Abstract
Intracranial artery calcification (IAC) is an important risk factor for cerebral infarction and a key biomarker for intracranial artery stenosis. Small dense low-density lipoprotein cholesterol (sd-LDL-c) was independently associated with increased cardiovascular events and coronary calcification. Our study assessed whether sd-LDL-c is an independent factor for IAC in acute ischemic stroke (AIS) patients. This cross-sectional study involved a total of 754 patients with AIS (mean age: 65 ± 13.2 years). All the patients had received brain computed tomography angiography (CTA) examination to evaluate IAC. Serum sd-LDL-c levels and other biochemical parameters were analyzed. Admission NIHSS score and mRS score at discharge were collected. After 60-days 85 patients died during hospitalization and follow-up. Partial correlation analysis showed that serum sd-LDL-c levels were associated with admission NIHSS score and IAC score after adjusted age and gender. Logistic regression analysis showed that serum sd-LDL-c levels independently predicted NIHSS scores (β = 1.537, 95%CI: 0.134-2.878, p = 0.042) and IAC scores (β = 1.355, 95%CI: 0.319-2.446, p = 0.015). The average level of sd-LDL-c in patients who died was also significantly increased compared to survival patients (1.04 ± 0.59 vs 0.88 ± 0.44 mmol/L, p = 0.017). However, multivariate logistic regression analysis showed serum sd-LDL-c levels could not predict all-cause mortality and prognosis in AIS patients. Our study found that sd-LDL-c as a strong atherogenic lipid particle can independently predict admission NIHSS scores and the severity of cerebral artery calcification in AIS patients. However, its prognostic value in AIS patients still needs further study in the future.
Similar content being viewed by others
Introduction
It has been reported that arterial calcification is a passive process of calcium deposition in cells and tissues due to an imbalance in the calcium and phosphorus metabolism in the body1,2. As a degenerative disease that progresses with aging, arterial calcification was considered as a sign of vascular aging. However, recent studies have shown that arterial calcification is a complex process with multiple etiologies, pathways and mechanisms3,4,5. Arterial calcification can be regarded as a biological phenomenon. The degree of arterial calcification is related to the burden of an atherosclerotic plaque6. Moreover, there is a significant correlation between lipid metabolism disorders and coronary artery calcification7.
Intracranial arterial calcification (IAC) is associated with ischemic stroke, cognitive decline and other vascular events8. IAC is an important risk factor for cerebral infarction and an important marker of intracranial artery stenosis. The result of the Rotterdam study showed that IAC is a major risk factor for stroke in white people9. The strong atherogenic lipid component small dense low-density lipoprotein cholesterol (sd-LDL-c) is strongly associated with coronary artery calcification in healthy women and asymptomatic adults at intermediate risk of cardiovascular disease10,11. However, the relationship between sd-LDL-c and IAC has not been evaluated in general and more specific, in acute ischemic stroke (AIS) patients.
Zeljkovic et al. showed that increased sd-LDL-c level was related to increased morbidity and mortality in AIS12. However, Markaki et al. found that high cholesterol levels are associated with improved long-term prognosis in AIS patients13. The National Institutes of Health Stroke Scale (NIHSS) score reflects the severity of an acute cerebral infarction and is closely related to the prognosis of patients. In AIS patients, the relation of sd-LDL-c and other lipid components with NIHSS score at admission is not clear. Therefore, we designed this study and evaluated the relationship between sd-LDL-c and IAC in AIS patients. Furthermore, it was examined whether sd-LDL-c can independently predict NIHSS score and short-term prognosis in AIS patients.
Materials and Methods
Subjects
This cross-sectional study was performed at the Department of Neurology of the Renmin Hospital, Wuhan University in China. The present study received approval by the Institutional Review Board of the University Affiliated Hospital. All patients referred for AIS between January 2017 and December 2018 were screened. Patients underwent brain CT and/or magnetic resonance imaging (MRI), and the diagnosis of ischemic stroke was based on the consensus of at least two neurologists. The present study complied with the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Renmin Hospital of Wuhan University (Wuhan, China, No. 2016H11018). Written informed consent was obtained from each participant. We had signed consent from every participant to participate in the study. The results are all anonymous. No individual result is presented and only group statistics were provided.
