Abstract
A novel amorphous FeOOH modified BiVO4 photocatalyst (A-FeOOH/BiVO4) was successfully produced and characterized by various techniques. The results showed that amorphous FeOOH with about 2 nm thickness evenly covered on BiVO4 surface, which caused resultant A-FeOOH/BiVO4 exhibiting higher visible light photocatalytic performance for producing O2 from water than BiVO4. When the covered amount of amorphous FeOOH was 8%, the resultant photocatalyst possessed the best photocatalytic performance. To find the reasons for the improvement of photocatalytic property, electrochemical experiments, DRS, PL and BET, were also measured, the experimental results indicated that interface effect between amorphous FeOOH and BiVO4 could conduce to migration of photogenerated charge, and exhibit stronger light responded capacity. These positive factors promoted A-FeOOH/BiVO4 presenting improved the photocatalytic performance. In a word, the combination of amorphous FeOOH with BiVO4 is an effective strategy to conquer important challenges in photocatalysis field.
Similar content being viewed by others
Introduction
Semiconductor photocatalysis has achieved keen attention in utilizing solar power to solve environments deterioration and energy crisis1,2,3. Hence, numerous photocatalyst materials were developed, including TiO24,5,6, ZnO7, WO38,9,10, CdS11,12, SnS213, Ag based photocatalysts14,15,16, etc. However, the development of photocatalysts with high efficient is still a huge and continuous undertaking.
The Bi-based photocatalyst materials, such as Bi2O317, BiOCl18, BiOBr19, BiOI20, BiVO421,22,23, Bi2WO624, and Bi2MoO625, have obtained great attention. Among these as-prepared photocatalysts, BiVO4 with strong visible light response capacity and good stability has been extensively investigated in environmental remediation and water splitting26,27,28,29,30,31. Nevertheless, a big problem that affects the photocatalytic property of BiVO4 is its unsatisfied charge carrier separated efficiency. To conquer this problem, many researchers had developed some methods for modifying BiVO4. Cao et al. successfully prepared Au-BiVO4 photocatalyst, which could present much higher visible-light photocatalytic performance for wastewater treatment and clean energy product than the individual BiVO432. Except Au modification, Pd, AuPd and CoPd were used to modify BiVO4 to enhance its photocatalytic property33,34,35. However, these noble metals or noble metal alloys were high cost so that this method was difficult to be wide application. Hence, it was necessary to develop an economical and convenient method to modify BiVO4.
Recently, amorphous semiconductor materials have been exploited and exhibited specific photocatalytic property36,37. Compared to crystals, amorphous semiconductor materials exist a most remarkable advantage that it possesses much smaller band gap than their crystalline counterparts, which conduces them to present more expansive light absorption range, which is an important condition for conversing solar energy37. However, amorphous semiconductor materials exhibited the short-range atomic order, and existed a number of defects, which could become the charge recombination centers, causing themselves inactive or weak performance. So could numerous defects existed on amorphous semiconductor materials as the electrons capturer be applied for modifying other photocatalysts? This view is very significative and interesting. Hence, considering the wide light responded property and cost, Fe based semiconductors enter into our view. Among Fe based semiconductors, FeOOH exhibits extensive visible light response capacity, which caused FeOOH coupled with other photocatalysts to modify the visible-light-irradiation photocatalytic property, some FeOOH with certain crystalline phase was used to modify photocatalyst materials, such as β-FeOOH/TiO238, β-FeOOH/g-C3N439, α-FeOOH/AgVO340, etc. Actually, FeOOH contained various crystalline phases, including α-phase, β-phase, δ-phase, γ-phase, and amorphous phase. Among these different crystalline phase FeOOH, amorphous FeOOH could exhibit excellent oxygen evolution rate by photoelectrochemical and superior pseudocapacitive performance41,42. However, it is relative lack of report about amorphous FeOOH as a modifier to be used in photocatalysis. Hence, combined with above proposed view, it is essential to investigate the role of amorphous FeOOH modifier.
Herein, a novel amorphous FeOOH modified BiVO4 was successfully prepared, and photocatalytic performance for producing O2 from water was investigated. It could be found that, after amorphous FeOOH evenly covered the surface of BiVO4, as-prepared photocatalysts exhibited better migration of photogenerated charges, and stronger visible light responded activity. These positive factors promoted A-FeOOH/BiVO4 presenting improved the photocatalytic performance. Hence, this work shows an effective and simple modified method for designing and preparing highly efficient photocatalysis materials.
