Abstract
The gonadotropin releasing hormone agonist (GnRH-a) long-protocols and the GnRH-antagonist protocols are two commonly used protocols for in vitro fertilization (IVF), but their cost-effectiveness has not been studied, especially in China. A retrospective study involving 1638 individuals in GnRH-a long-protocol and 621 in GnRH-antagonist protocol were conducted and a decision tree model analysis was used to analyze the cost-effectiveness. Both direct and indirect costs were calculated. As a result, during the fresh embryo transplantation cycles, there was no significant difference in the rate of ongoing pregnancy between the two protocols, the average cost of per ongoing pregnancy in the GnRH-antagonist protocol was $ 16970.85, and that in the GnRH-agonist long-protocol was $19902.24. The probability of cumulative ongoing pregnancy per start cycle was estimated at 60.65% for the GnRH-antagonist protocol and 71.6% for the GnRH-agonist long-protocol (P < 0.01). Considering the cumulative ongoing pregnancy rate, the mean costs per ongoing pregnancy were estimated at $8176.76 and at $7595.28 with GnRH-antagonist protocol and GnRH-agonist long protocol, respectively. In conclusion, in fresh embryo transplantation cycle, the GnRH-antagonist protocol has economic advantage. However, the GnRH-agonist long protocol is more cost effective considering the cumulative ongoing pregnancy rate in the fresh embryo and frozen embryo transplantation cycles.
Similar content being viewed by others
Introduction
According to a World Health Organization (WHO) report, about 48.5 million couples are affected by infertility worldwide in 20101. With the refinement and development of assisted reproductive technology (ART), increasing number of infertile couples seek ART. The European Society of Human Reproduction and Embryology (ESHRE) reported that the world-wide number of babies born as a result of ART has reached an estimated total of 8 million since the world’s first, Louise Brown, was born in July 19782. Up to now, ART is the most important method to treat infertility in the world.
Study has shown that cumulative live birth rates are increased with the number of oocytes obtained3. Therefore, controlled ovarian hyperstimulation (COH) is an important process to obtain a set number of oocytes for IVF. The GnRH-agonists were introduced into IVF in the late 1980s and the GnRH-agonist long-protocol is still the most frequently used protocol in most centers worldwide4. The basic principle is to use gonadotropin-releasing hormone agonist (GnRH-a) to regulate pituitary and stimulate follicular growth with exogenous gonadotropin hormone, and avoid endogenous luteinizing hormone (LH) surge before oocyte retrieval5,6. Since 1990s, GnRH antagonists were used in COH, this protocol competitively blocks pituitary GnRH receptors, inducing a rapid, reversible suppression of gonadotrophin secretion and preventing and interrupting LH surges6,7,8. The GnRH-antagonist protocol have been widely adopted in IVF due to these advantages.
IVF is a protracted and costly process. In most developed countries, IVF is covered by insurance or subsidized. This is not the case in developing countries9. In China, the cost of IVF is on patients. High costs discourage low-income infertility couples from seeking ART treatment.
While both protocols are commonly used today, little is known about their cost-effectiveness, especially in China. Studies have shown that hormonal stimulation covered the main part of the costs per cycle10. Moolenaar et al. reported that most economic studies about ART were performed in countries from mainland Europe (38%) and the United States (34%)11. In China, there has been few economic researches on COH protocols. Herein, we performed a retrospective study on comparing the cost-effectiveness of the two protocols using a single center data in China. Choosing a cost-effective protocol, can not only ease the financial pressure on couples, but also provide reference for medical decision-making.
Methods
Patients
Individuals who came to the Reproductive Center of Women’s Hospital, Zhejiang University School of Medicine for their first cycle of IVF treatment from 1 January 2015 to 31 December 2017 were included. Inclusion criteria were as follows: 20 < age ≤ 38 years, regular ovulatory cycles every 21–35 days, total antral follicle count (AFC) ≥ 5, first cycle of IVF treatment, COH planned using the GnRH-agonist long-protocol or the GnRH-antagonist protocol, IVF fertilization. Exclusion criteria were the use of donor oocytes or frozen-thawed oocytes for fertilization, other protocols for COH, ICSI fertilization. Patient demographics are presented in Table 1.
