Abstract
Splenic immune function was enhanced in diet-induced-obese (DIO) mice caused by Escherichia coli. The changes in spleen function on apoptosis were still unknown. Two hundred mice in groups Lean-E. coli and DIO-E. coli were intranasal instillation of E. coli. And another two hundred mice in groups Lean-PBS and DIO-PBS were given phosphate-buffered saline (PBS). Subsequently, spleen histology was analyzed. Then the rates of spleen cell (SC) apoptosis, and expression of the genes and proteins of Bcl-2, Bax, caspase-3 and caspase-9 were quantified in each group at 0 h (uninfected), 12 h, 24 h, and 72 h postinfection. The SC apoptosis rates of the DIO-E. coli groups were lower than those of the DIO-PBS groups at 12, 24 and 72 h (p < 0.05). Anti-apoptotic Bcl-2 expression gene and protein of the DIO-E. coli groups were higher than those of the DIO-PBS groups (p < 0.05). Gene expressions of pro-apoptotic Bax, caspase-3 and caspase-9 of the DIO-E. coli groups were lower than those of DIO-PBS groups at 12, 24 and 72 h (p < 0.05). The SC apoptosis rates of the Lean-E. coli groups were higher than those of the Lean- PBS groups at 12 h and 24 h (p < 0.05). Interestingly, the SC apoptosis rates in the DIO-E. coli groups were lower than those of the Lean-E. coli groups at 12 h (p < 0.05). In conclusion, our results suggested that the DIO mice presented stronger anti-apoptotic abilities than Lean mice in non-fatal acute pneumonia induced by E. coli infection, which is more conducive to protecting the spleen and improving the immune defense ability of the body.
Similar content being viewed by others
Introduction
Obesity is a complex metabolic condition that influences several physiologic systems, including immune function. Alterations in one of these physiologic systems alone or in combination can influence the response of the lungs to inflammatory stimuli dramatically1. Obesity increases people’s susceptibility to the bacterial infections, severe illness and death caused by bacteria-induced lung injury2,3.
Interestingly, recent studies have shown that obesity played a protective part in pneumonia4,5. Preliminary study of Gu reported that obesity could alleviate oxidative damage and inflammation of mice spleen under the condition of non-fatal acute pneumonia induced by Escherichia coli6. These results suggested that obesity could enhance the immune response of mice spleen against pulmonary infection.
Apoptosis is an important process for normal development and maintenance of host homeostasis in multicellular organisms7,8. It can be initiated or inhibited by various environmental stimuli as well as physiologic and pathologic conditions (e.g., oxidative stress)9. Apoptosis affects the stability of the immune system via regulation of gene expression and/or protein activity9. Obesity and infection can induce the apoptosis of spleen cells (SCs). For example, Wang et al.10 reported that mice fed a high-fat diet (HFD) had increased apoptosis of T regulatory cells in the spleen compared with that in control mice, that expression of B-cell lymphoma-2-associated X protein (Bax) and caspase-3 was increased, and that B-cell lymphoma (Bcl)-2 expression was decreased, in diet-induced obesity (DIO)-group mice. Furthermore, increased apoptosis of SCs in lipopolysaccharide (LPS)-treated rats was found, and that Bcl-2 expression decreased and Bax expression increased11.
The spleen is the largest immune organ in humans. It participates in humoral and cellular immune responses through its role in the generation, maturation and storage of lymphocytes12. Changes in SC apoptosis may affect the immune response of obese people suffering from a bacterial infection.
Until now, very little information has been available on SC apoptosis between obese and normal mice that have E. coli infection. To fill this knowledge gap, we observed the pathologic histology of the spleen, and measured the rate of apoptosis and expression of apoptosis-related genes and proteins (Bcl-2, Bax, caspase-3, caspase-9) in mice with E. coli infection. Our results could provide new experimental evidence for understanding apoptosis of spleen cells in diet-induced-obese mice in non-fatal acute pneumonia induced by E. coli infection.