Experimental
The synthesis of catalysts
To obtain BiVO4 material, in a beaker, Bi(NO3)3·5H2O (5 mmol) dissolved in HNO3 solution (5 mL 3 mol·L−1) and ethylene glycol (20 mL) mixed solution. Then in other beaker, NH4VO3 (5 mmol) and 0.25 g sodium dodecylbenzenesulfonate (SDBS) were dissolved in hot water (20 mL). After stirred for 30 min, above two solution mixed, and the pH of solution was adjusted to 5 using NaOH solution. Stirring for 60 min, obtained suspension solution was placed into high pressure reactor with PTFE liner, maintained at 180 °C for 24 h. After filtration, wash and desiccation, BiVO4 was prepared.
Amorphous FeOOH/BiVO4 was prepared as follows: BiVO4 (400 mg) was mixed into 40 mL deionized water containing FeCl3·6H2O and NH4HCO3 (The molar ratio of FeCl3·6H2O and NH4HCO3 is 1:3). Stirring for 60 min, the solid powder was obtained through centrifugation, wash and desiccation. According to the theoretical content of amorphous FeOOH in amorphous FeOOH/BiVO4, the obtained powder catalysts were marked as A-FeOOH/BiVO4(2 wt%), A-FeOOH/BiVO4(5 wt%), A-FeOOH/BiVO4(8 wt%) and A-FeOOH/BiVO4(10 wt%), respectively.
Characterizations and photocatalytic experiment
Supporting Materials showed their details.
Results and Discussion
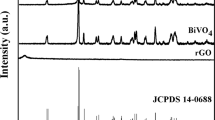
Figure 1 demonstrates the XRD of resultant photocatalysts. As can be observed, resultant BiVO4 exhibited highly crystalline, and there were some mainly diffraction peaks at 2θ of 18.6°, 28.9°, 30.5°, 34.4°, 35.3°, 39.4°, 42.3°, 46.1°, 46.6°, 47.3°, 53.3°, 58.3° and 59.9o, which indexed (101), (013), (112), (200), (020), (211), (105), (123), (204), (024), (301), (303) and (224) diffraction planes of monoclinic BiVO443. For amorphous FeOOH, there was no obvious diffraction peak to be found. Hence, in A-FeOOH/BiVO4(8 wt%), XRD diffraction peaks of BiVO4 could only be detected. The XRD results exposed that amorphous FeOOH had little impact the crystal phase of BiVO4. Moreover, no other diffraction peak was found, meaning that resultant samples possessed the high purity.
Whereafter, the XPS of A-FeOOH/BiVO4(8 wt%) was further investigated. As revealed in Fig. 2A, Bi, V, O and Fe could be found in the survey XPS spectra, according with the composition of material. Bi 4 f XPS spectrum (Fig. 2B) presented 164.3 eV and 159.1 eV two peaks that attributed to Bi 4f5/2 and Bi 4f7/2 in BiVO444,45. V 2p XPS spectrum (Fig. 2C) had two peaks at 516.3 eV and 524.3 eV, matching with V 2p1/2 and V 2p3/2 in BiVO446. Figure 2D (Fe 2p XPS spectrum) shows 724.2 (Fe 2p1/2) and 710.8 eV (Fe 2p3/2) that were consistent with FeOOH47. Hence, the results of XPS further confirmed that sample contained FeOOH and BiVO4, consistent to the XRD.
The morphologies of BiVO4 and A-FeOOH/BiVO4(8 wt%) were seen through SEM. As revealed in Fig. 3, BiVO4 and A-FeOOH/BiVO4(8 wt%) exhibited similar star-shaped particles with the size of about 3.5 μm, which demonstrated that introduced amorphous FeOOH did not influence on the feature of BiVO4. In addition, the elemental compositions of the resultant A-FeOOH/BiVO4(8 wt%) were measured through EDS mapping analysis. The related element mapping images were exhibited in Fig. 3C. Clearly, Fe, V, Bi and O only appeared in observed view, meaning the successful preparation of A-FeOOH/BiVO4.