COH protocols
GnRH-agonist long-protocol: A short-acting GnRH-a (Triptorelin, Ferring AG, Germany) was administrated daily in the mid luteal phase of the preceding cycle. 14 days later, follicular ultrasonography, serum LH, FSH and E2 were examined and 150–300IU recombination follicle stimulating hormone (r-FSH, Gonal-F, Merck Serono, Switzerland) was initiated daily when FSH and LH < 5 IU/L and E2 < 50 pg/ml. GnRH-a was continued until trigger. During the stimulation, according to ovarian response evaluated by transvaginal ultrasonography and serum hormone level, dose of r-FSH was adjusted as needed, human menopausal gonadotropin (HMG) or recombinant luteinizing hormone(r-LH) or growth hormone (GH) was added as needed.
GnRH-antagonist protocol: 150–300IU r-FSH was initiated on day 2 or 3 of the menstrual cycle until trigger. The dosage of r-FSH was adjusted and HMG or r-LH or GH was added according to the ovarian response evaluated by transvaginal ultrasonography and serum hormone level. 0.25 mg GnRH-A (Cetrorelix; Merck Serono, France) was used daily until trigger when the leading follicles reached a mean diameter of 14 mm.
For both protocols, if three follicles reached a mean diameter of 17 mm or two follicles reached a mean diameter of 18 mm, r-HCG (Ovidrel, Serono, Italy) was administered subcutaneously. Oocyte retrieval was performed 36 h after HCG injection by transvaginal ultrasound-guided single-lumen needle aspiration. Luteal phase support was initiated on day 1 after oocyte retrieval. Fresh embryo transplantation was carried out 72 h after oocyte retrieval. Fresh cycles were canceled if patients had endometrial thickness <7 mm, high risk of Ovarian hyperstimulation syndrome (OHSS) (E2 ≥ 5000 pg/ml on the trigger day, the number of oocytes obtained ≥20), no available embryos or other personal reasons. For those patients without fresh embryo transplantation or without ongoing pregnancy after fresh embryo transplantation, if excess frozen embryos were available and pregnancy test was negative, the frozen embryo transplantation (FET) was performed until no embryos remained or ongoing pregnancy was achieved. Clinical pregnancy was defined as a gestational sac observed by vaginal ultrasound. Ongoing pregnancy was defined as a pregnancy continuing 12 weeks without miscarriage.
Structure of the model
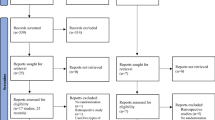
We constructed a decision tree model to analyze the cost-effectiveness of the GnRH-agonist long-protocol and GnRH-antagonist protocol in the fresh embryo transplantation and frozen embryo transplantation cycle (Fig. 1 and Fig. 2). Each route in the diagram represents possible steps in IVF. Each intersection is followed by a possible situation. Since the cumulative ongoing pregnancy rate was calculated by following utilization of all fresh and frozen embryos after the first IVF cycle, the number of transplants was based on available embryos. The terminal nodes of the model were: “No oocytes”, “No embryos”, “Ongoing pregnancy”, “No ongoing pregnancy”. Since each patient may have different situations after entering the cycle, we made relevant assumptions for the model for the convenience of calculation as shown in Table 2.
Transition probabilities
The transition probabilities for the various health states in this decision tree model were derived from the clinical data of included infertility patients. The transition probability of each step is shown in Fig. 1 and Fig. 2.
Cost analysis
This study was performed from a patient’s perspective. Both direct and indirect costs were included in the analysis. Direct medical costs included drug, ultrasound, laboratory, surgery and care costs. Medications included those used in ovulation stimulation trigger and luteal support. The cost of the drug was equal to the unit cost of the drug multiplied by the total amount used. When calculating the cost of IVF, it is prudent to include the treatment costs for complications, primarily OHSS. Therefore, the cost of treatment for OHSS was also calculated in the total cost of the patient. In this study, transportation costs were included as direct non-medical expenses. These were calculated as the average fare from each city in Zhejiang province to Hangzhou. Indirect costs included the cost of lost work. The cost of lost work was calculated according to the per capita disposable income. Intangible economic burden generally refers to the decline in the quality of life or other costs caused by illness in this case, IVF, and also included other costs not reflected in the direct and indirect costs. Thus, considering the difficulty in calculating these intangible costs, such costs were not included in this study. Cycle costs were relatively stable during the study period, so discounting is not considered. The specific costs are shown in Table 3.
Statistical analyses
The statistical software package SPSS22 was used for data analysis. When the measurement data matched the homogeneity of variance, the independent sample t test was used; when the data didn’t match the homogeneity of variance, the Mann-Whitney test was used. The chi-square test or Fisher’s exact probability method were used for the comparison of the rates, and P < 0.05 was considered statistically significant. Cost-effectiveness analysis and incremental cost-effectiveness analysis were used to evaluate the costs and effects between the two protocols. Sensitivity analyses were then performed to assess the stability of the model.
Ethical approval
The study was approved by the Ethics Committee of Women’s Hospital School of Medicine Zhejiang University. All patients meeting the inclusion criteria signed the informed consent. And this study complied with declaration of Helsinki/relevant guidelines for the study on humans.
Results
About 2259 infertility patients met inclusion criteria (1638 in GnRH-agonist long-protocol and 621 in GnRH-antagonist protocol). There were no differences in patient ages, BMI, duration of infertility, cycle day 3 LH levels, cycle day 3 E2 levels and AFC. The duration of gonadotrophin stimulation and dose of gonadotropin used, number of oocytes obtained and number of available embryos in GnRH-agonist long-protocol were higher than that in GnRH-antagonist protocol. The clinical pregnancy rate and ongoing pregnancy rate for GnRH-agonist long-protocol and GnRH-antagonist protocol in the fresh embryo transplantation cycle were not different (47.26% vs. 49.44%, P = 0.487; 38.77% vs. 37.22%, P = 0.613). When all available embryos had been utilized, the rate of cumulative ongoing pregnancy per start cycle was 60.65% for GnRH-antagonist protocol and 71.6% for GnRH-agonist long-protocol (P < 0.01). The OHSS rate was lower in GnRH-antagonist protocol (2.0% vs 4.9%, P = 0.001). The cumulative multiple pregnancy (17.6% vs 18.4%, P = 0.667) rate and the cumulative ectopic pregnancy rate (2.9% vs 4.0%, P = 0.26) in GnRH-agonist long-protocol was not different from that of GnRH-antagonist protocol. Data on patients’ demographic and infertility treatment-related characteristics are represented in Table 1.
In fresh embryo cycles, there were less amount of gonadotropin and few days of OHSS for GnRH-antagonist protocols. Therefore, the total cost was lower in GnRH-antagonist protocols (average cost $16970.85) than in GnRH-agonist long-protocol (average cost $19902.24) in fresh embryo cycles. In fresh embryo transfer cycles, there was no statistical difference in the ongoing pregnancy rate between the two protocols, therefore, the minimum cost method was used for analysis. When the cumulative ongoing pregnancy rate was taken into account in the long protocol group, the number of frozen embryo transfer cycle was higher, leading to a higher cumulative ongoing pregnancy rate, and consequently a higher cost. Using cost-effectiveness analysis, the mean costs per ongoing pregnancy were estimated at $8176.76 and at $7595.28 with GnRH-antagonist protocols and GnRH-agonist long-protocols, respectively. The incremental cost-effectiveness ratio (ICER) is an evaluation index commonly used in economics research. It refers to the ratio of cost difference and effect difference between different protocols. In this study, the ICER for GnRH-agonist long-protocol versus GnRH-antagonist protocol was estimated at $4375.95 for one more ongoing pregnancy (Table 4).
Sensitivity analysis is to change the value of some parameters and analyze the degree of influence on the results. The cost of ovarian stimulation protocols account for the majority of the total costs10. In this study, the cost of drugs accounted for the largest proportion of the total cost of ovulation stimulation protocols. The reliability of the results was assessed by sensitivity analysis with drug cost fluctuation. The results of sensitivity analysis were consistent with the results of cost-effectiveness analysis, the cost per ongoing pregnancy in the GnRH-agonist long-protocol was lower than that in the GnRH-antagonist protocol, which means the results are stable.
Discussion
In this study, we used an economics research method to evaluate the cost-effectiveness of the GnRH-agonist long-protocol and the GnRH-antagonist protocol for IVF. We found that in the fresh embryo transplantation cycle, there was no significant difference in the ongoing pregnancy rate between the GnRH-agonist long-protocol and the GnRH-antagonist protocol, while the cost in the GnRH-antagonist protocol was lower, so GnRH-antagonist protocol has advantage according to the principle of cost-minimization analysis of pharmacoeconomics. When considering the cumulative ongoing pregnancy rate after each ovarian stimulation, the cumulative ongoing pregnancy rate and cost in the GnRH-agonist long-protocol were higher than the GnRH-antagonist protocol. Using the cost-effectiveness analysis method, it was found that the average cost of each ongoing pregnancy in the GnRH-agonist long-protocol was lower than the GnRH-antagonist protocol. Therefore, the GnRH-agonist long-protocol is more cost-effective.
Studies have shown that one of the primary reasons for dropout from infertility treatment is economic burdens12,13,14. China’s medical system does not provide insurance coverage for infertility diagnosis and treatment15. It can be an enormous economic burden to patients seeking ART. Therefore, no matter from the perspective of patients, or from the perspective of medical resource allocation, it is necessary to carry out economic analysis on IVF and consider the cost and effect of each step. Although GnRH-agonist long-protocols and GnRH-antagonist protocols have been widely used in IVF, there is still an ongoing debate about the results of the two protocols. Orvieto16 and Grow17 found the GnRH-agonist protocol has a superiority over the GnRH-antagonist protocol in live birth rate. Some studies also found no significant difference in the rates of live births or ongoing pregnancies between the two protocols18,19. However, these studies are only from the perspective of clinical results, not from the perspective of economics. In our research, we hope to be able to focus more on evaluating the cost-effectiveness of both protocols, not just the clinical outcomes, which are just a link in the evaluation. After consulting the literature, we found that there have been little economic studies on the two different ovarian stimulation protocols used in IVF. Wei Pan20 et al. conducted a retrospective analysis of the cost-effectiveness of GnRH-a protocols, GnRH-ant protocols and GnRH-a ultra-long protocols. They used the live birth rate as one outcome of the study. However, it is difficult to calculate the cost throughout pregnancy. In order to ensure that the results are more reliable, we used the ongoing pregnancy rate as the end point of this economic study.
The cost of IVF treatment for infertility (the cost per ongoing pregnancy) in this study was higher than the average hospitalization cost for 30 diseases in 2018 according to the national bureau of statistics21. IVF is a complex process involving ovarian stimulation, ovum retrieval, fertilization, embryo transfer and other processes. In addition to the cost of these processes, the total cycle costs of IVF should also include the transportation costs, lost wages and the cost of treating OHSS. However, it is difficult to accurately assess the transportation costs and lost wages and these indirect cost consisted a small percentage of the total costs, many studies did not include them in the total cycle cost analysis22. But, from the perspective of patients, these costs are indirect medically, yet direct economically to patients, so they are also included in this study. OHSS is a serious complication of IVF, and the treatment is expensive, which directly affects the total cycle costs. Studies have shown that the incidence of OHSS in the GnRH-antagonist protocol is lower than that in the GnRH-agonist long-protocol18,23,24. Accordingly, the cost of treating OHSS in the GnRH-antagonist protocol is lower, resulting in reduction in the total cycle cost. This may be one of the reasons why the cost in GnRH-antagonist protocol is lower than the GnRH-agonist long-protocol in the fresh embryo transfer cycle.
Economic analysis of IVF is still challenging, and there is no unified view on the result indicators of the analysis. Existing economic studies often used ongoing pregnancy, live birth rates or quality-adjusted life years (QALYs) as result of the study. However, it is worth considering that QALYs of both husband and wife or child is used when taking QALYs as a result of IVF25. Toftager26 et al. found that quality of life and psychosocial and physical well-being of patients used the GnRH-antagonist protocol was better than that used the GnRH-agonist protocol. But these are hard to quantify in terms of costs. And it’s difficult to calculate the impact on families and society of obtaining a healthy baby by IVF. Considering the different incidence of related complications during pregnancy, the treatment costs and nursing costs vary greatly. Therefore, in this study, we used the cumulative ongoing pregnancy rate as the effect of the study.
There are many economic analysis methods, but among the existing studies on economics in ART, 84% are cost-effectiveness analysis and 48% are model-based studies11. Economic model carried out before a trial is particularly useful in reducing unnecessary waste of research resources in evaluating techniques and interventions and improving the quality and efficiency of the research27. In our study, we developed a decision-tree model to evaluate the cost-effectiveness of the GnRH-agonist long-protocol and GnRH-antagonist protocol. The relevant transfer probability in the model was calculated using the data of infertile patients in the reproductive center of our hospital. In addition, we have made some reasonable assumptions for analysis, as shown in Table 2.