According to AHA/ASA stroke guidelines14, 913 patients with initial diagnosis of AIS were admitted to the Wuhan University affiliated Renmin Hospital. These patients were enrolled in the study: 18-85 years old, duration of onset of symptoms to hospital less than 48 hours, patients or their guardians signed the informed consent. Other types of strokes including transient ischemic attack and hemorrhagic stroke were excluded. 159 patients were excluded from the study: 30 patients did not undergo computed tomography angiography (CTA) examination, 63 patients had missing follow-up results, 31 patients had a history of chronic liver disease and abnormal liver function, and 35 patients had no NIHSS score at admission. Meanwhile, in order to eliminate the effect of drugs on lipid metabolism, we further excluded 96 statin users and using other lipid-lowering agents of 26 patients. A total of 632 patients were included in logistic regression analysis. The primary outcome was 60-day mortality after admitted to hospital.
Biochemical measurements
All patients included in the study underwent head CT, electrocardiogram (ECG), MRI, transthoracic echocardiography (TEE) and had standard laboratory examinations at admission. Blood samples were obtained from an antecubital vein and collected in vacutainer tubes containing EDTA. Patients were asked to fast for eight hours before blood draw, and liver enzyme levels, serum lipid profiles including total triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c), biochemical parameters of the kidney, fasting plasma glucose (FPG), homocysteine (Hcys) and glycosylated hemoglobin (HbA1c) levels were determined on an Olympus AU600 analyzer (Olympus, Tokyo, Japan). sd-LDL-c was measured by the method of Hirano et al.15 with minor modification16 using the commercially available assay kit (sd-LDL-c SEIKEN, Denka Seiken Co., Ltd, Tokyo, Japan). Apolipoproteins (apo) A-1, and B were measured by immunoturbidimetry (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). The following variables were collected in the acute phase of ischemic stroke: age, gender, cause of ischemic stroke (according to TOAST criteria)17, and confirmed or new detected risk factors such as hypertension, diabetes mellitus (DM), coronary heart disease, smoking, long-term alcohol consumption, atrial fibrillation (AF) and BMI. Hyperlipidemia was defined as serum TC was over 5.2 mmol/L or/and serum TG was over 1.7 mmol/L. Those with hyperlipidemia were subgrouped into combined hyperlipidemia, hypertriglyceridemia, and hypercholesterolemia. Combined hyperlipidemia was defined as both serum TC was over 5.2 mmol/L and serum TG was over 1.7 mmol/L; hypertriglyceridemia was defined as serum TG was over 1.7 mmol/L; hypercholesterolemia was defined as serum TC was over 5.2 mmol/L.
Assessment of IAC score
All patients underwent multidetector brain CTA using a 64-slice spiral CT device (GE Healthcare, Milwaukee, WI, USA) with the following parameters:120 kVp, 140 mA, 0.9-mm section thickness, 0.9-mm slice acquisition interval, and intravenous administration of 80 mL of iohexol at a rate of 5.0 mL/s. Bone window CT images covered the whole brain (from the skull base to the vertex) to identify IAC. IAC foci were defined as hyperdense foci with a median density greater than 130 Hounsfield units. We used the 5-point scale semi-quantitative scoring system proposed by Babiarz et al. to evaluate IAC18. The highest calcification score for each cerebral artery was selected and the calcification score of all evaluated arteries were added to obtain the patient’s total calcification score. The severity of IAC was graded based on the total calcification score: 0 was absent, 1–4 was mild, 5–8 was moderate, and 9–12 was severe19. Two experienced neurologists independently reviewed the images from CT angiography and graded the degree of cerebral artery calcification.