Subsequently, to further observe the microstructure of A-FeOOH/BiVO4, transmission electron microscopy were also analyzed. As revealed in Fig. 4A, A-FeOOH/BiVO4(8 wt%) exhibited the similar star-shaped particles, which was consistent with the SEM. Clearly, amorphous FeOOH could not be observed at low resolution TEM image. As a result, the HRTEM of A-FeOOH/BiVO4 (Fig. 4B) was provided. The lattice fringe spacing of 0.237 nm attributed to BiVO4 (220) was obviously observed. Moreover, the thickness of ultrathin amorphous FeOOH nanolayers was about 2 nm, and evenly adhered on the surface of BiVO4. Amorphous FeOOH nanolayers did not reveal a lattice spacing, demonstrating traditional amorphous structure.
The photocatalytic property over resultant photocatalysts was measured through producing O2 from slitting water. Before producing O2, to improve oxygen evolution rate, the sacrificial agent need be added. The previous report indicated that BiVO4 could exhibit better photocatalytic performance for producing O2 in the NaIO4 solution than that in the AgNO3 solution48. Meanwhile, to verify this result, we also measured the photocatalytic performance for producing O2 over A-FeOOH/BiVO4 photocatalyst in the same concentration NaIO4 or AgNO3 solution. As shown in Fig. S1, A-FeOOH/BiVO4 photocatalyst in the NaIO4 solution could present high oxygen evolution rate (OER) than A-FeOOH/BiVO4 photocatalyst in the AgNO3 solution. Hence, in this system, we selected NaIO4 solution as the sacrificial agent. Then the results of oxygen evolution over all of photocatalysts were shown in Fig. 5A. For a series of resultant A-FeOOH/BiVO4 photocatalysts, O2 could be persistently produced with reaction time prolonging. Compared with pure BiVO4, resultant A-FeOOH/BiVO4 photocatalysts could clearly display the improvement of photocatalytic capacity for producing O2. When loading amount of amorphous FeOOH was 8%, the sample had the optimal catalytic rate of O2 evolution. Then Fig. 5B gives their OER. Their OER were 162.3, 691.7, 1077.1, 1206.3 and 962.8 μmol h−1 g−1 for pure BiVO4, A-FeOOH/BiVO4(2 wt%), A-FeOOH/BiVO4(5 wt%), A-FeOOH/BiVO4(8 wt%) and A-FeOOH/BiVO4(10 wt%), respectively. The OER of A-FeOOH/BiVO4(8 wt%) was around 7.4 folds more than pure BiVO4. In addition, the O2 production rates of different materials in previous reports have been in Table 149,50,51,52, and compared with this work. Obviously, as-prepared A-FeOOH/BiVO4(8 wt%) in this work could present excellent advance.
Furthermore, as we known, the stability is a very significant index to appraise its ability. Thus, recycling tests for producing O2 over A-FeOOH/BiVO4(8 wt%) were investigated. As demonstrated in Fig. 6A, the A-FeOOH/BiVO4(8 wt%) presented relative stable OER, after 6 times cycled experiments, as-prepared A-FeOOH/BiVO4(8 wt%) still presented 70% photocatalytic activity of fresh sample. Besides, the XRD of used A-FeOOH/BiVO4(8 wt%) was measured in Fig. 6B. Its XRD did not have obviously change in comparison to fresh A-FeOOH/BiVO4(8 wt%). And the morphology of used sample was also observed in Fig. 6C,D. It can be observed that used sample exhibited a certain aggregate in comparison to fresh sample, which might result in the declined photocatalytic property. Besides, the whole morphology did not present great change.
Obviously, it was found that covered amorphous FeOOH could effectively modify BiVO4 to improve catalytic activity. Why? To find the reason for promoting effect, some analyzed instruments were tested to investigated light response capacity, the photoinduced charge separated rate and surface area of as-prepared photocatalysts, which are deemed as the main factors to effect on the photocatalytic performance53,54,55.
Figure 7 presents the UV-Vis absorption spectrum of resulted BiVO4 and A-FeOOH/BiVO4(8 wt%). Pure BiVO4 presented remarkable light absorption between 200 and 800 nm. The absorption edge was 525 nm. Band gap was 2.36 eV. After covered by amorphous FeOOH, the light absorption capacity of as-obtained A-FeOOH/BiVO4(8 wt%) gained the obvious enhancement. Hence, the enhanced photocatalytic property might be anticipated.