Materials and Methods
Ethical approval of the study protocol
Protocols on the care and study of animals were approved by the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and the Ethics Committee of Sichuan Agricultural University (Chengdu, China), respectively. All methods were performed in accordance with the relevant guidelines and regulations.
Treatment of experimental animals
Four hundred male Kunming mice (3 weeks) were purchased from Dashuo Animals (Chengdu, China) and were housed under specific pathogen-free conditions. During experimentation, mice had access ad libitum to steriled commercial diets from Dashuo Animal Center (Chengdu, China) for 8 weeks4,13. Ambient temperature was maintained at 22–24 °C, and mice were subjected to a 12-h light–dark cycle. After 1 week of acclimatization to their environment, mice were divided randomly into two groups: Lean (n = 200) and DIO (n = 200). The body weights of mice in each feeding groups were measured every week, by which DIO mice could be obtained after 8 weeks4.
E. coli
E. coli was obtained from the Veterinary Medical Laboratory of Sichuan Agricultural University (Chengdu, China). Previously, we demonstrated that mice infected with E. coli (4 × 109 CFU/mL) via the intranasal route had typical pulmonary inflammation but did not die. E. coli was cultured at 37 °C for 20 h in lysogeny broth medium to obtain a bacterial liquid. The latter was centrifuged and suspended in PBS to produce inocula.
Intranasal infection
Mice were divided further into four groups: Lean- PBS (n = 100), Lean-E. coli (n = 100), DIO-PBS (n = 100) and DIO-E. coli (n = 100). Mice from Lean-E. coli and DIO-E. coli groups underwent intranasal instillation with 40 μL of a suspension containing ~109 CFU of E. coli. Mice from Lean- PBS and DIO-PBS groups were given the same dose of PBS4,5. Two mice which died within 6 h of intranasal instillation were removed.
Histopathology and staining
At 0 h (uninfected), 12 h, 24 h, and 72 h postinfection, eight mice in each group were sacrificed, the spleen tissues were analyzed and photographed. Spleen tissues were fixed in 4% paraformaldehyde, and processed routinely in paraffin. Then, they were dehydrated, embedded in paraffin, sectioned (thickness, 4~5 μm) and stained with hematoxylin and eosin (H&E) or TUNEL assay. The DNA fragmentation indicative of apoptosis was tested using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay14. Slides were stained by TUNEL using an apoptosis detection kit according to manufacturer ((Boster, Wuhan, China) instructions. Slides were viewed under a light microscope (BX 43; Olympus, Tokyo, Japan) and photographed.
Splenocyte preparation
At 0 h (pre-infection), 12 h, 24 h, and 72 h (post-infection), excised spleens were made into cell homogenates and centrifuged at 1000 × g for 5 min at 4 °C to precipitate them. The cell concentrations were adjusted to ~106/mL by PBS and were stored at 4 °C. Then, 5 µL of annexin V-fluorescein isothiocyanate (V-FITC; 51-66121E; BD Pharmingen, San Jose, CA, USA) and propidium iodide (PI; 5 µL) were added to 100 µL of the cell suspensions. After that, spleen cell suspensions were incubated for 15 min at 22 °C in the dark. 400 µL of binding buffer (BD Pharmingen, USA, 559763) was added to the cell suspensions. Then, the cell suspensions were Vortex oscillation mixied.
Determination of the apoptosis rate by flow cytometry
At 0 h (uninfected), 12 h, 24 h, and 72 h postinfection, eight mice in each group were sacrificed, the spleen tissues were analyzed. Apoptosis rates in the spleen were examined by flow cytometry using BD FACSCalibur™ (BD Biosciences, San Diego, CA, USA) within 1 h. Fluorescence-activated cell sorting was done with a sample size of 10,000 cells gated on the basis of forward and sideward scatter. Data were stored and processed using Flowjo (BD Biosciences).