This is a retrospective study on the data available from infertility diagnosis and treatment in our reproductive center. The relevant probability and costs in the model are calculated based on the data of our single center. These results may differ from those of other centers, but can provide some guidance for patients and clinicians. In the future, large samples, multi-center prospective randomized controlled trials are needed to more thoroughly explore more economical and effective treatment protocols.
In conclusion, if fresh embryo transfers are considered, the pregnancy outcomes between GnRH-agonist long protocols and GnRH-antagonist protocols are similar, but GnRH-antagonist protocols have lower cost. Therefore, in the fresh embryo transfer cycle, the GnRH-antagonist protocol has economic advantage and is worth recommending. However, GnRH-agonist long-protocol have higher success rates and higher costs when cumulative ongoing pregnancy rates are taken into account. The cost per ongoing pregnancy in the GnRH-agonist long-protocol cycles was lower than that in the GnRH-antagonist protocol cycles. Thus, cost-effectiveness analysis shows that the GnRH-agonist long-protocol is more cost-effective than the GnRH-antagonist protocol and may represent a cost-effective option from the perspective of patients. However, further large sample sizes and multi-center randomized controlled trials are needed.
References
Mascarenhas, M. N., Flaxman, S. R., Boerma, T., Vanderpoel, S. & Stevens, G. A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med 9, e1001356, https://doi.org/10.1371/journal.pmed.1001356 (2012).
European Society of Human Reproduction and Embryology. More than 8 million babies born from IVF since the world’s first in 1978., (2018).
Polyzos, N. P. et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including approximately 15,000 women. Fertility and sterility 110, 661–670. e661, https://doi.org/10.1016/j.fertnstert.2018.04.039 (2018).
Coccia, M. E., Comparetto, C., Bracco, G. L. & Scarselli, G. GnRH antagonists. European journal of obstetrics, gynecology, and reproductive biology 115(Suppl 1), S44–56, https://doi.org/10.1016/j.ejogrb.2004.01.033 (2004).
Fleming, R. C. J. R. Induction of multiple follicular growth in normally menstruating women with endogenous gonadotropin suppression. Fertility and sterility 45, 226–230 (1986).
Huirne, J. A., Homburg, R. & Lambalk, C. B. Are GnRH antagonists comparable to agonists for use in IVF? Human reproduction (Oxford, England) 22, 2805–2813, https://doi.org/10.1093/humrep/dem270 (2007).
Diedrich, K. et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Human reproduction (Oxford, England) 9, 788–791 (1994).
Hall, J. E. et al. Evidence of differential control of FSH and LH secretion by gonadotropin-releasing hormone (GnRH) from the use of a GnRH antagonist. The Journal of clinical endocrinology and metabolism 67, 524–531, https://doi.org/10.1210/jcem-67-3-524 (1988).
Ombelet, W. & Campo, R. Affordable IVF for developing countries. Reproductive biomedicine online 15, 257–265 (2007).
Bouwmans, C. A. et al. A detailed cost analysis of in vitro fertilization and intracytoplasmic sperm injection treatment. Fertility and sterility 89, 331–341, https://doi.org/10.1016/j.fertnstert.2007.03.003 (2008).
Moolenaar, L. M. et al. Economic evaluation studies in reproductive medicine: a systematic review of methodologic quality. Fertility and sterility 99, 1689–1694, https://doi.org/10.1016/j.fertnstert.2012.12.045 (2013).
Domar, A. D., Smith, K., Conboy, L., Iannone, M. & Alper, M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertility and sterility 94, 1457–1459, https://doi.org/10.1016/j.fertnstert.2009.06.020 (2010).
Mourad, S. M. et al. Determinants of patients’ experiences and satisfaction with fertility care. Fertility and sterility 94, 1254–1260, https://doi.org/10.1016/j.fertnstert.2009.07.990 (2010).
van Dongen, A. J., Verhagen, T. E., Dumoulin, J. C., Land, J. A. & Evers, J. L. Reasons for dropping out from a waiting list for in vitro fertilization. Fertility and sterility 94, 1713–1716, https://doi.org/10.1016/j.fertnstert.2009.08.066 (2010).
Zheng, X. Y. & Disease, Q. Y. burden of infertility in China. Chin J Public Health 28, 257–260 (2012).
Orvieto, R. & Patrizio, P. GnRH agonist versus GnRH antagonist in ovarian stimulation: an ongoing debate. Reproductive biomedicine online 26, 4–8, https://doi.org/10.1016/j.rbmo.2012.11.001 (2013).
Grow, D. et al. GnRH agonist and GnRH antagonist protocols: comparison of outcomes among good-prognosis patients using national surveillance data. Reproductive biomedicine online 29, 299–304, https://doi.org/10.1016/j.rbmo.2014.05.007 (2014).
Wang, R. L., Lin, S. R., Wang, Y., Qian, W. P. & Zhou, L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. PloS one 12, e0175985, https://doi.org/10.1371/journal.pone.0175985 (2017).
Al-Inany, H. G. et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. The Cochrane database of systematic reviews 4, Cd001750, https://doi.org/10.1002/14651858.CD001750.pub4 (2016).
Pan, W. et al. Decision analysis about the cost-effectiveness of different in vitro fertilization-embryo transfer protocol under considering governments, hospitals, and patient. Medicine 98, e15492, https://doi.org/10.1097/md.0000000000015492 (2019).
National Health Commission. China health statistics yearbook 2018 115 (China union medical university press (2018).
Collins, J. An international survey of the health economics of IVF and ICSI. Human reproduction update 8, 265–277 (2002).
Lambalk, C. B. et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Human reproduction update 23, 560–579, https://doi.org/10.1093/humupd/dmx017 (2017).
Chappell, N. & Gibbons, W. E. The use of gonadotropin-releasing hormone antagonist post-ovulation trigger in ovarian hyperstimulation syndrome. Clinical and experimental reproductive medicine 44, 57–62, https://doi.org/10.5653/cerm.2017.44.2.57 (2017).
ESHRE Capri Workshop Group. Economic aspects of infertility care: a challenge for researchers and clinicians. Human reproduction (Oxford, England) 30, 2243–2248, https://doi.org/10.1093/humrep/dev163 (2015).
Toftager, M. et al. Quality of life and psychosocial and physical well-being among 1,023 women during their first assisted reproductive technology treatment: secondary outcome to a randomized controlled trial comparing gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist protocols. Fertility and sterility 109, 154–164, https://doi.org/10.1016/j.fertnstert.2017.09.020 (2018).
Torgerson, D. J. & Byford, S. Economic modelling before clinical trials. BMJ (Clinical research ed. 325, 98 (2002).
Acknowledgements
We would like to express our gratitude to Professor Dong Heng-jin from Zhejiang University, School of Public Health for his guidance on analytical methods and Sun Sai-jun from our hospital for her statistical help. We are very grateful to Dr. Kallen Amanda from Yale School of Medicine and Dr. Yiping Shen from Boston Children’s Hospital for their revision and suggestions on the language grammar of our article. This study was supported by the National Natural Science Foundation of China (no. 81300464), the Medical and Health Science and Technology Plan Project of Zhejiang Province (2015KYA123), Zhejiang University Education Foundation Global Partnership Fund and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Miaomiao Jing performed analysis and wrote the manuscript. Chenxi Lin, Xiaoyu Tu, Qi Chen, Xiufang Wang, Youbing Zheng collected clinical data. Wenjun Zhu collated and analyzed the data. Runju Zhang conceived the study and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jing, M., Lin, C., Zhu, W. et al. Cost-effectiveness analysis of GnRH-agonist long-protocol and GnRH-antagonist protocol for in vitro fertilization. Sci Rep 10, 8732 (2020). https://doi.org/10.1038/s41598-020-65558-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65558-0
This article is cited by
-
Long pentraxin 3 and vitamin D receptor mRNA expression pattern of cumulus granulosa cells isolated from PCOS oocytes at different stages of nuclear maturation
Reproductive Biology and Endocrinology (2024)
-
Perinatal outcomes of singletons following double vitrification-warming procedures: a retrospective study using propensity score analysis
BMC Pregnancy and Childbirth (2023)
-
The (decision) tree of fertility: an innovative decision-making algorithm in assisted reproduction technique
Journal of Assisted Reproduction and Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.