Photoluminescence (PL) property could analyze the separation and transfer efficiency of photoinduced charges56. Therefore, the PL of BiVO4 and A-FeOOH/BiVO4 (8%) were measured in Fig. 8. The emission band intensity of A-FeOOH/BiVO4 (8%) was clearly declined in comparison of pure BiVO4. This result implied that covered amorphous FeOOH could effectively reduce the recombined efficiency of photoinduced electrons and holes, conducing to improve photocatalytic property57.
Subsequently, electrochemistry measurements (photocurrent and EIS measurement) were used to assess the separated and transfer efficiency of photoinduced charges. Currently, the stronger photocurrent manifests the more effective separation and transfer rate of photo-charges58,59. As illustrated in Fig. 9A, pure BiVO4 and A-FeOOH/BiVO4(8 wt%) could produce certain intensity photocurrent signals. And the order of photocurrent intensity from strong to weak was A-FeOOH/BiVO4(8 wt%) > pure BiVO4. Evidently, resultant A-FeOOH/BiVO4(8 wt%) exhibited the better photocurrent intensity, revealing that the separated rate of photogenerated charges for A-FeOOH/BiVO4(8 wt%) photocatalyst was significantly improved by covering amorphous FeOOH.
Then EIS techniques were further used to estimate charge separation property. Figure 9B gives the EIS of pure BiVO4 and A-FeOOH/BiVO4(8 wt%). Small frequency semicircle radius exposes a better charge transfer rate. As demonstrated in Fig. 9B, the semicircle radius of A-FeOOH/BiVO4(8 wt%) was shorter than that of pure BiVO4, meaning that A-FeOOH/BiVO4(8 wt%) possessed a higher separation and transport rate of photogenerated charges.
Besides, the BET surface area of pure BiVO4 and A-FeOOH/BiVO4(8 wt%) were 4.9 and 5.2 m2 g−1, respectively. The addition of amorphous FeOOH had little effect on the surface area of photocatalyst. Additionally, the surface hydrophilic property of photocatalyst was performed to measure interact with the water. As presented in Fig. 10A,B, water contact angle (CA) of BiVO4 (69.55°) and A-FeOOH/BiVO4(8 wt%) (48.35°) was measured. This result meant that the covered amorphous FeOOH made BiVO4 possess water favorable wetting capacity, providing a good chance to oxidate H2O in aqueous environment60.
Hence, combined with above analyzed results, it was found that, the main reason that as-prepare A-FeOOH/BiVO4 possessed the better photocatalytic performance than BiVO4 could be explained that the former exhibited higher efficiency for separation of photogenerated charges, and stronger strong visible responded activity compared with the latter. Hence, the remarkable improvement of photocatalytic capacity for producing O2 was obtained.
Whereafter, to speculate the photocatalytic mechanism, the energy structure of BiVO4 might be calculated61
Herein Eg, EVB, ECB, X, Ee are band gap of photocatalyst, the valence band potential, conduction band potential, electro negativity of component atoms, hydrogen scale (4.5 eV), respectively. Here, for BiVO4, X is 6.15 eV62, Eg is 2.36 eV (Fig. 7). Hence, the CB and VB potentials of BiVO4 were respective 0.47 and 2.83 eV.
Finally, in Fig. 11, a probable photocatalytic mechanism for A-FeOOH/BiVO4 was presented. BiVO4 could generate electrons and holes under light irradiation. Due to quantum-tunneling effect (QTE)63,64, formed charges from the conduction band of BiVO4 could transfer through amorphous FeOOH, or cationic vacancy network in amorphous FeOOH phase65. Then NaIO4 as the electrons sacrificial agent consumed electrons. Because the cationic vacancy might be also activated by trapping hole66, then holes remained on the VB of BiVO4 would migrate to amorphous FeOOH surface to produce O2. Hence, in this process, the existence of amorphous FeOOH could boost the separation of photoinduced charges for A-FeOOH/BiVO4 system, obtaining the enhancement of photocatalytic performance.
Conclusions
We successfully produced a novel amorphous FeOOH modified BiVO4, and investigated it photocatalytic performance for producing O2 from water. It could be found that, amorphous FeOOH modified BiVO4 exhibited higher migration rate of photogenerated charges, and strong visible responded capacity than BiVO4, which resulted that amorphous FeOOH modified BiVO4 could present better photocatalytic property than BiVO4, and kept excellent performance and structure stability. Hence, this work provides a simple and inexpensive modified method for design and synthesis of effective photocatalysts.