Quantitative real-time polymerase chain reaction (qRT-PCR)
At 0 h (uninfected), 12 h, 24 h, and 72 h postinfection, eight mice in each group were sacrificed, the spleen tissues were analyzed. Gene expressions in spleen tissues were measured using qRT-PCR. Total RNA was isolated from the spleen using RNAiso Plus (9108/9109; Takara Bio, Tokyo, Japan). Complimentary (c) DNA was synthesized from total RNA in the spleen by a PrimeScript™ RT reagent kit (RR047A; Takara Bio) according to manufacturer instructions. And then, the cDNA was used as a template for qRT-PCR analysis. For qRT-PCR reactions, 10 μL mixtures were made by using SYBR® Premix Ex Taq™ II (DRR820A, Takara Bio), containing 5 μL Tli RNaseH Plus, 0.4 μL of forward and 0.4 μL of reverse primer, 3.4 μL RNAase-free water and 0.8 μL cDNA. Gene expressions of Bax, Bcl-2, Caspase-3, and Caspase-9 were quantified, β-actin (Sangon Biotech, Shanghai, China) was used as an internal standard.
The following mouse primers (forward and reverse, respectively) were used: Bcl-2 AGCCTGAGAGCAACCCAAT and AGAGGATGACCACCACAAAG; Bax, ATGCGTCCACCAAGAAGC and CAGTTGAAGTTGCCATCAGC; caspase-3, ACATGGGAGCAAGTCAGTGG and CGTCCACATCCGTACCAGAG; caspase-9, GAGGTGAAGAACGACCTGAC and AGAGGATGACCACCACAAAG; β-actin, TGCTGTGTTCCCATCTATCG and TTGGTGACAATACCGTGTTCA.
These gene primers were designed by Primer 5 and obtained from Sangon Biotech. Cycling conditions were: 95 °C for 3 min followed by 44 cycles of 10 s at 95 °C, 30 s at 60 °C, and 10 s at 72 °C. Cycling was done using the LightCycler® 480 Real-Time PCR system (Roche, Basel, Switzerland). Transcripts were quantified by the 2−ΔΔCt method.
Western blotting
At 12 h postinfection, three mice in each group were sacrificed, the spleen tissues were observed. Spleen tissues were lysed and proteins extracted with RIPA lysis buffer. Then, spleen total lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 12% gels and transferred to nitrocellulose membranes for 30 min in electrophoretic transfer cells. Subsequently, these nitrocellulose membranes were blocked in 2% non-fat dry milk for 1 h and incubated overnight at 4 °C with the primary antibodies Bax (ab32503), Bcl-2 (ab182858), caspase-3 (ab184787) and caspase-9 (ab202068), which were all from Abcam (Cambridge, UK). The nitrocellulose membranes were washed thrice (15-min each) with TBS-Tween (TBST) and incubated with secondary antibodies (7074; Cell Signaling Technology, Danvers, MA, USA) for 1.5 h, and washed thrice (15-min each) with TBST. The blots were visualized by ECLTM Chemiluminescence reagent (Beyotime technology, P0018A) and captured on the X-ray film. Protein expression was processed using Image-Pro® Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Statistical analyses
The significance of difference between two groups was analyzed by the independent samples t test, whereas the significant differences among four groups within 72 h experiment were analyzed by variance analyses (LSD or Dunnett’s T3). The results were expressed as means ± standard deviation. The analyses were performed using SPSS 17.0 software (IBM Corp, Armonk, NY, USA) for Windows. Statistical significance was considered at p < 0.05.
Ethical approval of the study protocol
Protocols on the care and study of animals were approved by the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and the Ethics Committee of Sichuan Agricultural University (Chengdu, China), respectively.
Results
Spleen histopathology
Spleens of Lean-E. coli groups and DIO-E. coli groups had normal histology with clear white pulp and red pulp at 0 h, but slight hyperemia of red pulp were found in the DIO-E. coli groups (Fig. 1). At 12 hours, the red marrow areas of Lean-E. coli groups were congested. In the areas, the number of multinucleated giant cells increased, the arrangement of white marrow lymphocytes were loose, and the spleen nodules were empty. While the red marrow areas of DIO-E. coli groups were congested, and there were macrophages and multinucleated giant cells in the areas. At 24 hours, a small amounts of plasma cells, neutrophils, macrophages, megakaryocytes and vacuoles were found in the marginal area of spleen of Lean-E. coli groups. And vacuoles were found in the sinuses of spleen. At 72 hours, the spleen of Lean-E. coli groups and DIO-E. coli groups showed red pulp hyperemia.
