osteocalcin and DMP1 in the ETB-KO appliance group compared to the ETB-WT appliance group after 35 days of OTM. It has been shown that bone formation after the application of orthodontic force is increased predominantly by stimulating the differentiation of osteoblasts, and to a lesser extent by an increase in the number of these cells50. This is in concordance with our results, which show no considerable difference in osteoblast volume between the groups, but significant changes in osteoblast activity. Osteocalcin plays an important role in the maturation of mineral species51, and modulates osteogenic differentiation of MSCs52; DMP1 has a similar role in osteoblast differentiation and matrix mineralisation. Interestingly, it has also been reported that recombinant DMP1 induces the osteogenic differentiation of human periodontal ligament cells53, and appears to play an important role in the osteogenic differentiation of dental follicle stem cells54.
In a study on Bone Marrow-Derived Stem Cells (BMSCs) it was shown, using specific antagonists, that both receptors ETA and ETB are involved in the differentiation of BMSCs into active osteoblasts, and the osteogenesis of BMSCs was attenuated by blocking ETA and/or ETB receptors. The findings of this study reveal that both ETA and ETB receptors and downstream AKT and ERK signalling are involved in ET-1 primed lineage specification of MSCs24. Similarly, a study on ETB-KO mice showed less fibroblast activation and myofibroblast formation in response to bleomycin or ET-125. Therefore, in our study, the lower expression levels of osteoblast activity markers, DMP1 and osteocalcin could be a result of attenuated osteoblast maturation and/or osteogenic differentiation of MSCs, a process that is mediated through both receptors ETA and ETB. In the absence of ETB in the ETB-KO appliance group, osteogenesis is attenuated, resulting in a lower alveolar bone volume and a decreased amount of OTM (Fig. 8).
Possible mechanisms in PDL space are represented in this Figure, ultimately leading to decreased alveolar bone volume and a lower amount of OTM in the ETB-KO rats. The crossed-out triangles indicate the absence of ETB receptors. ET-1—Endothelin-1; ETA—Endothelin receptor A; ETB—Endothelin receptor B; EC—Endothelial cell; PDLSC—Periodontal Ligament Stem Cell.
The bone resorption process was studied by determining osteoclast volume and osteoclast activity using cathepsin K, a marker of bone resorption. A considerable increase in osteoclast volume was determined in both appliance groups compared to the control groups after 35 days of OTM. Similar results were obtained in a previous study, where osteoclast volume increased in all animal appliance groups11. In the late stage of OTM, we expected the upregulation of the cathepsin K gene expression level in the ETB-KO and ETB-WT appliance groups, whereas the osteoclast volume increased significantly in both appliance groups43. However, a significant downregulation of the gene expression level of cathepsin K appeared in the ETB-KO appliance group compared to ETB-KO control group. Several lines of evidence suggest that changes in osteoclast function and volume/number do not always happen simultaneously55. In a previous study it was shown that the absence of ETB in osteoclast specific ETB-KO mice impairs the formation of mature osteoclasts and impairs bone resorption activity with no influence on the expression of osteoclastogenic genes56. ET-1/ETB axis was shown to enhance osteoclast differentiation via co-stimulation of RANKL and M-CSF signalling and ETB deficiency impaired bone resorption activity and formation of mature osteoclasts56. However, in the present study, there were no significant differences between the ETB-KO and ETB-WT appliance groups in terms of osteoclast volume and osteoclast activity. The applied force due to the coil spring used in the OTM model induced osteoclast activity independently of the presence or absence of ETB or increased circulatory ET-1.