References
Li, X. et al. Engineering heterogeneous semiconductors for solar water splitting. Journal of Materials Chemistry A 3, 2365–2534. (2015).
Zhu, Z., Kao, C. T., Tang, B. H., Chang, W. C. & Wu, R. J. Efficient hydrogen production by photocatalytic water-splitting using Pt-doped TiO2 hollow spheres under visible light. Ceramics International 42, 6749–6754 (2016).
Kisch, H. Semiconductor photocatalysis for chemoselective radical coupling reactions. Accounts of Chemical Research 50, 1002–1010 (2017).
Wen, J. Q. et al. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chinese Journal of Catalysis 36, 2049–2070 (2015).
Huang, Z. A. et al. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2. Applied Catalysis B: Environmental 164, 420–427 (2015).
Pan, D., Han, Z., Miao, Y., Zhang, D. & Li, G. Thermally stable TiO2 quantum dots embedded in SiO2 foams: Characterization and photocatalytic H2 evolution activity. Applied Catalysis B: Environmental 229, 130–138 (2018).
Pirhashemi, M., Habibi-Yangjeh, A. & Pouran, S. R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. Journal of Industrial and Engineering Chemistry 62, 1–25 (2018).
Cui, L. F. et al. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Applied Surface Science 391, 201–210. (2017).
Kumar, S. G. & Koteswara Rao, K. S. R. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Applied Surface Science 391, 124–148 (2017).
Li, L. et al. Nanotube array-like WO3 photoanode with dual-Layer oxygen-Evolution cocatalysts for photoelectrocatalytic overall water splitting, ACS Applied Energy. Materials 1, 6871–6880 (2018).
Chen, J. Z. et al. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angewandte Chemie International Edition 54, 1210–1214 (2014).
Wang, L. et al. Photosensitization of CdS by acid red-94 modified alginate: Dual ameliorative effect upon photocatalytic hydrogen evolution. Applied Surface Science 492, 598–606 (2019).
Zhang, Y. C., Li, J., Zhang, M. & Dionysiou, D. D. Size-tunable hydrothermal synthesis of SnS2 nanocrystals with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI). Environmental Science & Technology 45, 9324–9331 (2001).
Hu, C., Lan, Y. Q., Qu, J. H., Hu, X. X. & Wang, A. M. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. Journal of Physical Chemistry B 110, 4066–4072 (2006).
Han, L., Wang, P., Zhu, C. Z., Zhai, Y. M. & Dong, S. J. Facile solvothermal synthesis of cube-like Ag@AgCl: a highly efficient visible light photocatalyst. Nanoscale 3, 2931–2935 (2011).
Liu, R., Wang, P., Wang, X. F., Yu, H. G. & Yu, J. G. UV- and visible-light photocatalytic activity of simultaneously deposited and doped Ag/Ag(I)-TiO2 photocatalyst. Journal of Physical Chemistry C 116, 17721–17728 (2012).
Ai, Z. H., Huang, Y., Lee, S. C. & Zhang, L. Z. Monoclinic α-Bi2O3 photocatalyst for efficient removal of gaseous NO and HCHO under visible light irradiation. Journal of Alloys and Compounds 509, 2044–2049 (2011).
Zhang, K. L., Liu, C. M., Huang, F. Q., Zheng, C. & Wang, W. D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Applied Catalysis B: Environmental 68, 125–129 (2006).
Gao, M. C. et al. Surface decoration of BiOBr with BiPO4 nanoparticles to build heterostructure photocatalysts with enhanced visible-light photocatalytic activity. Separation and Purification Technology 170, 183–189 (2016).
Li, Y. Y., Wang, J. S., Yao, H. C., Dang, L. Y. & Li, Z. J. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. Journal of Molecular Catalysis A:Chemical 334, 116–122 (2001).
Lin, F. et al. Low-cost dual cocatalysts BiVO4 for highly efficient visible photocatalytic oxidation. RSC Advances 7, 15053–15059 (2017).
Zhang, L., Chen, D. R. & Jiao, X. L. Monoclinic structured BiVO4 nanosheets: hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties. Journal of Physical Chemistry B 110, 2668–2673 (2006).