Apoptosis rates of SCs by flow cytometry
The rates of SC apoptosis were tested by detecting the total percentage of early (Annexin-V- positive and PI-negative) and late (Annexin-V-positive and PI-positive) apoptotic cells using flow cytometry. The rates of SC apoptosis in the DIO-PBS groups were significantly higher than those of the Lean-PBS groups across all time points (p < 0.05) (Fig. 2A–E). The SC apoptosis rates of the Lean-E. coli groups were significantly higher than those of the Lean- PBS groups at 12 h and 24 h (p < 0.05) and, conversely, those of the DIO-E. coli groups were significantly lower than those of the DIO-PBS groups (p < 0.05). The apoptosis rates of SC of the DIO-E. coli groups were significantly lower than those of the Lean-E. coli groups at 12 h and 24 h (p < 0.05).
Changes in the apoptosis rate in mouse spleens during E. coli infection. (A–D) Assessment of SC apoptosis in DIO-group and lean-group mice 12 h after infection. (A) Lean-PBS, (B) Lean-E. coli, (c) DIO-PBS, (D) DIO- E. coli. (e). Assessment of SC apoptosis in DIO-group and lean-group mice during infection. Values are expressed as means ± SD (n = 8 mice per group). Asterisks indicate significant difference between Lean-E.coli and Lean-PBS, as well as DIO-E. coli and DIO-PBS (p < 0.05). Hash indicate significant difference between DIO-PBS and Lean-PBS, as well as DIO-E. coli and Lean -E. coli (p < 0.05).
SCs apoptosis by TUNEL assay
As shown in Fig. 3, apoptotic cells with brown-stained nuclei were found in the spleen by the TUNEL assay. And the apoptotic cells showed nuclear condensation and irregular shapes.
Expression of the mRNA of bcl-2, bax, caspase-3 and caspase-9 by qRT-PCR
According to qRT-PCR, expression of the pro-apoptotic gene Bax of the DIO-groups were higher than those of Lean-groups at 0 h, respectively (p < 0.05). Meanwhile, compared with Lean-groups, a lower ratio of Bcl-2: Bax and higher expressions of caspase-3 and caspase-9 were found in the DIO-groups at 0 h (p < 0.05). After intranasal instillation of E. coli, the mRNA levels of Bcl-2 and Bcl-2:Bax ratio of the DIO-E. coli groups were significantly higher than those of the DIO-PBS groups. And the mRNA levels of Bax, caspase-3, and caspase-9 of the DIO-E. coli groups were significantly lower than those of the DIO-PBS groups (p < 0.05) (Fig. 4). The mRNA levels of Bcl-2 and the Bcl-2:Bax ratios of the Lean-E. coli groups were lower than those of the Lean- PBS groups at 12 h. And the mRNA levels of Bax, caspase-3 and caspase-9 of the Lean-E. coli groups were higher than those of the Lean- PBS groups (p < 0.05). Interestingly, the mRNA levels of Bcl-2 and the Bcl-2: Bax ratios in the DIO-E. coli groups were higher than those of the Lean-E. coli groups at 12 h, while the mRNA levels of Bax and caspase-3 were lower than those of the Lean-E. coli groups at 12 h (p < 0.05).
Changes in expression of apoptotic genes in mice after E. coli infection. (A) Bcl-2, (B) Bax, (C) Bcl-2: Bax ratio, (D) caspase-3 and (E) caspase-9. Values are expressed as means ± SD (n = 8 mice per group). Asterisks indicate significant difference between Lean-E.coli and Lean-PBS, as well as DIO-E. coli and DIO-PBS (p < 0.05). Hash indicate significant difference between DIO-PBS and Lean-PBS, as well as DIO-E. coli and Lean -E. coli (p < 0.05).