In the present study, the amount of OTM in the ETB-KO appliance group was significantly less than in the ETB-WT appliance group. Because of the diminished alveolar bone volume and lower osteoblast activity, a higher amount of OTM would initially be expected. However, we observed a lower amount of OTM, and similar results were also reported in Plut et al.42. During OTM, due to the lower osteoblast activity, bone formation at the tension site cannot keep up with bone resorption at the compression site, and the consequences are a widening of the PDL space and increased tooth mobility57. Tooth movement requires a coupling of bone resorption and bone formation. Due to the delayed bone formation in the absence of ETB there may be a further aggravation of the uncoupling of bone formation and bone resorption, expressing as suppressed bone turnover and resulting in lower amount of OTM. In rats, molars naturally drift distally. Therefore, there is predominately bone resorption on the distal side and bone formation on the mesial side of molar roots58. In the present study, the distal drift was smaller in the ETB-KO control group than in the ETB-WT control group, but there was no significant difference. These results suggest a different mechanism of alveolar bone turnover under physiological conditions in comparison to OTM, shown in the ETB-KO groups.
In the ETB-KO control group the serum ET-1 level was significantly higher than in the ETB-WT control group. It was also significantly elevated in the ETB-KO appliance group in comparison to ETB-WT appliance group. This is in concordance with many studies that confirmed the role of ETB as a ET-1 clearance receptor32,37,38,39,59. The lower amount of OTM in this study could be assigned to several processes, among them attenuated osteoblast maturation and increased ET-1 due to a lack of circulatory ET-1 clearance. High ET-1 levels are normally almost exclusively cleared by endothelial ETB, which was lacking in our animal model. It has been shown that chronic exposure of ET receptors to increased plasma ET-1 levels results in a significantly reduced density of ETA in mice aortas, correlating with a reduction in functional response to ET-160. Similarly, ETB-KO mice were found to have a 45% lower ETA density and significantly reduced expression (lower ETA mRNA) in peripheral tissues with no change in receptor affinity. A potential mechanism of reduced ETA density in ETB-KO models was proposed as the compensatory downregulation of the receptor in response to higher circulating ET-1 levels as a result of a lack of ETB clearing receptors or some other role of ETB in the development of cells expressing ETA receptors60,61. It is therefore possible that in our study high serum ET-1 in the ETB-KO groups resulted in downregulation of the ETA receptors, which already have an established role in bone modelling, resulting in diminished alveolar bone volume and lower amount of OTM (Fig. 8).
Many studies report interactions (cross talk) between ETA and ETB receptors, which means that the activation or inhibition of one receptor subtype can alter the function of another receptor subtype. For example, using ETB receptor-deficient rats it was reported that both ETA and ETB are involved in ET-1-induced DNA synthesis in astrocytes, accompanied by MAPK activation62. Similarly, it was shown that both ETA and ETB regulate lung myofibroblast proliferation, indicating possible interactions between receptor subtypes63. In addition, endothelin receptors (ETA and ETB) also exist in the form of homo- and heterodimers64, and for many receptor heterodimers, co-expression of both receptor subtypes is crucial for functional receptor activity, pharmacological proprieties, maturation, and proper cell-surface trafficking65,66. Another example of cooperation between receptor subtypes are HEK 293 cells which, transfected with both receptors, display a considerably prolonged increase in intracellular Ca2+ concentration in response to ET-1 or the selective ETB receptor agonist Sarafotoxin 6c, in comparison to more transitory responses of cells transfected with either receptor subtype alone67. It is therefore possible that similar interactions between the two receptor subtypes are also necessary for normal bone modelling during the late stage of OTM.
Another potential explanation for reduced OTM is anti-apoptotic activity of elevated ET-1 in the two ETB-KO groups. Specifically, apoptosis of osteocytes is critical for osteoclast activation and resorption at the compression site of the tooth during OTM68,69. Moreover, in a study of OTM with micro-osteoperforations it was shown that the rate of OTM is increased by increased apoptosis and cell proliferation of PDL cells70. Therefore, the lower amount of OTM in the absence of ETB could be a result of several different processes, including the anti-apoptotic activity of ET-1 on numerous cells in periodontal tissues. One limitation of the study, however, are only two time points in the experiment. Thus, no exact mechanism of the action can be elucidated. We rather propose possible mechanisms through which ETB-KO modulated bone modelling during OTM (Fig. 8).