Zhou, S. et al. High-performance photoelectrochemical water splitting of BiVO4@Co-MIm prepared by a facile in-situ deposition method. Chemical Engineering Journal 371, 885–892 (2019).
Zhu, S. B., Xu, T. G., Fu, H. B., Zhao, J. C. & Zhu, Y. F. Synergetic effect of Bi2WO6 photocatalyst with C60 and enhanced photoactivity under visible irradiation. Environmental Science &Technology 41, 6234–6239 (2007).
Zhang, L. W., Xu, T. G., Zhao, X. & Zhu, Y. F. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Applied Catalysis B: Environmental 98, 138–146 (2010).
Zhou, L. et al. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. Journal of Molecular Catalysis A: Chemical 252, 120–124 (2006).
Ke, D. N., Peng, T. Y., Ma, L., Cai, P. & Jiang, P. Photocatalytic water splitting for O2 production under visible-light irradiation on BiVO4 nanoparticles in different sacrificial reagent solutions, Applied. Catalysis A: General 350, 111–117 (2008).
Sun, S. M., Wang, W. Z., Li, D. Z., Zhang, L. & Jiang, D. Solar light driven pure water splitting on quantum sized BiVO4 without any cocatalyst, ACS. Catalysis 4, 3498–3503 (2014).
Wang, Q. et al. Synthesis of MFe2O4 (M=Ni, Co)/BiVO4 film for photolectrochemical hydrogen production activity. Applied Catalysis B: Environmental 214, 158–167 (2017).
Wang, X., Liao, D., Yu, H. & Yu, J. Highly efficient BiVO4 single-crystal photocatalyst with selective Ag2O-Ag modification: orientation transport, rapid interfacial transfer and catalytic reaction. Dalton Transactions 47, 6370–6377 (2018).
Wang, Q. et al. FeF2/BiVO4 heterojuction photoelectrodes and evaluation of its photoelectrochemical performance for water splitting. Chemical Engineering Journal 337, 506–514 (2018).
Cao, S. W. et al. Preparation of Au-BiVO4 heterogeneous nanostructures as highly efficient visible-light photocatalysts. ACS Applied Materials &Interfaces 4, 418–423 (2012).
Ge, L. Synthesis and characterization of novel visible-light-driven Pd/BiVO4 composite photocatalysts. Materials Letters 62, 926–928 (2008).
Zhang, J. et al. Novel AuPd bimetallic alloy decorated 2D BiVO4 nanosheets with enhanced photocatalytic performance under visible light irradiation. Applied Catalysis B: Environmental 204, 385–393 (2017).
Zhang, K. et al. Co–Pd/BiVO4: High-performance photocatalysts for the degradation of phenol under visible light irradiation. Applied Catalysis B: Environmental 224, 350–359 (2018).
Lin, Z., Du, C., Yan, B., Wang, C. & Yang, G. Two-dimensional amorphous NiO as a plasmonic photocatalyst for solar H2 evolution. Nature Communications 9, 4036 (2018).
Kang, Y. et al. An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Advanced Materials 27, 4572–4577 (2015).
Chowdhury, M., Ntiribinyange, M., Nyamayaro, K. & Fester, V. Photocatalytic activities of ultra-small β-FeOOH and TiO2 heterojunction structure under simulated solar irradiation. Materials Research Bulletin 68, 133–141 (2015).
Zheng, Y., Zhang, Z. S. & Li, C. H. Beta-FeOOH-supported graphitic carbon nitride as an efficient visible light photocatalyst. Journal of Molecular Catalysis A: Chemical. 68, 133–141 (2015).
Sun, M., Senthil, R. A., Pan, J., Osman, S. & Khan, A. A facile synthesis of visible-light driven rod-on-rod like α-FeOOH/α-AgVO3 nanocomposite as greatly enhanced photocatalyst for degradation of rhodamine B. Catalysts 8, 392 (2018).
Chemelewski, W. D., Lee, H. C., Lin, J. F., Bard, A. J. & Mullins, C. B. Amorphous FeOOH oxygen evolution reaction catalyst for photoelectrochemical water splitting. Journal of the American Chemical Society 136, 2843–2850 (2014).