Expression of the proteins of bcl-2, bax, caspase-3 and caspase-9 by western blotting
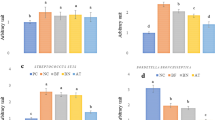
As shown in Fig. 5, at 12 h, expressions of Bcl-2 protein of the Lean-E. coli groups were lower than those of Lean- PBS groups, and expressions of Bcl-2 protein of the DIO-E. coli groups were higher than those of DIO-PBS groups (p < 0.05). At 12 h, expressions of the proteins of Bax, caspase-3 and caspase-9 of the Lean-E. coli groups were higher than those of the Lean- PBS groups, and expressions of these same proteins of the DIO-E. coli groups were lower than those of the DIO-PBS groups (p < 0.05). Interestingly, expression of Bcl-2 protein of the DIO-E. coli groups were higher than those of the Lean-E. coli groups (p < 0.05). Expression of the proteins of Bax, caspase-3 and caspase-9 of the DIO-E. coli groups were lower than those of the Lean-E. coli groups (p < 0.05). Moreover, the Bcl-2: Bax ratios were decreased significantly in lean-groups mice while increased in DIO-groups mice at 12 h. Besides, the Bcl-2: Bax ratios in the DIO-E. coli groups were higher than those of the Lean-E. coli groups (p < 0.05).
Changes in protein expression of Bcl-2, Bax, caspase-3, and caspase-9 in mouse spleens at 12 h. (A) Western blots. (B) Quantitative analysis of Bcl-2 expression. (C) Quantitative analysis of Bax expression. (D) Bcl-2:Bax ratio. (E) Quantitative analysis of caspase-3 expression. (f) Quantitative analysis of caspase-9 expression. Values are expressed as means ± SD (n = 8 mice per group). Asterisks indicate significant difference between Lean-E.coli and Lean-PBS, as well as DIO-E. coli and DIO-PBS (p < 0.05). Hash indicate significant difference between DIO-PBS and Lean-PBS, as well as DIO-E. coli and Lean -E. coli (p < 0.05).
Discussion
Obesity is a chronic, low-grade inflammatory reaction and influences non-specific and specific immune responses mediated by humoral and cell-mediated mechanisms15. The spleen, as the largest peripheral lymphatic organ in humans and rodents, is central to the efficient functioning of the immune response16,17. The spleen can synthesize antibodies in its white pulp and remove antibody-coated bacteria and antibody-coated blood cells by way of blood and lymph-node circulations. Hence, changes in spleen structure affect the immune status of the host.
A significant relationship between HFD intake and splenic disorganization in mice has been documented18. Yamano et al.19 noted an increased percentage of splenic red pulp and macrophages in mice fed a HFD, and elemental iron was deposited mainly in red pulp. During splenomegaly, many immune cells are released to participate in immune defense when the host is infected11,20. In the present study, the spleen structures showed differences among different treatment groups. Structural disorganization due to comorbidities (obesity and infection) may alter microenvironments within the spleen, which then showed different immune responses in Lean-mice and DIO- mice18. We will do further verification in the next test.
Apoptosis plays a major part in determining cellular fate, and is involved in physiologic and pathologic processes21. Apoptosis is actively involved in immunosuppression in various circumstances22. Obesity can induce apoptosis in skeletal muscle23, adipose tissue24, the liver25 and heart26. However, the effect of obesity on SC apoptosis has been reported rarely. In the present study, after 8 weeks of consuming a HFD, compared with lean mice, an increase in SC apoptotis was detected in DIO-group mice by flow cytometry and the TUNEL assay, data that are in accordance with previous studies10,27. Bacterial infection can induce the apoptosis of macrophages28, neutrophils29, monocytes and T cells30. Furthermore, Schreiber et al.31 found that pneumonia could be induced in animals by Streptococcus pneumoniae or Pseudomonas aeruginosa, and that lymphocyte apoptosis in the spleen occurred according to flow cytometry. In the present study, after infection, there were increased SC apoptosis of lean-groups mice and decreased SC apoptosis of DIO-groups mice. Furthermore, SC apoptosis in DIO-groups mice were lower than those of lean-groups mice after infection. Besides, in our previous test, DIO-group mice exhibited stronger spleen antioxidant capacities after intranasal instillation of 109 CFUs/mL of E. coli6. Thus, we hypothesized there were three possible reasons. Firstly, obese mice suffering from E. coli infection might leaded to increased production of splenic lymphocytes. Secondly, due to spleen cells being exchanged with blood cells during infection32, the decrease in the number of apoptotic spleen cells in obese mice might cause more spleen cells (lymphocytes, macrophages, red blood cells) to be released into blood and then migrated to the lungs, thereby leading to the recruitment of lung lymphocytes and promotion of the immune response. Thirdly, the results may be related to the decrease in oxidative activity and inflammation in spleens of obese mice. However, whether different concentrations of bacterial suspension can account for such controversial results or not will be further tested.