Statistical analyses
SPSS version 22.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Continuous variables with a normal distribution were compared using the Student’s t-test and ANOVA. Categorical variables were analyzed using the chi-squared test. Patients were divided in tertiles based on their serum sd-LDL-c levels. Partial Spearman correlation coefficients were used to examine the association between serum sd-LDL-c with IAC scores and admission NIHSS scores after adjustment for age and gender. To assess the association between serum sd-LDL-c and severe IAC, binary logistic regression analysis was used. Further analyses were performed to calculate multivariable-adjusted ORs (95% CIs) of severe IAC for the highest tertile versus the lowest tertile of serum sd-LDL-c levels after adjustment for covariates. To determine whether sd-LDL-c and other cholesterol components or ratio predicted short-term all-cause mortality risk of AIS patients, Cox regression model was applied after adjustment for the covariates. Differences were considered significant when P < 0.05.
Result
A total of 754 AIS patients, with an average age of 65 ± 13.2years, including 515 males were enrolled in this study. After 60-days 85 patients died during hospitalization and follow-up. These patients included 632 hypolipemic drugs users and 122 non-hypolipemic drugs users. The average sd-LDL-c level of all patients was 0.90 ± 0.46 mmol/L. There was no difference of sd-LDL-c level between hypolipemic drugs users and non-hypolipemic drugs users (0.89 ± 0.47 vs 1.07 ± 0.37 mmol/L, p = 0.067). We divided non-hypolipemic drugs users into tertiles (0.12-0.64 mmol/L, 0.65-1.07 mmol/L, > 1.07 mmol/L) based on their sd-LDL-c level. Analysis of variance showed that the NIHSS score, IAC score and mortality rate in the highest tertile of sd-LDL-c were significantly higher than in the other two tertiles (Table 1). The average level of sd-LDL-c in all patients who died was also significantly increased compared to survival patients (1.04 ± 0.59 vs 0.88 ± 0.44 mmol/L, P = 0.017).
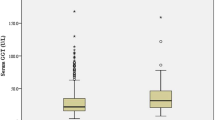
We analyzed the correlation between sd-LDL-c level and IAC score and admission NIHSS score in non-hypolipemic drugs users. After adjusting for age and sex, partial correlation analysis showed that sd-LDL-c was correlated with IAC score (r = 0.131, P = 0.006) and admission NIHSS score (r = 0.316, P < 0.001, Fig. 1).
Univariate linear logistic regression analysis showed that the level of sd-LDL-c predicted admission NIHSS score (β = 2.248, 95%CI: 1.136-3.519, p < 0.001)and IAC score (β = 1.209, 95%CI: 0.498-1.766, p < 0.001). After adjusting for the variables, sd-LDL-c was still independently correlated with admission NIHSS score (β = 1.537, 95%CI: 0.134-2.878, p = 0.042, Table 2) and IAC score (β = 1.355, 95%CI: 0.319-2.446, p = 0.015, Table 2). Cox logistic regression analysis showed that sd-LDL-c was associated with short-term mortality risk in AIS patients without adjusting for variables (OR = 1.936, 95%CI: 1.134-3.306, p = 0.016, Table 2). However, after adjustment for DBP, HbA1c, admission NIHSS score, and infarction type, etc., sd-LDL-c was no longer an independent predictor of the short-term mortality in AIS patients (Table 2).
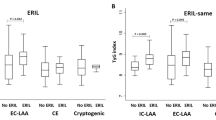
We defined patients with an IAC score above 4 as having severe calcification. The level of sd-LDL-c in patients with severe IAC was significantly increased compared to non-severe IAC patients (1.09 ± 0.55 vs 0.86 ± 0.44 mmol/L, P < 0.001). However, there was no significant difference in admission NIHSS score (6.67 ± 5.78 vs 6.53 ± 6.45, p = 0.873), mortality rate (8.1% vs 12.4%, p = 0.198) between severe intracranial calcification patients and non-severe calcification patients. We assessed the predictive value of TG, TG/HDL-c, sd-LDL-c, LDL-c, ApoB and ApoB/ApoA1 for severe intracranial calcification, admission NIHSS score and mRS score at discharge. After adjusting for age, sex, hypertension, DM, AF, HbA1c variables and so on, logistic regression analysis showed that only sd-LDL-c predicted the severity of IAC (OR = 2.857, 95%CI: 1.549-5.395, p = 0.001, Fig. 2a). However, there was no significant difference in the risk of severe calcification between tertiles groups.