Liu, J., Zheng, M., Shi, X., Zeng, H. & Xia, H. Amorphous FeOOH quantum dots assembled mesoporous film anchored on grapheme nanosheets with superior electrochemical performance for supercapacitors. Advanced Functional Materials 26, 919–930 (2016).
Xu, H. et al. Synthesis, characterization and photocatalytic activities of rare earth-loaded BiVO4 catalysts. Applied Surface Science 256, 597–602 (2009).
Zhang, B. B., Wang, L., Zhang, Y. J., Ding, Y. & Bi, Y. P. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angewandte Chemie International Edition 57, 2248–2252 (2018).
Xu, D. et al. MOF-derived Co3O4 thin film decorated BiVO4 for enhancement of photoelectrochemical water splitting. Applied Surface Science 491, 497–504 (2019).
Chen, R. et al. BiVO4/α-Fe2O3 catalytic degradation of gaseous benzene: preparation, characterization and photocatalytic properties. Applied Surface Science 427, 141–147 (2018).
Huang, J., Ding, Y., Luo, X. & Feng, Y. Solvation effect promoted formation of p–n junction between WO3 and FeOOH: A high performance photoanode for water oxidation. Journal of Catalysis 333, 200–206 (2016).
Wang, J. & Osterloh, F. E. Limiting factors for photochemical charge separation in BiVO4/Co3O4, a highly active photocatalyst for water oxidation in sunlight. Journal of Materials Chemistry A 2, 9405–9411 (2014).
Xin, G., Guo, W. & Ma, T. Effect of annealing temperature on the photocatalytic activity of WO3 for O2 evolution. Applied Surface Science 256, 165–169 (2009).
Wang, Z. et al. BiVO4 nano–leaves: Mild synthesis and improved photocatalytic activity for O2 production under visible light irradiation. CrystEngComm 13, 2500–2504 (2011).
Kudo, A., Ueda, K., Kato, H. & Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catalysis Letters 53, 229–230 (1998).
Zhu, J. et al. Hierarchical hollow spheres composed of ultrathin Fe2O3 nanosheets for lithium storage and photocatalytic water oxidation. Energy & Environmental Science 6, 987–993 (2013).
Ananpattarachai, J., Kajitvichyanukul, P. & Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. Journal of Hazardous Materials 168, 253–261 (2009).
Liao, G. Z., Chen, S., Quan, X., Yu, H. T. & Zhao, H. M. Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. Journal of Materials Chemistry 22, 2721–2726 (2012).
Liu, B. et al. Doping high-surface-area mesoporous TiO2 microspheres with carbonate for visible light hydrogen production. Energy & Environmental Science 7, 2592–2597 (2014).
Humayun, M. et al. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Applied Catalysis B: Environmental 180, 219–226 (2016).
Guo, Y., Ao, Y., Wang, P. & Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Applied Catalysis B: Environmental 254, 479–490 (2019).
Lv, D. et al. One-pot combustion synthesis of BiVO4/BiOCl composites with enhanced visible-light photocatalytic properties. Separation and Purification Technology 174, 97–103 (2017).
Shi, L., Ma, J., Yao, L., Cui, L. & Qi, W. Enhanced photocatalytic activity of Bi12O17Cl2 nano-sheets via surface modification of carbon nanotubes as electron carriers. Journal of Colloid and Interface Science 519, 1–10 (2018).
Fan, Y. et al. Three-dimensional branched crystal carbon nitride with enhanced intrinsic peroxidase-like activity: A hypersensitive platform for colorimetric detection. ACS Applied Materials &Interfaces 11, 17467–17474 (2019).
Kumar, A. et al. Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. Journal of Photochemistry and Photobiology A: Chemistry 337, 118–131 (2017).
Selvarajan, S., Suganthi, A., Rajarajan, M. & Arunprasath, K. Highly efficient BiVO4/WO3 nanocomposite towards superior photocatalytic performance. Powder Technology 307, 203–212 (2017).
Yu, Z. et al. Noninvasively modifying band structures of wide-band gap metal oxides to boost photocatalytic activity. Advanced Materials 30, 1706259 (2018).
Huang, L. et al. Dual cocatalysts loaded type I CdS/ZnS core/shell nanocrystals as effective and stable photocatalysts for H2 evolution. Journal of Physical Chemistry C 117, 11584–11591 (2013).
Richters, J.-P., Voss, T., Kim, D. S., