Abstract
The effects of different concentrations (0, 50,100, 1000 and 2500 mg/L) of engineered aluminum and nickel oxide nanoparticles (Al2O3 and NiO NPs) on plant growth, oxidative stress and antioxidant activities in the hydroponically grown tissues of Nigella arvensis L. were investigated. The plant biomass was significantly increased under 50 and 100 mg/L of Al2O3 NPs or 50 mg/L of NiO NPs treatment, but was significantly decreased at higher concentrations of these nanoparticles. Assays of several enzymatic antioxidants such as ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) in roots and shoots indicate a general increase of activities after exposure to 50–2,500 mg/L of Al2O3 NPs and NiO NPs. The results are corroborated by an increased 2,2-diphenyl-1-picryl hydrazyl (DPPH) scavenging activity, total antioxidant capacity, total reducing power, total iridoids content, total saponin content, and total phenolic content in treated plants by Al2O3 NPs compared to the control plants. By contrast, the antioxidant activities, formation of secondary metabolites, and other related physiological parameters such as the total antioxidant capacity, DPPH scavenging activity and total saponin content were inhibited after the concentration of NiO NPs was increased to 100 mg/L. Total phenols, saponins, iridoids and total antioxidant content and DPPH scavenging activity were increased in plants treated with 100–2,500 mg/L Al2O3 NPs. Overall, these two nanoparticles displayed different effects in the shoots and roots of plants at different concentrations, which may be due to their physico-chemical properties.
Similar content being viewed by others
Introduction
Rapid development of nanotechnology has greatly expanded the applications of engineered nanoparticles (ENPs) in commercial and industrial products1. Increased application and potential accumulation of ENPs in the environment and their unknown interactions with different organisms, aggravated by some reports of greater toxicity at nanoscale than the bulk scale, caused broad concerns about the environmental health and safety of ENPs2,3. Previous studies have demonstrated some disruptive effects of some ENPs on the natural environment including water, air, and soil quality4. Plants are one of the most essential components of the ecosystem and interact with ENPs closely5. These ENPs could be taken up by plants, and enter into the food chain through dietary consumption, ultimately affecting human health6.
Many previous investigations explored the potential applications of ENPs in agriculture7,8,9,10. However, the majority of previous studies in ENPs - plant interactions focused on the potential toxicity of nanoparticles to higher plants. Both positive and negative or insignificant effects of ENPs on plants have been reported11. In general, the phytotoxicity of ENPs is mediated through the production of reactive oxygen species (ROS) in plant cells12. Even though ROS are normally produced within plants as a byproduct of metabolic processes in chloroplasts and other organelles13,14, excessive production of ROS can disrupt plant photosynthesis and other physiological and biochemical processes, eventually triggering the defense mechanisms in plants such as greater activities of certain antioxidants15. In addition, accumulation of ENPs can stimulate defense mechanisms through antioxidant enzymes to scavenge the deleterious ROS16.
The amount of ROS and antioxidant responses vary with metal oxide nanoparticle types, exposure conditions and plant species15. For example, La2O3 NPs at 2,000 mg/L of induced significant ROS production and cell death in cucumber (Cucumis sativus L.)17. However, no significant effects was observed when cucumber was exposed to 0.2–200 mg/L CeO2 NPs17. Cerium oxide nanoparticles (CeO2NPs) increased H2O2 content in Zea mays and Brassica rapa18,19 but decreased the H2O2 content in Oryza sativa20,21. Hu et al.22 showed that SOD and CAT activities significantly increased in Salvinia natans treated with 50 mg/L ZnO NPs22. Kim et al.23 also reported high activities of SOD, POD and CAT following ZnO and CuO NPs (100 mg/L) applications to cucumber plants23.
Among the most abundant nanoparticles produced in the industrial and commercial sectors are aluminum oxide nanoparticles (Al2O3 NPs) and nickel oxide nanoparticles (NiO NPs). Al2O3 NPs are widely used in various military and commercial products24,25. For example, they have been used in catalyst support and microelectronics26 and in semiconductor materials, glass products, rocket fuel, explosives, wear-resistant coatings for ships, packaging materials, cosmetic fillers, plastics and sensors27,28,29. The phytotoxicity of Al2O3 NPs was reported in tobacco and wheat30,31. Lee et al.32 investigated the phytotoxicity of Al2O3 NPs (∼150 nm) to Arabidopsis thaliana at concentrations of 400, 2,000 or 4,000 mg/L and no toxic effects were observed32. Some positive effects of Al2O3 NPs were reported on the root growth of ryegrass, radish, lettuce and rape plants33. Riahi-Madvar et al.31 showed that antioxidant enzyme activities of wheat plant decreased the damaging effects of Al2O3 NPs by scavenging the induced ROS31.
Nickel oxide nanoparticles (NiO NPs) have many novel properties compared to their bulk counterpart and are widely used in catalytic materials, sensors, magnetic materials, battery electrode, diesel–fuel additives and electrochromic films34,35. It has been reported that NiO NPs can be easily transported into plants inducing cytotoxic and genotoxic effects36. Feisal et al.37 reported that NiO NPs at 0.025–2.0 mg/mL led to oxidative stress, mitochondrial dysfunction, lipid peroxidation and apoptosis/necrosis in tomato seedling roots37. The activity of antioxidative enzymes, including CAT, and SOD was improved. Dewez et al.38 indicated that both NiO NPs and bulk NiO at 1000 mg/L increased ROS generation and had high inhibitory effect on the PSII quantum yield, reducing the photosynthetic electron transport performance in Lemna gibba L.38.