The “intrinsic” mitochondria-dependent apoptotic pathway (MDAP) is regarded as one of the major pathways that triggers apoptosis directly. It starts from within the cell and results in the release of several pro-apoptotic factors from the intermembrane space of mitochondria9. The MDAP is regulated mainly by anti-apoptotic proteins and pro-apoptotic proteins, such as Bcl-2 and Bax33,34,35. The Bcl-2: Bax ratio determines cell survival by regulating apoptosis: a high Bcl-2: Bax ratio inhibits apoptosis and vice versa34,36,37. Zhang et al. reported apoptosis of endothelial cells in mice fed a HFD by downregulation of Bcl-2 expression38. A similar picture has been observed in skeletal muscle23 and the heart26. The present study showed that expressions of the mRNA and protein of anti-apoptotic Bcl-2 reduced, whereas expressions of those of pro-apoptotic Bax increased. Also, the Bcl-2:Bax ratio of DIO-groups mice spleen decreased. Infection with intracellular pathogens also induces the apoptosis of host cells by regulation of Bcl-2 and Bax39. Xiao et al.11 found that increased SC apoptosis in LPS-treated rats could be promoted by down-regulation of Bcl-2 expression and up-regulation of Bax expression. Interestingly, in the present study, expressions of gene and protein of the Bcl-2 and Bcl-2: Bax ratios in SCs in DIO-group mice increased, whereas expressions of the Bax gene and protein decreased, after E. coli infection. Directly opposite effects were noted in lean mice, which contributed to alleviation of SC apoptosis in obese mice after infection.
In the MDAP, caspase-9 participates in its initiation, and caspase-3 participates in its execution, and both have important roles in apoptosis40,41. Expressions of caspase-3 and caspase-9 were higher of obese rat hearts than those of lean rat hearts26. We showed that expressions of caspase-3 and caspase-9 of the DIO-groups mice spleens were higher than those of lean-groups mice at 0 h. Splenic apoptosis increased for active caspase-3 and caspase-9 in gram-positive or gram-negative pneumonia31. In the present study, expressions of protein caspase-3 and protein caspase-9 of the DIO-E. coli groups decreased. While after E. coli infection, expressions of protein caspase-3 and protein caspase-9 of the Lean-E. coli groups increased. Therefore, our findings strongly suggested that SC apoptosis of the DIO-groups mice were lower than those of lean-groups mice after E. coli infection. These data suggested a beneficial effect of obesity upon infection. In conclusion, the SC apoptosis was more active in obese mice than in lean mice. Interestingly, the DIO-groups mice could alleviate SC apoptosis during pneumonia through increased expression of the anti-apoptotic Bcl-2 gene and decreased expression of the pro-apoptotic Bax, caspase-3, caspase-9 genes. These results demonstrated, for the first time, potential changes in splenic immune function in obese mice with pneumonia through alteration of SC apoptosis. Of course, further studies on apoptotic pathways or possible mechanisms are demanded to clarify the relationship between obesity and infection.
Data availability
No additional unpublished data are available. All authors share the data underlying the findings of their manuscripts. Data sharing allows researchers to verify the results of an article, replicate the analysis, and conduct secondary analyses.
Change history
31 January 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-024-52877-9
References
Mancuso, P. Obesity and lung inflammation. J. Appl. Physiol. 108, 722–728 (2010).
Kornum, J. B. et al. Obesity and risk of subsequent hospitalisation with pneumonia. Eur. Respir. J. 36, 1330–1336 (2010).
Rozzini, R. & Trabucchi, M. Pneumonia and mortality beyond hospital discharge in elderly patients. Chest 139, 473–474 (2011).
Wan, T. M. et al. Diet-induced obese mice exhibit altered immune responses to acute lung injury induced by Escherichia coli. Obesity 24, 2101–2110 (2016).
Wang, F. Y. et al. Histopathological changes caused by inflammation and oxidative stress in diet-induced-obese mouse following experimental lung injury. Sci. Rep. 8, 14250 (2018).
Gu, X. C. Obesity enhances antioxidant capacity and reduces cytokine levels of the spleen in mice to resist splenic injury challenged by Escherichia coli. J. Immunol. Res. 5948256 (2020).
Cohen, J. J. Apoptosis and its regulation. Adv. Exp. Med. Biol. 406, 11–20 (1996).
Wyllie, A. H., Kerr, J. F. R. & Currie, A. R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68, 251–306 (1980).
Zhao, H. M. Extrinsic and intrinsic apoptosis signal pathway review. Apoptosis & Medicine (2012).
Wang, B. et al. Increased oxidative stress and the apoptosis of regulatory T cells in obese mice but not resistant mice in response to a high-fat diet. Cell. Immunol. 288, 39–46 (2014).
Xiao, K. et al. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res. 359, 605–618 (2015).
Sandford, E. E. et al. Spleen transcriptome response to infection with avian pathogenic Escherichia coli in broiler chickens. BMC Genomics 12, 469 (2011).
Arcari, D. P. et al. Antiobesity effects of yerba maté extract (Ilex paraguariensis) in high-fat diet–induced obese mice. Obesity 17, 2127–2133 (2009).
Yuan, G. P. et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int. J. Clin. Exp. Patho. 7, 2905–2914 (2014).
Martí, A., Marcos, A. & Martínez, J. A. Obesity and immune function relationships. Obes. Rev. 2, 131–140 (2001).
Tarlinton, D., Light, A., Metcalf, D., Harvey, R. P. & Robb, L. Architectural defects in the spleens of Nkx2-3-deficient mice are intrinsic and associated with defects in both B cell maturation and T cell-dependent immune responses. J. Immunol. 170, 4002–4010 (2003).
Balogh, P., Horváth, G. & Szakal, A. K. Immunoarchitecture of distinct reticular fibroblastic domains in the white pulp of mouse spleen. J. Histochem. Cytochem. 52, 1287–1298 (2004).
Da Silva, A. M., Corrêa, C. L., Neves, R. H. & Machado-Silva, J. R. A high-fat diet associated with acute schistosomiasis mansoni causes disorganization in splenic architecture in mice. Exp. Parasitol. 132, 193–199 (2012).
Yamano, N. et al. A long-term high-fat diet changes iron distribution in the body, increasing iron accumulation specifically in the mouse spleen. J. Nutr. Sci. Vitaminol. 61, 20–27 (2015).
Rungruang, T. et al. The effect of vitamin C on morphology and histology of liver and spleen of Plasmodium-infected mice. Int. Food Res. J. 20, 1639–1643 (2013).
Mukhopadhyay, S., Panda, P. K., Sinha, N., Das, D. N. & Bhutia, S. K. Autophagy and apoptosis: where do they meet? Apoptosis 19, 555–566 (2014).
Sun, E. W. & Shi, Y. F. Apoptosis: the quiet death silences the immune system. Pharmacol. Ther. 92, 135–145 (2001).
Sishi, B. et al. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 96, 179–193 (2011).
Sergeev, I. N. & Song, Q. M. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 58, 1342–1348 (2014).
Monteiro, M. E. L., Xavier, A. R., Oliveira, F. L., Filho, P. J. S. & Azeredo, V. B. Apoptosis induced by a low-carbohydrate and high-protein diet in rat livers. World J. Gastroentero. 22, 5165–5172 (2016).