Conclusion
Our results showed for the first time that ETB is involved in bone modelling in the late stage of OTM. ETB-KO resulted in lower osteoblast activity and therefore decreased alveolar bone volume and lower amount of OTM. This could be due to the role of ETB in osteoblast maturation and/or the differentiation of mesenchymal precursor cells, the adaptive downregulation of ETA as a response to high levels of circulating ET-1 or anti-apoptotic activity of ET-1. Further research is needed to explain the exact mechanism by which ETB modulates bone modelling in OTM, and which of the proposed mechanisms is predominant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nelson, J. B. et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology 53, 1063–1069. https://doi.org/10.1016/s0090-4295(98)00658-x (1999).
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 100, 10954–10959. https://doi.org/10.1073/pnas.1830978100 (2003).
Bagnato, A. & Natali, P. G. Endothelin receptors as novel targets in tumor therapy. J. Transl. Med. 2, 16. https://doi.org/10.1186/1479-5876-2-16 (2004).
Zhao, Y., Liao, Q., Zhu, Y. & Long, H. Endothelin-1 promotes osteosarcoma cell invasion and survival against cisplatin-induced apoptosis. Clin. Orthop. Relat. Res. 469, 3190–3199. https://doi.org/10.1007/s11999-011-1939-2 (2011).
Von Schroeder, H. P., Veillette, C. J., Payandeh, J., Qureshi, A. & Heersche, J. N. M. Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone 33, 673–684 (2003).
Clines, G. A. et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol. Endocrinol. 21, 486–498 (2007).
Van Sant, C. et al. Endothelin signaling in osteoblasts: Global genome view and implication of the calcineurin/NFAT pathway. Mol. Cancer Ther. 6, 253–261. https://doi.org/10.1158/1535-7163.MCT-06-0574 (2007).
Clines, G. A. et al. Regulation of postnatal trabecular bone formation by the osteoblast endothelin A receptor. J. Bone Miner. Res. 26, 2523–2536 (2011).
Liu, Z.-Y. et al. Less vertebral bone mass after treatment with macitentan in mice: A pilot study. BioMed Res. Int. https://doi.org/10.1155/2019/2075968 (2019).
Sprogar, S., Meh, A., Vaupotic, T., Drevensek, G. & Drevensek, M. Expression levels of endothelin-1, endothelin-2, and endothelin-3 vary during the initial, lag, and late phase of orthodontic tooth movement in rats. Eur. J. Orthod. 32, 324–328. https://doi.org/10.1093/ejo/cjp091 (2010).
Sprogar, S., Vaupotic, T., Cör, A., Drevensek, M. & Drevensek, G. The endothelin system mediates bone modeling in the late stage of orthodontic tooth movement in rats. Bone 43, 740–747. https://doi.org/10.1016/j.bone.2008.06.012 (2008).
Drevensek, M., Sprogar, S., Boras, I. & Drevensek, G. Effects of endothelin antagonist tezosentan on orthodontic tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 129, 555–558. https://doi.org/10.1016/j.ajodo.2005.12.016 (2006).
Davidovitch, Z. Tooth movement. Crit. Rev. Oral Biol. Med. 2, 411–450 (1991).
Shanfeld, J., Jones, J., Laster, L. & Davidovitch, Z. Biochemical aspects of orthodontic tooth movement I Cyclic nucleotide and prostaglandin concentrations in tissues surrounding orthodontically treated teeth in vivo. Am. J. Orthod. Dentofac. Orthop. 90, 139–148 (1986).
Reitan, K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 53, 721–745 (1967).
Rygh, P. Ultrastructural changes in tension zones of rat molar periodontium incident to orthodontic tooth movement. Am. J. Orthod. 70, 269–281 (1976).
Krishnan, V. & Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 129, 469.e461-e432. https://doi.org/10.1016/j.ajodo.2005.10.007 (2006).