The aims of this work were to investigate the impact of Al2O3 and NiO NPs on the growth and antioxidative responses of Nigella arvensis at different concentrations. N. arvensis is a grassy plant with small black seeds known as the “seed of blessing”. The plant belongs to the genus of Nigella and family of Ranculaceae. The seeds have been used to enhance flavor in cakes and bread. They are used as curative material for treating various diseases in some parts of the world. The seeds also demonstrate some therapeutic (for worm infestation), antiallergic, antiviral and anti-inflammatory properties39,40. In this study, we investigated the roles of Al2O3 and NiO NPs on plant growth, intracellular ROS generation and levels of antioxidant responses for example activity of different antioxidant enzymes including CAT, APX, SOD and POD, as well as the alteration of secondary metabolites such as total phenolic, total iridoid and total saponin content, total antioxidant capacity, total reducing power and scavenging activity against DPPH free radical in the treated medicinal plant of N. arvensis.
Results and Discussion
Biomass assay
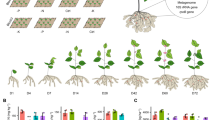
The results (Fig. 1A) showed that Al2O3 NPs up to 100 mg/L stimulated the growth of N. arvensis, but exerted toxicity at higher concentrations. The highest shoot and root dry weight (1.2 ± 0.02 and 0.46 ± 0.03 g) and the lowest shoot and root weight (0.55 ± 0.03 and 0.28 ± 0.002 g) was observed at 100 and 2500 mg/L Al2O3 NPs respectively. NiO NPs demonstrated significantly stronger effects than Al2O3 NPs on the growth of N. arvensis. Maximum shoot and root dry weights (1.12 ± 0.03 and 0.37 ± 0.009 g respectively) were obtained at 50 mg/L, and the lowest shoot and root dry weight (0.56 ± 0.04 and 0.15 ± 0.007 g) were found at 2500 mg/L NiO NPs (Fig. 1B). Consistent with our results, Asztemborska et al.41 also reported an increase in plant biomass of Allium cepa L., Zea mays, Lepidium sativum and Kalanchoe daigremontiana upon exposure to low concentrations of Al2O3 NPs, but a 33% decrease of root dry mass was noticed at the highest Al2O3 NPs concentration (1000 mg/kg)41.
H2O2 content and antioxidant enzymes activity
ROS is normally formed as a by-product of plant cellular metabolism. Various environmental stresses can lead to overproduction of ROS in plants, which can cause progressive oxidative damage. After exposure to Al2O3 NPs, H2O2 content (a product of the superoxide dismutase reaction) in the roots of N. arvensis increased at 50 and 100 mg/L but decreased significantly at 1000 and 2500 mg/L to a comparable level of the control plants, suggesting that excessive amounts of ROS were scavenged by antioxidant enzymes at higher concentrations. However, H2O2 content in the shoot increased with the increase of Al2O3 NPs up to 1000 mg/L, indicating higher Al stress at higher concentrations (Fig. 2A). Formation of ROS can be a result of interactions between Al and plant cells42,43. Pakrashi et al.44 indicated that leached out Al3+ and electrostatic interactions between Al2O3 NPs and algae cells might synergistically alter surface functional moieties on algal cells, resulting in oxidative stress and cell membrane damage44.
In N. arvensis treated with NiO NPs, H2O2 content in both root and shoot tissues increased significantly at 2500 mg/L compared to the control and other treatments. Exposure to 2500 mg/L NiO NPs increased the root H2O2 content by 59% compared to control, which functions as a signaling molecule in the induction and regulation of antioxidants. The H2O2 content in the shoots exposed to NiO NPs increased significantly by 34% at 2500 mg/L compared to the control, but was unaffected in other treatments (Fig. 2B). Other studies with Vicia narbonensis L.45 and Zea mays19 treated with titanium oxide (TiO2) and cerium oxide (CeO2) NPs demonstrated little effects of these NPs on H2O2 accumulation in plant tissues, indicating that the induction of H2O2 in plants vary with the properties (e.g. composition) of metallic nanoparticles.
The activities of antioxidant enzymes in plants increase under environmental stresses46. Enhanced activities of antioxidant enzymes can increase plant tolerance to oxidative stress47. Figure 3 shows the activities of antioxidant enzymes in the roots and shoots of N. arvensis in the presence of 0–2500 mg/L Al2O3 and NiO NPs. The CAT activity in the shoots and roots of N. arvensis increased with increasing ENPs levels for both nanoparticles (p < 0.01) (Fig. 3A), but the patterns of increase differed between these two ENPs. The CAT activity in plant tissues significantly increased after exposure to different concentrations of Al2O3 NPs compared to the control plants, however, the differences between treatments with different concentrations of Al2O3 NPs are generally insignificant. By contrast, the CAT activity displayed a dose-response relationship with NiO NPs, with higher NiO NPs leading to greater CAT activity in both roots and shoots (Fig. 3A). The exposure to CAT is one of the most important enzymes that scavenge ROS in plant cells. CAT partakes in the main defense system against the increase of H2O2 and can regulate the H2O2 levels in cells by converting it to water and oxygen48. The increased activity with increasing ENPs concentrations correlated with the decreased H2O2 content and lipid peroxidation in plants (Fig. 2), underscoring the importance of CAT in alleviating ENPs-induced oxidative stress. Furthermore, higher activity of CAT can be attributed to higher activities of SOD and higher production of H2O2. In agreement with our observations in roots and shoots, Laware and Raskar, (2014) found that the CAT activity in plants was increased upon exposure to 300 mg/L TiO2 NPs49. Also consistent with our previous results, CAT activity in tomato roots was not affected by up to 250 mg/L NiO NPs treatment, but its activity increased significantly in treatments with 250 to 2000 mg/L of NiO NPs37. Interestingly, TiO2 at 100 and 200 mg/L concentrations actually reduced the CAT activity compared with the control49, indicating that ENP composition plays a role in the induction of oxidative stress in plants.