In addition, the predictive value of six lipid components or cholesterol ratios for admission NIHSS scores and mRS scores at discharge were assessed. Similarly, sd-LDL-c (OR = 2.457, 95% CI: 1.152-3.775, p < 0.001, Fig. 2b) and ApoB/ApoA1 (OR = 2.415, 95% CI: -0.165-5.008, p = 0.05, Fig. 2b) independently predicted admission NIHSS score after adjusting for relevant variables. However, only TG/HDL-c (OR = 0.095, 95% CI: -0.180-1.426, p = 0.015, Fig. 2c) independently predicted the mRS score at discharge.
Discussion
In this study, we assessed the relationship between sd-LDL-c levels and IAC, NIHSS scores, short-term all-cause mortality and mRS scores at discharge in AIS patients. The level of sd-LDL-c in patients who died and those with severe IAC were significantly increased. Although there seemed to be a relation between sd-LDL-c and mortality risk and mRS score, sd-LDL-c did not independently predict all-cause mortality and prognosis. sd-LDL-c independently predicted the NIHSS score and IAC score in AIS patients, but there was no significant difference in IAC in patients with different levels of sd-LDL-c.
As a marker of intracranial atherosclerosis, IAC is an easily measurable and promising biomarker reflecting the severity of intracranial vascular disease. Chen et al. were the first to demonstrate that the incidence of IAC was higher in Chinese patients with ischemic stroke in a cross-sectional study20. In a Danish population, Ovesen et al. showed that the severity of intracranial atherosclerosis detected during emergency assessment (graded by the number of cerebral artery calcification) predicts an increased risk of stroke recurrence21. The Rotterdam study quantified the volume of IICA calcification measured by CT scan, and determined that IAC was the main risk factor for stroke in white people9. Although IAC is often observed, there is currently lack of a standard method to quantify the severity of IAC. Therefore, using severity of IAC in clinical practice is limited.
In our study subjects, sd-LDL-c can independently predict the severity of IAC. sd-LDL-c, as a lipid particle, can easily enter the arterial wall due to its small size. It binds to glycoproteins in the arterial wall and causes oxidative and inflammatory reactions, thereby damaging the intima of blood vessels22. In previous studies, we found that sd-LDL-c can independently predict the progression of arteriosclerosis23. At the present study, although the cholesterol levels of AIS patients who used lipid-lowering drugs before admission was lower than that of those who did not, there was no difference in sd-LDL-c level between them. The age, IAC score and the proportion of severe IAC were significantly higher in patients who used lipid-lowering drugs than those who did not. It has been suggested that statins may increase the proportion of calcium in coronary atherosclerotic plaque24.Simultaneously, a meta-analysis also showed that lowering LDL had no inhibitory effect on coronary artery calcification25.We found that LDL could not independently predict the degree of IAC. These information suggest that lowering traditional cholesterol level and inhibiting vascular calcification may be two different pathophysiological mechanisms.
Hyperlipidemia is a well-known risk factor for cardiovascular disease. However, findings of previous observational studies attempting to explain the effects of lipid levels on the prognosis of stroke are variable13,26,27. Large artery atherosclerosis is the main subtype of cerebral infarction in the TOAST classification system. However, the proportion of cerebral infarction caused by large artery atherosclerosis in different populations varies28. In our study population, the level of sd-LDL-c in patients who had died was significantly increased. Although sd-LDL-c is correlated with mortality risk and mRS score at discharge, it was not an independent predictor of all-cause mortality and short-term prognosis. sd-LDL-c can independently predict the NIHSS score at admission. Zeljkovic et al. conducted a study with a short-term follow-up of 200 patients with new onset of cerebral infarction and found that sd-LDL-c independently predicted the occurrence and short-term mortality of AIS12. Our study showed a different result. This may be explained by different subtypes of cerebral infarction in the study population and the length of follow-up.