Sims, M. R. Endothelin-1 expression in the microvasculature of normal and 3-hour continuously loaded rat molar periodontal ligament. Eur. J. Orthod. 23, 647–662. https://doi.org/10.1093/ejo/23.6.647 (2001).
Friedl, G. et al. Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: Transcriptionally controlled early osteo-chondrogenic response in vitro. Osteoarthrit. Cartil. 15, 1293–1300 (2007).
Zhang, L. et al. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/β-catenin pathway. Biochim. Biophys. Acta Gen. Subj. 1860, 2211–2219 (2016).
Shen, T. et al. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 7, 7872 (2014).
Hu, L.-W., Wang, X., Jiang, X.-Q., Xu, L.-Q. & Pan, H.-Y. In vivo and in vitro study of osteogenic potency of endothelin-1 on bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 357, 25–32 (2017).
Liang, L., Zhou, W., Yang, N., Yu, J. & Liu, H. ET-1 promotes differentiation of periodontal ligament stem cells into osteoblasts through ETR, MAPK, and Wnt/β-catenin signaling pathways under inflammatory microenvironment. Mediat. Inflamm. https://doi.org/10.1155/2016/8467849 (2016).
Lee, M. S. et al. Endothelin-1 differentially directs lineage specification of adipose- and bone marrow-derived mesenchymal stem cells. FASEB J. 33, 996–1007. https://doi.org/10.1096/fj.201800614R (2019).
Akashi, K. et al. Knockout of endothelin type B receptor signaling attenuates bleomycin-induced skin sclerosis in mice. Arthritis Res. Ther. 18, 113–113 (2016).
Someya, A. et al. Effect of YM598, a selective endothelin ETA receptor antagonist, on endothelin-1-induced bone formation. Eur. J. Pharmacol. 543, 14–20. https://doi.org/10.1016/j.ejphar.2006.06.035 (2006).
Park, M. H. & Lee, D. H. Endothelin 1 protects HN33 cells from serum deprivation-induced neuronal apoptosis through Ca(2+)-PKCalpha-ERK pathway. Exp. Mol. Med. 40, 92–97. https://doi.org/10.3858/emm.2008.40.1.92 (2008).
Araki, M. et al. Endothelin-1 as a protective factor against beta-adrenergic agonist-induced apoptosis in cardiac myocytes. J. Am. Coll. Cardiol. 36, 1411–1418. https://doi.org/10.1016/s0735-1097(00)00822-6 (2000).
McWhinnie, R. et al. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L278-286. https://doi.org/10.1152/ajplung.00111.2006 (2007).
Bagnato, A. et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: Evidence for an autocrine role in tumor growth. Cancer Res. 59, 720–727 (1999).
Okazawa, M., Shiraki, T., Ninomiya, H., Kobayashi, S. & Masaki, T. Endothelin-induced apoptosis of A375 human melanoma cells. J. Biol. Chem. 273, 12584–12592 (1998).
Dupuis, J., Goresky, C. A. & Fournier, A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: Exclusive role of ETB receptors. J. Appl. Physiol. 1985(81), 1510–1515. https://doi.org/10.1152/jappl.1996.81.4.1510 (1996).
Nelson, J. B. et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 56, 663–668 (1996).
Bagnato, A. & Rosanò, L. The endothelin axis in cancer. Int. J. Biochem. Cell Biol. 40, 1443–1451. https://doi.org/10.1016/j.biocel.2008.01.022 (2008).
Lahav, R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol 49, 173–180. https://doi.org/10.1387/ijdb.041951rl (2005).
Anguelova, E. et al. Functional endothelin ET B receptors are selectively expressed in human oligodendrogliomas. Brain Res. Mol. Brain Res. 137, 77–88. https://doi.org/10.1016/j.molbrainres.2005.02.015 (2005).
Ozaki, S. et al. ETB-mediated regulation of extracellular levels of endothelin-1 in cultured human endothelial cells. Biochem. Biophys. Res. Commun. 209, 483–489. https://doi.org/10.1006/bbrc.1995.1527 (1995).
Fukuroda, T. et al. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem. Biophys. Res. Commun. 199, 1461–1465. https://doi.org/10.1006/bbrc.1994.1395 (1994).
Böhm, F., Pernow, J., Lindström, J. & Ahlborg, G. ETA receptors mediate vasoconstriction, whereas ETB receptors clear endothelin-1 in the splanchnic and renal circulation of healthy men. Clin. Sci. 104, 143–151. https://doi.org/10.1042/CS20020192 (2003).
Gariepy, C. E., Williams, S. C., Richardson, J. A., Hammer, R. E. & Yanagisawa, M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J. Clin. Investig. 102, 1092–1101. https://doi.org/10.1172/JCI3702 (1998).
Drevensek, M., Volk, J., Sprogar, S. & Drevensek, G. Orthodontic force decreases the eruption rate of rat incisors. Eur. J. Orthod. 31, 46–50. https://doi.org/10.1093/ejo/cjn078 (2009).
Plut, A. et al. Bone remodeling during orthodontic tooth movement in rats with type 2 diabetes. Am. J. Orthod. Dentofac. Orthop. 148, 1017–1025. https://doi.org/10.1016/j.ajodo.2015.05.031 (2015).
Meh, A. et al. Effect of cetirizine, a histamine (H(1)) receptor antagonist, on bone modeling during orthodontic tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 139, e323-329. https://doi.org/10.1016/j.ajodo.2009.11.013 (2011).
Feng, J. Q. et al. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J. Bone Miner. Res. 17, 1822–1831 (2002).
Bagnato, A. et al. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Can. Res. 64, 1436–1443 (2004).
Lahav, R., Suvà, M.-L., Rimoldi, D., Patterson, P. H. & Stamenkovic, I. Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Can. Res. 64, 8945–8953 (2004).
Gariepy, C. E., Cass, D. T. & Yanagisawa, M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc. Natl. Acad. Sci. USA 93, 867–872. https://doi.org/10.1073/pnas.93.2.867 (1996).
Sánchez-Mejías, A., Fernández, R. M., López-Alonso, M., Antiñolo, G. & Borrego, S. New roles of EDNRB and EDN3 in the pathogenesis of Hirschsprung disease. Genet. Med. 12, 39–43. https://doi.org/10.1097/GIM.0b013e3181c371b0 (2010).
van Leeuwen, E. J., Maltha, J. C. & Kuijpers-Jagtman, A. M. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur. J. Oral Sci. 107, 468–474 (1999).
Pavlin, D., Dove, S., Zadro, R. & Gluhak-Heinrich, J. Mechanical loading stimulates differentiation of periodontal osteoblasts in a mouse osteoinduction model: Effect on type I collagen and alkaline phosphatase genes. Calcif. Tissue Int. 67, 163–172 (2000).
Tsao, Y.-T. et al. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 18, 159 (2017).
Nakamura, A. et al. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng. Part C Methods 15, 169–180 (2009).
Ahmad, A. R. et al. Recombinant human dentin matrix protein 1 (DMP1) induces the osteogenic differentiation of human periodontal ligament cells. Biotechnol. Rep. 23, e00348 (2019).
Rad, M. R. et al. The role of dentin matrix protein 1 (DMP1) in regulation of osteogenic differentiation of rat dental follicle stem cells (DFSCs). Arch. Oral Biol. 60, 546–556 (2015).
Bollerslev, J. et al. Ultrastructural investigations of bone resorptive cells in two types of autosomal dominant osteopetrosis. Bone 14, 865–869. https://doi.org/10.1016/8756-3282(93)90316-3 (1993).
Sun, J.S., Oh, S.O., Shin D.M. & Chang I. Critical role of endothelin in osteoclast differentiation and function. J. Bone Miner Res. 32(Suppl 1), S88. https://doi.org/10.1002/jbmr.3363 (2017).
Wise, G. & King, G. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 87, 414–434 (2008).
Vignery, A. & Baron, R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat. Rec. 196, 191–200. https://doi.org/10.1002/ar.1091960210 (1980).
Kelland, N. F. et al. Endothelial cell-specific ETB receptor knockout: Autoradiographic and histological characterisation and crucial role in the clearance of endothelin-1. Can. J. Physiol. Pharmacol. 88, 644–651. https://doi.org/10.1139/Y10-041 (2010).
Télémaque-Potts, S. et al. Elevated systemic levels of endothelin-1 and blood pressure correlate with blunted constrictor responses and downregulation of endothelinA, but not endothelinB, receptors in an animal model of hypertension. (2002).
Davenport, A. P. & Kuc, R. E. Down-regulation of ETA receptors in ETB receptor-deficient mice. J. Cardiovasc. Pharmacol. 44, S276–S278 (2004).
Sasaki, Y., Hori, S., Oda, K., Okada, T. & Takimoto, M. Both ETA and ETB receptors are involved in mitogen-activated protein kinase activation and DNA synthesis of astrocytes: Study using ETB receptor-deficient rats (aganglionosis rats). Eur. J. Neurosci. 10, 2984–2993 (1998).
Prefontaine, A., Calderone, A. & Dupuis, J. Role of endothelin receptors on basal and endothelin-1-stimulated lung myofibroblast proliferation. Can. J. Physiol. Pharmacol. 86, 337–342 (2008).
Boesen, E. I. Endothelin ETB receptor heterodimerization: Beyond the ETA receptor. Kidney Int. 74, 693–694 (2008).
Bulenger, S., Marullo, S. & Bouvier, M. Emerging role of homo-and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 26, 131–137 (2005).
Prinster, S. C., Hague, C. & Hall, R. A. Heterodimerization of g protein-coupled receptors: Specificity and functional significance. Pharmacol. Rev. 57, 289–298 (2005).
Dai, X. & Galligan, J. J. Differential trafficking and desensitization of human ETA and ETB receptors expressed in HEK 293 cells. Exp. Biol. Med. 231, 746–751 (2006).
Kennedy, O. D., Laudier, D. M., Majeska, R. J., Sun, H. B. & Schaffler, M. B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 64, 132–137 (2014).
Moin, S. et al. Osteocyte death during orthodontic tooth movement in mice. Angle Orthod. 84, 1086–1092 (2014).
Sugimori, T. et al. Micro-osteoperforations accelerate orthodontic tooth movement by stimulating periodontal ligament cell cycles. Am. J. Orthod. Dentofac. Orthop. 154, 788–796 (2018).
Funding
This work was supported by the P3-0293 research program financed by the Slovenian Research Agency and Ad futura—Slovenian human resources development and scholarship fund.
Author information
Authors and Affiliations
Contributions
S.D.I. has carried the most of experimental work, inspected and manually corrected raw data, conducted statistical analyses and written the first draft of the manuscript. B.F. evaluated part of the data and statistical analysis, prepared the figures, and participate in writing final manuscript. G.D. has enabled the approval of the study by the Veterinary Administration of the Republic of Slovenia, participated in study design, coordinating and participating the execution of the animal experiments, corrected the working version of the manuscript, and improved the final manuscript. M.Y. established ETB knockout rats strain, participated in the study design and did the final reading of the manuscript. A.P. was involved in data evaluation, figures preparation and improved the manuscript. S.H. has done the histomorphometric analysis and histomorphometric data analysis. I.P.Ž. performed ELISA analysis and prepared those materials for publishing. J.M. has performed the gene expression study and its analysis. M.D. has participated in study design, coordinated the execution of the measurements, corrected the working versions of the manuscript, improved the final manuscript and is a corresponding author. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahimi Disha, S., Furlani, B., Drevensek, G. et al. The role of endothelin B receptor in bone modelling during orthodontic tooth movement: a study on ETB knockout rats. Sci Rep 10, 14226 (2020). https://doi.org/10.1038/s41598-020-71159-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71159-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.