The POD activities in N. arvensis roots and shoots were elevated after exposure to both Al2O3 and NiO NPs. The POD activity in plant roots increased by 1.32 and 1.47 fold at 1000 and 2500 mg/L of Al2O3NPs (p < 0.05) compared to the control. Similarly, NiO NPs markedly increased the POD activity in plant root, with a 3.7 fold increase at 1000 mg/L. Interestingly, 2500 mg/L of NiO NPs significantly decreased the POD activity in plant roots by 24% compared with the 1000 mg/L treatment (P < 0.05).The enhancement of POD activity in plant shoot was relatively mild compared with that in plant roots. Treatment with 1000 and 2500 mg/L NiO NPs resulted in 1.9 and 2.2 fold increase of POD activity in plant shoots (Fig. 3B). In addition to reducing H2O2 accumulation during oxidative stress, POD also affects lignin and ethylene synthesis, as well as the decomposition of indole-3-acetic acid (IAA). It also involves in plant resistance against pathogens and wound healing48. The increase in POD activities against studied ENPs implies the protective ability of N. arvensis against oxidative stress. In agreement with our studies, increased POD activity in Glycine max and Cucumis sativus treated with CuO NPs, in Triticum aestivum treated with Ag NPs, and in Vicia narbonensis treated with TiO2 NPs was also reported23,45,50,51.
SOD plays an important role against ROS-mediated toxicity by catalyzing the dismutation of free hydroxyl radicals to H2O2 and O2. Al2O3 and NiO NPs displayed different effects on SOD activity in the shoots and roots of N. arvensis. The presence of NiO NPs up to 1000 mg/L increased the SOD activity in roots, but decreased the SOD activity at 2500 mg/L to a similar level in the control plant. The SOD activities in the shoots also increased with NiO NPs, but the increase was significant only at the highest concentration of 2500 mg/L (p < 0.01). The addition of Al2O3 NPs had no significant effects on SOD activities of N. arvensis roots. When N. arvensis was treated with 1000 mg/L of Al2O3 NPs, the SOD activity in plant shoots was significantly enhanced by 49% (p < 0.05), while 2500 mg/L of this ENP reduced shoot SOD activity by 27% (p < 0.01) compared with the 1000 mg/L treatment (Fig. 3C). Significant increase in SOD activity may be due to either direct effect of these ENPs on the SOD gene expression or an indirect effect mediated through an increase in the level of O2−• radicals. Rajeshwari et al.52 found similar impact of Al2O3 NPs on SOD activity in Allium cepa root tips and a maximum increase was found at 100 mg/L52. Feisal et al.37 revealed that in NiO NPs treated tomato roots, SOD activity increased with increasing NiO NPs in comparison to control but its activity was decreased at 1500 and 2000 mg/L NiO NPs that confirms our results37. Therefore, reduced SOD activity in this study under the highest Al2O3 and NiO NPs concentrations may reflect the low ROS scavenging capacity and increased damage to plants11.
As a member of the ascorbic acid-glutathione cycle, APX plays a crucial role in eliminating hazardous H2O2 from plant cells. There was a progressive increase in APX activity with increasing NiO NPs in the shoots and roots of N. arvensis. The APX slightly increased with the increase of NiO NPs from 50–100 mg/L, but substantially increased in 1000 and 2500 mg/L NiO NPs treated plants. In plants treated with 2500 mg/L of NiO NPs, APX activity in the roots and shoots of N. arvensis was 3.49 and 2.54-fold of their respective controls (0 mg/L) (Fig. 3D). The APX activity in plants exposed to Al2O3 NPs exhibited different patterns in comparison to its activity under NiO NPs exposure. The enzyme activities were increased gradually with increasing level of Al2O3 NPs, and reached the maximum at 1000 mg/L. The APX activity decreased at the Al2O3 NPs concentration of 2500 mg/L (Fig. 3D).
By comparing the activities of these four enzymatic antioxidants (CAT, POD, SOD and APX), it is evident that the accumulation of studied ENPs induced a strong antioxidant response in N. arvensis. Furthermore, these results showed differential responses of the antioxidant enzymes to different ENPs in different plant tissues, probably stemming from the different physico-chemical properties of these two ENPs such as their size, shape, surface chemistry.
Antioxidant compounds
Secondary metabolites such as the total phenols, saponins and iridoids were measured in the shoots of N. arvensis. Phenols as secondary metabolites of plants are known to be involved in the antioxidant activity in plants growing under heavy metal stress and are typically increased by metal stress53. Also, phenols are oxidized by peroxidase and play a role in scavenging H2O2 molecules54. In this study, both ENPs enhanced the total phenol contents in N. arvesis shoot compared with the control and there was no significant difference between the treatment groups of between 50–2500 mg/L NiO NPs. As shown in Fig. 4A, the total phenol content was significantly increased by 96% and 79% in the presence of 2500 mg/L of Al2O3 and NiO NPs respectively in comparison to the control plants. The effects were more pronounced in the shoots of Al2O3 NPs treated plants than NiO NPs treated plants (Fig. 4A). Similar effects were also recorded in Eichhornia crassipes55 and Bacopa monnieri56 under Ag NPs, which supports the conviction that ENPs induce the production of total phenolic compounds in plants.