Lu, M. C. et al. More activated cardiac mitochondrial-dependent apoptotic pathway in obese zucker rats. Obesity 15, 2634–2642 (2012).
Avula, C. P., Zaman, A. K., Lawrence, R. & Fernandes, G. Induction of apoptosis and apoptotic mediators in balb/C splenic lymphocytes by dietary n-3 and n-6 fatty acids. Lipids 34, 921–927 (1999).
Zhou, Z. Y., Wu, S. Y. & Xue, Z. M. Bacterial infection and the apoptosis of mouse macrophage. Shanghai Laboratory Animal Science 22, 249–253 (2002).
Ebner, F. et al. The RNA-binding protein tristetraprolin schedules apoptosis of pathogen-engaged neutrophils during bacterial infection. J. Clin. Invest. 127, 2051–2065 (2017).
Daigneault, M. et al. Monocytes regulate the mechanism of T-cell death by inducing Fas-mediated apoptosis during bacterial infection. Plos Pathog. 8, e1002814 (2012).
Schreiber, T. et al. Both gram-negative and gram-positive experimental pneumonia induce profound lymphocyte but not respiratory epithelial cell apoptosis. Shock 26, 271–276 (2006).
Bronte, V. & Pittet, M. J. The spleen in local and systemic regulation of immunity. Immunity 39, 806–818 (2013).
Fan, T. J., Xia, L. & Han, Y. R. Mitochondrion and apoptosis. Acta Bioch. Bioph. Sin. 33, 7–12 (2001).
Adams, J. M. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322–1326 (1998).
Starkov, A. A., Polster, B. M. & Fiskum, G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J. Neurochem. 83, 220–228 (2010).
Mcclintock, D. S. et al. Bcl-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol. Cell. Biol. 22, 94–104 (2002).
Hanada, M., Aimé-Sempé, C., Sato, T. & Reed, J. C. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270, 11962–11969 (1995).
Zhang, T. et al. Atherosclerosis-associated endothelial cell apoptosis by MiR-429-mediated down regulation of Bcl-2. Cell. Physiol. Biochem. 37, 1421–1430 (2015).
Perfettini, J. L. et al. Role of Bcl-2 family members in caspase-independent apoptosis during chlamydia infection. Infect. Immun. 70, 55–61 (2002).
Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15, 269–290 (1999).
Rudel, T. Caspase inhibitors in prevention of apoptosis. Herz 24, 236–241 (1999).
Acknowledgements
We thank Arshad Makhdum, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This manuscript was supported by Sichuan Science and Technology Program (2018NZ0002), National key research and development project (2018YFD0501800), and the China Scholarship Council in 2019 (201906915019).
Author information
Authors and Affiliations
Contributions
The authors’ contributions are as follows: Zhihua Ren and Xuchu Gu and Jing Fang were the principal investigator and contributed to the study design and the study design and interpretation of the findings and wrote the manuscript. Dongjie Cai contributed to the study design and data analyses. Zhicai Zuo contributed to the study design. Shuang Liang, and Hengmin Cui contributed to the subject briefings and data collection. Junliang Deng conducted the western blotting experiments. Xiaoping Ma, and Yi Geng contributed to the subject briefings and data collection. Ming Zhang prepared the figures and tables. Yue Xie, Gang Ye, Liping Gou, and Yanchun Hu contributed to the study design and carried out data analyses. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
This article has not been published elsewhere in whole or in part. All authors have read and approved the content, and agree to submit for consideration for publication in the journal. The authors declared no conflict of interest. All work complies with the Ethical Policies of Scientific Reports and has been conducted under internationally accepted ethical standards after relevant ethical review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-52877-9"
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ren, Z., Gu, X., Fang, J. et al. RETRACTED ARTICLE: Effect of intranasal instillation of Escherichia coli on apoptosis of spleen cells in diet-induced-obese mice. Sci Rep 10, 5109 (2020). https://doi.org/10.1038/s41598-020-62044-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62044-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.