The NIHSS score has been widely used in evaluating the severity of stroke in patients. TG, LDL-c, HDL-c, TG/HDL, ApoB and other lipid components or proportions are to some degree correlated with the NIHSS score29,30,31. Yasuhiro et al. semi-quantitatively detected sd-LDL-c in peripheral blood of new diagnosed AIS patients32. The results showed that sd-LDL-c was associated with adverse prognosis and mRS scores. However, sd-LDL-c was not related to the NIHSS score. We quantitatively measured sd-LDL-c and found that it was independently correlated with the NIHSS score. sd-LDL-c is a strong atherogenic factor and can penetrate into the arterial wall more easily and cause oxidative stress, which can lead to the onset of AIS and aggravate the degree of an infraction.
This study has the following limitations. First, the short follow-up of only 60 days affects the accuracy of sd-LDL-c in predicting prognosis. Second, up to now, there is no accurate and widely accepted quantitative measurement for IAC score. As in other study methods, IAC was semi-quantitatively determined and graded in our study. Third, the number of patients included in the study is small. The relationship between sd-LDL-c level and IAC, NIHSS scores and prognosis in AIS patients needs to be examined in large-scale prospective studies.
Conclusion
In conclusion, we systematically evaluated the relationship between serum sd-LDL-c levels and other lipid components or ratios with IAC score and admission NIHSS score in the Chinese AIS patients. sd-LDL-c as a strong atherogenic lipid particle is demonstrated that could independently predict admission NIHSS scores and the severity of cerebral artery calcification at the present study. However, its prognostic value in AIS patients still needs further study in the future.
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Renmin Hospital of Wuhan University (Wuhan, China, No.2016H11018).
References
Shanahan, C. M., Crouthamel, M. H., Kapustin, A., Giachelli, C. M. & Towler, D. A. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 109(6), 697–711 (2011).
Burke, A. P. et al. Pathophysiology of calcium deposition in coronary arteries. Herz 26(4), 239–244 (2001).
Evrard, S., Delanaye, P., Kamel, S., Cristol, J. P. & Cavalier, E. Vascular calcification: from pathophysiology to biomarkers. Clin. Chim. Acta 438, 401–414 (2015).
Reiss, A. B. et al. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 278, 49–59 (2018).
Andrews, J., Psaltis, P. J., Bartolo, B. A., Nicholls, S. J. & Puri, R. Coronary arterial calcification: a review of mechanisms, promoters and imaging. Trends Cardiovasc. Med. 28(8), 491–501 (2018).
Sangiorgi, G. et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 31(1), 126–133 (1998).
Verweij, S. L. et al. Elevated lipoprotein (a) levels are associated with coronary artery calcium scores in asymptomatic individuals with a family history of premature atherosclerotic cardiovascular disease. J. Clin. Lipidol. 12(3), 597–603. e1 (2018).
Wu, X. H., Chen, X. Y., Wang, L. J. & Wong, K. S. Intracranial artery calcification and its clinical significance. J. Clin. Neurol. 12(3), 253–261 (2016).
Bos, D. et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol. 71(4), 405–411 (2014).
Mackey, R. H. et al. Hormone Therapy, Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am. J. Cardiol. 90(8), 71–76 (2002).
Prado, K. B., Shugg, S. & Backstrand, J. R. Low-density lipoprotein particle number predicts coronary artery calcification in asymptomatic adults at intermediate risk of cardiovascular disease. J. Clin. Lipidol. 5(5), 408–413 (2011).
Zeljkovica, A. et al. LDL and HDL subclasses in acute ischemic stroke: Prediction of risk and short-term mortality. Atherosclerosis 210, 548–554 (2010).
Markaki, I., Nilsson, U., Kostulas, K. & Christina, S. High cholesterol levels are associated with improved long-term survival after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 23(1), e47–e53 (2014).
Powers, W. J. et al. AHA/ASA Guideline. Stroke 46(10), 3020–35 (2015).
Hirano, T., Ito, Y., Saegusa, H. & Yoshino, G. A novel and simple method for quantification of small, dense LDL. J. Lipid Res. 44, 2193–201 (2003).