Saponin is a class of amphipathic glycosides, which have one or more hydrophilic glycoside moieties combined with a lipophilic triterpene derivative57. The total saponin content of plants treated with Al2O3 and NiO NPs at various concentrations is shown in Fig. 4B. Al2O3 NPs significantly increased the total saponin production in N. arvensis at all tested concentrations, whereas its content dropped 5 and 1.6 times at 100 and 2500 mg/L NiO NPs, compared with the control. This result agrees with a previous report that low concentrations of Ag NPs increased total saponin content in Calendula officinalis58, but higher Ag NPs (0.8 to 1.6 mM) decreased the total saponin content in the same plant.
Iridoids are a type of monoterpenes, which are typically found in plants as glycosides, often bound to glucose. The iridoids produced by plants act primarily as a defense against biotic and abiotic stresses. A marked increase (39% compared with controls, Fig. 4C) in total iridoids concentration was observed in plant shoots treated with 2500 mg/L Al2O3 NPs. However, shoot iridoids content steadily increased in the presence of up to 1000 mg/L NiO NPs before decreased in plants treated with 2500 mg/L NiO NPs.
Total antioxidant capacity, DPPH scavenging and reducing power activities
The total antioxidant capacity of N. arvensis exposed to both ENPs is shown in Fig. 5A. The total antioxidant activity increased significantly at 100 and 1000 mg/L Al2O3 NPs. Plant exposure to 0–1000 mg/L of NiO NPs resulted in a decrease of the total antioxidant capacity compared with plants grown in ENPs-free medium (Fig. 5A). By contrast, the total antioxidant capacity was significantly increased at 2500 mg/L NiO NPs (88.5% increase over control).
In this study, DPPH free radical scavenging activity in shoot of N. arvensis exposed to NiO NPs increased with increasing NiO NPs concentrations after three weeks of treatment (Fig. 5B). When N. arvensis was exposed to 200 µL of 2500 mg/L of NiO NPs, the DPPH free radical scavenging activity reached the maximum of 35.79% of inhibition (Fig. 5B). This result implies that N. arvensis has a high capability to cope with the oxidative stress induced by NiO NPs. In plants exposed to Al2O3 NPs, DPPH scavenging activity increased in different levels with increasing Al2O3 NPs concentrations from 50 to 1000 mg/L (Fig. 5B). The highest DPPH free radical scavenging activity in plant shoot was 4.93 fold of the control at 1000 mg/L Al2O3 NPs. The DPPH free radical scavenging activity were decreased by 17% in extracts containing 2500 mg/L of Al2O3 NPs compared with 1000 mg/L treated plants. Similar to our results, Khan et al.59 evaluated the effects of nine types of metal ENPs including monometallic and bimetallic alloy ENPs such as AgCu, AuCu and AgAu on DPPH free radical scavenging activity of Silybum marianum58. They showed an increase in DPPH percentage after 28 days of exposure for all ENPs suspensions. Javed et al.60 demonstrated that DPPH free radical scavenging activity in Stevia rebaudiana Bertoni shoots was 74.8% and 68.6% higher under 10 mg/L and 1 mg/L ZnO NPs treatments60. Their results also showed that the lowest antioxidant activities were obtained from extracts containing 1000 mg/L ZnO NPs in MS medium, which can be related to the toxic effects of this treatment by generating oxidative stress and imbalance of anti-oxidative activities60.
The reduction of Fe (III) is often used as an indicator of electron-donating activity, an important process in phenolic antioxidant reaction61. The presence of reductant (antioxidant) in the plant extracts causes the reduction of the Fe3+/ferricyanide complex to the ferrous form. Therefore, the concentration of Fe2+ was monitored by measuring the formation of Perl’s Prussian blue at 700 nm62. In this study, the total reducing power of plants exposed to both Al2O3 and NiO NPs was affected similarly as the DPPH free radical scavenging activity (Fig. 5C). Although these effects were mostly not statistically significant (p > 0.05), there were significant (p < 0.05) increases in the total reducing power of shoot extract treated with 1000 mg/L of Al2O3 NPs (by 43%) and those treated with 2500 mg/L of NiO NPs (by 32%) when compared to the control plants. Therefore, with Al2O3 NPs treatments, the lowest antioxidant activities were obtained from extracts containing 2500 mg/L NPs treatments, which also had lower DPPH free radical scavenging activity and total reducing power activity than the control group.
Conclusion
In summary, this study demonstrated the concentration dependent effects of NiO and Al2O3 NPs on the growth and antioxidant activities of N. arvensis. NiO NPs exhibited greater effects than Al2O3 NPs on N. arvensis growth. Significantly enhanced activities of antioxidant enzymes (CAT, POD, APX and SOD) and antioxidant compounds (total iridoids, total saponin, and total phenolic) along with DPPH scavenging activity, total antioxidant capacity and total reducing power were observed in plants treated with 50 to 1000 mg/L of NiO and Al2O3 NPs in hydroponic systems. However, adverse effects of NiO and Al2O3 NPs on these phytochemical assays appeared when Hoagland medium was supplemented with 1000 or 2500 mg/L of NiO and Al2O3 NPs. The concentration and composition-dependent responses of plants observed in this study provide new insights into the effects of ENPs in the mineral nutrition, antioxidant activities and alteration of metabolic pathways of medicinal plants.
Materials and Methods
Nanomaterials, chemicals and seeds
Al2O3 and NiO NPs were purchased from Iranian Nanomaterial Company. According to the supplier, the diameter of NiO NPs fell in the range 5–8 nm. Most NiO NPs were spherical and has the purity of 99.5%. The specific surface area was in the range of 50–100 m2/g. The average diameter of Al2O3 NPs was about 5 nm. The purity of Al2O3 NPs was 99.99% and its specific surface area was 150 m2/g. Seeds of N. arvensis were purchased from Pakanbazr Company (Isfahan, Iran).
Preparation of particles and cultures
Seeds of N. arvensis were sterilized for 10 min in 10% sodium hypochlorite solution before germination. They were germinated on sand soaked with 0.1 strength modified Hoagland solution (0.5 mM MgSO4, 2.5 mM Ca (NO3)2, 0.5 mM KH2PO4, 2.5 mM KCl, 25 μM H3BO3, 5 μM MnSO4, 0.4 μM ZnSO4, 0.2 μM CuSO4, 0.25 μM Na2MoO4, 50 μM Fe-EDTA, pH 5.5). After 10 days, the solution was replaced with Hoagland solutions containing different concentrations of Al2O3 or NiO NPs (0, 50, 100, 1000 and 2500 mg/L). Prior to the replacement, the ENP suspensions (100 mL) were first sonicated in an ultrasonic water-bath for 90 min. All experiments were carried out in a greenhouse under semi-controlled conditions: day/night photoperiod (16/8 h), a light intensity of 100 μM/m²/s, day/night temperature (24/20 ± 1 °C) and day/night relative humidity (70/75%). Hydroponic system was adopted for this study to avoid the compounding effect of soil particles and microorganisms in soil on the physiological effect of chosen nanoparticles. In addition, hydroponic system is gain popularity in urban agriculture for vegetable growth due to dwindling global arable lands. Therefore, the results hold great importance for sustainable applications of nanotechnology in agriculture. A wide range of concentrations are used for both ENPs so that the physiological responses of plants in the presence of mild to severe contamination can be investigated. All treatments had three replicates and the experiment lasted for three weeks. Afterwards, the plants were harvested, and rinsed with tap water. The shoot and root biomass were measured after they were oven dried at 65 °C for three days.
Hydrogen peroxide (H2O2) content
Hydrogen peroxide level was measured following the method described by Sergiev et al.63. Fresh roots or shoots (0.5 g) were homogenized in ice bath with 5 mL of 0.1% (W/V) trichloroacetic acid (TCA). The mixtures were centrifuged at 12,000 g for 15 min. 0.5 mL of the obtained supernatant was added to the mixture containing 0.5 mL of potassium phosphate buffer (10 mM, pH 7.0) and 1 mL of KI solution (1 M). The content of H2O2 was determined with a spectrophotometer (Bausch & Lomb 70) at 390 nm.
Enzyme extractions and assays
To prepare extracts for the analysis of enzyme activities, fresh plant tissues were ground to fine powders in liquid nitrogen and extracted at a ratio 1:3 (w/v) fresh weight to extraction buffer containing 1 mM EDTA, 3 mM DTT and 5% PVP. The homogenate thus obtained was centrifuged for 20 min at 14,000 rpm and the supernatant was either used for enzyme assays or protein extraction, which is described below in details.
Catalase (CAT) activity
Catalase activity was determined according to Aebi’s (1984) method64. 0.1 mL of enzyme extract was mixed with 2.9 mL of 50 mM phosphate buffer (pH 7.0) containing 30 mM H2O2 to make a total volume of 3 mL. CAT activity was estimated based on the decreased absorbance of H2O2 at 240 nm (Shimadzu-UV mini- 1240). The CAT activity was determined based on the molar extinction coefficient of H2O2 (39.4 M−1cm−1) and is expressed as µM H2O2 per mg fresh weight per min.
Ascorbate peroxidase (APX) activity
Ascorbate peroxidase was assayed based on the method reported by Nakano and Asada (1981)65. The reaction complex contained potassium phosphate (50 mM, pH 7.0), EDTA (0.2 mM), ascorbic acid (0.5 mM), 2% H2O2, and 100 µL of enzyme extract with a total volume of 3 mL. A reduction of absorbance (at 290 nm for 1 min) was recorded. The activity of enzyme was estimated using the extinction coefficient of 2.8 mM−1 cm−1. One unit APX activity was defined as 1 mM ascorbate oxidized per mL per min at 25 °C.
Peroxidase (POD) activity
Peroxidase activity was measured using the guaicol oxidation method of Chance and Machly (1955)66. The reaction mixture (3 mL in final volume) contains potassium phosphate buffer (10 mM, pH 7.0), guaicol (8 mM) and 100 µL enzyme extract. The reaction was initiated by adding 10 µL of 40 mM H2O2. The absorbance was determined spectrophotometerically (Bausch & Lomb 70) within 1 min at 470 nm. POD activity was calculated using the extinction coefficient of 26.6 mM−1 cm−1.
Superoxide Dismutase (SOD) activity
Superoxide dismutase activity was determined following the Giannopolitis and Ries (1977) protocol by spectrophotometer method67. In this method, reaction solution contained 13 mM methionine, 75 μM nitroblue tetrazolium (NBT), 2 μM riboflavin, 50 mM phosphate buffer (pH = 7.8) and 0–50 μL of extracted enzyme. Reaction was started by placing the tubes against two fluorescent lamps (15 W). The reaction was terminated after 10 min by turning off the lamps. Absorption was measured at 560 nm with a spectrophotometer (Bausch & Lomb 70). The illuminated and non-illuminated reactions without supernatant were placed as calibration standards. Ultimately, one unit of enzyme activity was equivalent to the amount of the enzyme needed for 50% reduction of the NBT photochemical reaction.
Preparation of extract and antioxidant assays
N. arvensis leaf extracts were prepared by drying and then grounding leaves from plants exposed to various concentrations of ENPs. One gram of leaf powder from each treatment was ground and extracted by 3 ml of acidic methanol reagent (99:1 methanol: HCl) in 25 °C for 24 h and the extract was centrifuged at 10,000 rpm for 15 min. The supernatant was collected and then stored in an airtight container in the refrigerator (4 °C) for the measurement of all antioxidant compounds. The spectrophotometric assays were performed in triplicates.
Determination of total phenolic content
The total phenols were determined based on the Folin–Ciocalteu method68. An aliquot (0.5 mL) of plant extract (1 mg/mL) was mixed with 2.5 mL of Folin–Ciocalteu reagent (diluted 1:10) and 2 mL of NaHCO3 (7.5%). Then, the test solution was maintained at 45 °C for 15 min. The absorbance was recorded at 765 nm with a spectrophotometer (Bausch & Lomb 70) against a blank sample. Total phenols were determined as Gallic acid (standard) equivalents (mg GA/g extract).
Total saponins assay
Total saponin contents in leaves were estimated using the colorimetric method reported by Hiai et al.69 with minor modifications69. 50 µL of plant extract was added to different test tubes containing 0.25 mL of vanillin reagent (8% w/v in ethanol 99.9%). Those test tubes were placed in ice-cold water bath and 2.5 mL of 72% (v/v) sulfuric acid was slowly added to each tube. After 3 min, the tubes were heated to 60 °C for 10 min using a water bath and then cooled to room temperature. Absorbance was measured at 544 nm using a spectrophotometer against the reagent blank. Diosgenin was used as a standard and the content of total saponins was expressed as Diosgenin equivalents (mg DE/g extract).
Estimation of total iridoid content
The total iridoid content was determined according to the colorimetric method described by Haag-Berrurier et al.70 with slight modifications70. A 100 µL aliquot of plant extract solution was incubated with 900 µL of reagent solution containing 82 mL methanol, 100 mg vanillin and 8 mL concentrated sulfuric acid. Absorbance was read spectrophotometrically at 538 nm. Catalpol was used as a standard compound for the establishment of the calibration curve. Total iridoids was expressed as Catalpol equivalents (mg CE/g extract).
Total antioxidant capacity
The capacity of total antioxidant of the methanolic extracts of samples was determined using a previously reported method by Prieto et al.71 with slight modifications71. An aliquot of 0.3 mL of sample extracts solution was mixed with 2.7 mL of reagent solution containing sulfuric acid (0.6 M), sodium phosphate (28 mM) and ammonium molybdate (4 mM). The tubes containing reaction solution were incubated at 95 °C for 90 min and then cooled to room temperature. All samples were run in triplicates and their absorbance was read at 695 nm by a spectrophotometer. The standard reference was ascorbic acid and the total antioxidant capacity was expressed as mg of ascorbic acid equivalent per gram of the dry extract.
DPPH radical scavenging activity analyze
The free radical scavenging activity of plants was calculated using the method described by Sarker72. Briefly, serial dilutions were carried out with the stock solution (1 mg/mL) of the extracts. Diluted solutions (1 mL of each samples) were reacted with 1 mL of a freshly prepared DPPH (2,2-diphenyl-1-picryl hydrazyl) methanol solution (80 µg/mL) for 30 min in the dark at room temperature. Absorbance values of these solutions were determined with a spectrophotometer at 517 nm. Methanol was used as a blank. Control sample was prepared containing the same amount of methanol and DPPH without test compounds. Inhibitions of DPPH radical in percent (I%) were estimated as follow:
where AControl is defined as the absorbance of the control reaction (comprising all reagents without the test compound) and Asample is the absorbance of the test compounds.
Reducing power activity
Total reducing power activity of samples was investigated according to the method described by Aman et al.73 with slight modifications73. An aliquot of 2.5 mL of stock solution of each sample was mixed with phosphate buffer (2.5 mL, 0.2 M, PH 6.6) and potassium ferricyanide (2.5 mL, 1%). The tubes containing the reaction solutions were then incubated at 50 °C for 20 min. Afterwards, 2.5 mL of 10% trichloroacetic acid was added to each tube and then 2.5 mL of the reaction mixture was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 0.1%). The absorbance of samples was measured at 700 nm with a spectrophotometer. Ascorbic acid was used as a positive control and the results were expressed as mg ascorbic acid equivalent per gram.
Statistical analysis
The data represent mean of three replicates ± standard error (S.E). One-way ANOVA was employed to confirm the variability of data and validity of results with different rates of NPs addition. Duncan’s multiple range test (DMRT) was employed to determine the significant differences between treatments to a significance level of P < 0.05 or very significant as P ≤ 0.001. Statistical analyses were performed using SPSS (24) software.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Scrinis, G. & Lyons, K. The emerging nano-corporate paradigm: nanotechnology and the transformation of nature, food and agri-food systems. Int. J. Sociol. Agr. Food 15, 22–44 (2007).
Nel, A., Xia, T., Mädler, L. & Li, N. Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006).
Thwala, M., Klaine, S. J. & Musee, N. Interactions of metal‐based engineered nanoparticles with aquatic higher plants: A review of the state of current knowledge. Environ. Toxicol. Chem (2016).
Hashimoto, Y., Takeuchi, S., Mitsunobu, S. & Ok, Y.-S. Chemical speciation of silver (Ag) in soils under aerobic and anaerobic conditions: Ag nanoparticles vs. ionic Ag. J. Hazard. Mater. 322, 318–324 (2017).
Yanık, F. & Vardar, F. Toxic effects of aluminum oxide (Al2O3) nanoparticles on root growth and development in Triticum aestivum. Water. Air. Soil. Pollut 226, 296 (2015).
Pittol, M., Tomacheski, D., Simões, D. N., Ribeiro, V. F. & Santana, R. M. C. Macroscopic effects of silver nanoparticles and titanium dioxide on edible plant growth. Environ. Nanotechnol. Monit. Manage 8, 127–133 (2017).
Sharon, M., Choudhary, A. K. & Kumar, R. Nanotechnology in agricultural diseases and food safety. J. Phytol 2 (2010).
Khot, L. R., Sankaran, S., Maja, J. M., Ehsani, R. & Schuster, E. W. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35, 64–70 (2012).
Sekhon, B. S. Nanotechnology in agri-food production: an overview. Nanotechnol. Sci. Appl 7, 31–53 (2014).
Manjunatha, S., Biradar, D. & Aladakatti, Y. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci 29, 1–13 (2016).
Hatami, M. & Ghorbanpour, M. Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turk. J. Biol 38, 130–139 (2014).
Melegari, S. P., Perreault, F., Costa, R. H. R., Popovic, R. & Matias, W. G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol 142, 431–440 (2013).
Rico, C., Peralta-Videa, J. & Gardea-Torresdey, J. In Nanotechnology and Plant Sciences 1–17 (Springer, 2015).
Ma, C., White, J. C., Dhankher, O. P. & Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol 49, 7109–7122 (2015).
Du, W. et al. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem (2016).
Rai, P. K. et al. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int 119, 1–19 (2018).
Ma, Y. et al. Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicol 9, 262–270 (2015).
Ma, X., Wang, Q., Rossi, L. & Zhang, W. Cerium Oxide Nanoparticles and Bulk Cerium Oxide Leading to Different Physiological and Biochemical Responses in Brassica rapa. Environ. Sci. Technol (2015).
Zhao, L. et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS nano 6, 9615–9622 (2012).
Rico, C. M. et al. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ. Sci. Technol 47, 14110–14118 (2013).
Rico, C. M. et al. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ. Sci. Technol 47, 5635–5642 (2013).
Hu, C., Liu, X., Li, X. & Zhao, Y. Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ. Sci. Pollut. Res 21, 732–739 (2014).
Kim, S., Lee, S. & Lee, I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water, Air, Soil Pollut 223, 2799–2806 (2012).
Handy, R. D., Owen, R. & Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicol 17, 315–325 (2008).
Jakubiak, M., Giska, I., Asztemborska, M. & Bystrzejewska-Piotrowska, G. Bioaccumulation and biosorption of inorganic nanoparticles: factors affecting the efficiency of nanoparticle mycoextraction by liquid-grown mycelia of Pleurotus eryngii and Trametes versicolor. Mycol. Prog 13, 525–532 (2014).
Stanley, J. K., Coleman, J. G., Weiss, C. A. & Steevens, J. A. Sediment toxicity and bioaccumulation of nano and micron‐sized aluminum oxide. Environ. Toxicol. Chem 29, 422–429 (2010).
Hanemann, T. & Szabó, D. V. Polymer-nanoparticle composites: from synthesis to modern applications. Materials 3, 3468–3517 (2010).
Schrand, A. M. et al. Metal‐based nanoparticles and their toxicity assessment. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol 2, 544–568 (2010).
Sadiq, I. M., Pakrashi, S., Chandrasekaran, N. & Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res 13, 3287–3299 (2011).
Burklew, C. E., Ashlock, J., Winfrey, W. B. & Zhang, B. Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PloS one 7, e34783 (2012).
Riahi-Madvar, A., Rezaee, F. & Jalali, V. Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran. J. Plant Physiol 3, 595–603 (2012).
Lee, C. W. et al. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem 29, 669–675 (2010).
Lin, D. & Xing, B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150, 243–250 (2007).
Salimi, A., Sharifi, E., Noorbakhsh, A. & Soltanian, S. Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys. Chem 125, 540–548 (2007).
Venkateswara Rao, K. & Sunandana, C. Effect of fuel to oxidizer ratio on the structure, micro structure and EPR of combustion synthesized NiO nanoparticles. J. Nanosci. Nanotechnol 8, 4247–4253 (2008).
Magaye, R. & Zhao, J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environ. Toxicol. Pharmacol 34, 644–650 (2012).
Faisal, M. et al. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J. Hazard. Mater 250, 318–332 (2013).
Oukarroum, A., Barhoumi, L., Samadani, M. & Dewez, D. Toxic effects of nickel oxide bulk and nanoparticles on the aquatic plant Lemna gibba L. Biomed. Res. Int 2015 (2015).
Marbut, M. M., Al-Kadhi, N. A. S. & Al-Mzaein, K. A. Extraction of Flavonoid compounds from Nigella Arvensis Linn seeds & to study their physiological effects on female reproductive system. Tikrit Med. J 13, 64–69 (2007).
Facciola, S. Cornucopia: a source book of edible plants. (1990).
Asztemborska, M., Steborowski, R., Kowalska, J. & Bystrzejewska-Piotrowska, G. Accumulation of aluminium by plants exposed to nano-and microsized particles of Al2O3. Int. J. Environ. Res 9, 109–116 (2015).
Anane, R. & Creppy, E. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase+ catalase and vitamins E and C. Hum. Exp. Toxicol 20, 477–481 (2001).
Rao, K. S. J. & Stein, R. First evidence on induced topological changes in supercoiled DNA by an aluminium D-aspartate complex. JBIC J. Biol. Inorg. Chem 8, 823–830 (2003).
Pakrashi, S. et al. Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat. Toxicol 132, 34–45 (2013).
Castiglione, M. R., Giorgetti, L., Cremonini, R., Bottega, S. & Spanò, C. Impact of TiO2 nanoparticles on Vicia narbonensis L.: potential toxicity effects. Protoplasma. 251, 1471–1479 (2014).
Zeng, D. & Zhao, H. Activity test and mechanism study of an environmentally friendly wheat seed coating agent. Agric Sci 4, 334 (2013).
Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7, 405–410 (2002).
Song, G. et al. Effects of CuO nanoparticles on Lemna minor. Bot Stud 57, 1–8, https://doi.org/10.1186/s40529-016-0118-x (2016).
Laware, S. & Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci 3, 749–760 (2014).
Nair, P. M. G. & Chung, I. M. A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biol. Trace Elem. Res 162, 342–352 (2014).
Barbasz, A., Kreczmer, B. & Oćwieja, M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3). Acta. Physiol. Plant 38, 1–11 (2016).
Rajeshwari, A. et al. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip—effects of oxidative stress generation and biouptake. Environ Sci. Pollut Res 22, 11057–11066 (2015).
Dudjak, J., Lachman, J., Miholová, D., Kolihová, D. & Pivec, V. Effect of cadmium on polyphenol content in young barley plants (Hordeum vulgare L.). Plant Soil Environ 50, 471–477 (2004).
Singh, Y. & Malik, C. Phenols and their antioxidant activity in Brassica juncea seedlings growing under HgCl2. stress. J. Microbiol. Biotech. Res 1, 124–130 (2011).
Rani, P. U., Yasur, J., Loke, K. S. & Dutta, D. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta. Physiol. Plant 38, 1–9 (2016).
Krishnaraj, C. et al. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47, 651–658 (2012).
Zu, Y. et al. Effects of arsenic treatments on saponin content and heterogeneity extracted from rhizome and main root of Panax notoginseng plants grown in shaded field. J. Geosci. Environ Prot 4, 15 (2016).
Ghanati, F. & Bakhtiarian, S. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop. J. Pharmaceut. Res 13, 1783–1789 (2014).
Khan, M. S., Zaka, M., Abbasi, B. H. & Shah, A. Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles. IET Nanobiotechnol (2016).
Javed, R., Usman, M., Yücesan, B., Zia, M. & Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem (2016).
Hinneburg, I., Dorman, H. D. & Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem 97, 122–129 (2006).
Amarowicz, R., Pegg, R., Rahimi-Moghaddam, P., Barl, B. & Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 84, 551–562 (2004).
Sergiev, I., Alexieva, V. & Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 51, 121–124 (1997).
Aebi, H. Catalase in vitro. Meth.Enzymol 105, 121–126 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880 (1981).
Chance, B. & Maehly, A. Assay of catalases and peroxidases. Met. Enzymol. 2, 764–775 (1955).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases I. Occurrence in higher plants. Plant physiol. 59, 309–314 (1977).
Singleton, V. L., Orthofer, R. & Lamuela-Raventos, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method. Enzymol 299, 152–178 (1999).
Hiai, S., Oura, H. & Nakajima, T. Color reaction of some sapogenin and saponins with vanilin and sulfuric acid. Planta Med. 29, 116–122 (1976).
Haag-Berrurier, M., Kuballa, B. & Anton, R. Dosage des glucoiridoïdes totaux dans la racine d’Harpagophytum procumbens. DC. Plant. Med. Phytotherap. 12, 197–206 (1978).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal.Biochem. 269, 337–341 (1999).
Sarker, S.D., Latif, Z. & Gray, A. I. (Eds.). Natural Products Isolation, 20 (Humana Press Inc., NJ, USA, 2006).
Aman, S. et al. Antioxidant activity of thymol: protective role in AAPH-induced hemolysis in diabetic erythrocytes. IJPSI. 2, 55–60 (2013).
Acknowledgements
We would like to thanks the graduate school of Razi University for providing research facilities for this study.
Author information
Authors and Affiliations
Contributions
A.C., designed the work and performed the experiments. F.Q., analyzed the data statically. N.K., guided the project design. The manuscript was written by A.C., and X.M., edited the text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chahardoli, A., Karimi, N., Ma, X. et al. Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L.. Sci Rep 10, 3847 (2020). https://doi.org/10.1038/s41598-020-60841-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60841-6
This article is cited by
-
Optimizing organically nano-fabricated Ni metal complexes for enhanced antioxidant and anticancer activity using response surface methodology
Future Journal of Pharmaceutical Sciences (2024)
-
Nano-elicitation and hydroponics: a synergism to enhance plant productivity and secondary metabolism
Planta (2024)
-
Ozonated water soaking improves the flower growth, antioxidant activity, and bioactive compound accumulation in Agastache rugosa
Chemical and Biological Technologies in Agriculture (2023)
-
Induction of bioactive constituents and antioxidant enzyme activities in Achillea fragrantissima (Forskal) callus cultures using ZnO nanoparticles
In Vitro Cellular & Developmental Biology - Plant (2023)
-
Nanomaterials in agriculture for plant health and food safety: a comprehensive review on the current state of agro-nanoscience
3 Biotech (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.