Hirano, T., Ito, Y. & Yoshino, G. Measurement of small dense low-density lipoprotein particles. J. Atheroscler. Thromb. 12, 67–72 (2005).
Adams, H. P. Jr. et al. for the TOAST Investigators. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke 24, 35–41 (1993).
Babiarz, L. S., Yousem, D. M., Bilker, W. & Wasserman, B. A. Middle cerebral artery infarction: relationship of cavernous carotid artery calcification. AJNR Am. J. Neuroradiol. 26, 1505–1511 (2005).
Mak, H. K. et al. Computed tomography evaluation of intracranial atherosclerosis in Chinese patients with transient ischaemic attack or minor ischaemic stroke-its distribution and associated vascular risk factors. J. Stroke Cerebrovasc. Dis. 18, 158–163 (2009).
Chen, X. et al. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovasc. Dis. 21(1-2), 91–97 (2006).
Ovesen, C. et al. Prevalence and long-term clinical significance of intracranial atherosclerosis after ischaemic stroke or transient ischaemic attack: a cohort study. BMJ open. 3(10), e003724 (2013).
Norata, G. D. et al. Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis 206(2), 556–562 (2009).
Li, G. et al. Small dense low density lipoprotein-cholesterol and cholesterol ratios to predict arterial stiffness progression in normotensive subjects over a 5-year period. Lipids Health Dis. 17, 27 (2018).
Nakazato, R. et al. Statins use and coronary artery plaque composition: results from the International Multicenter CONFIRM Registry. Atherosclerosis 225, 148–153 (2012).
Henein, M. Y. & Owen, A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int. J. Cardiol. 153, 31–35 (2011).
Deng, Q. et al. Association of serum lipids with clinical outcome in acute ischaemic stroke: A systematic review and meta-analysis. J. Clin. Neurosci. 59, 236–244 (2019).
Vauthey, C., De Freitas, G. R., Van Melle, G., Devuyst, G. & Bogousslavsky, J. Better outcome after stroke with higher serum cholesterol levels. Neurology 54(10), 1944–1949 (2000).
Kim, B. J. & Kim, J. S. Ischemic stroke subtype classification: an Asian viewpoint. JoS 16(1), 8 (2014).
Yeh, P. S. et al. Low levels of high-density lipoprotein cholesterol in patients with atherosclerotic stroke: a prospective cohort study. Atherosclerosis 228(2), 472–477 (2013).
Deng, Q. W. et al. Low triglyceride to high-density lipoprotein cholesterol ratio predicts hemorrhagic transformation in large atherosclerotic infarction of acute ischemic stroke. Aging 11(5), 1589 (2019).
Ryu, W. S., Schellingerhout, D., Jeong, S. W., Nahrendorf, M. & Kim, D. E. Association between serum lipid profiles and early neurological deterioration in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 25(8), 2024–2030 (2016).
Manabe, Y. et al. Estimation of the presence of small dense lipoprotein cholesterol in acute ischemic stroke. Neurol. Int. 7, 1 (2015).
Acknowledgements
This study was supported by research grants from the Hospital Talents Support Fund of Hubei Provincial Hospital of TCM (201612022B) and Natural Science Foundation of Hubei Province of China (2018CFB699). The authors are indebted to the other investigators, staff, and participants for expert technical assistance.
Author information
Authors and Affiliations
Contributions
G.L. and Y.B.D. contributed to the conception and design of the study. T.Y. and J.L. recruited the subjects. Fellow-up patients were performed by Q.L. and Q.C. T.Y. analyzed the data and wrote the initial draft of the paper. Z.C.L. participated in its design and supervised the study. All authors contributed to the writing, reviewing, and revising of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, T., Long, Q., Li, J. et al. Small dense low-density lipoprotein cholesterol is strongly associated with NIHSS score and intracranial arterial calcification in acute ischemic stroke subjects. Sci Rep 10, 7645 (2020). https://doi.org/10.1038/s41598-020-64715-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64715-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.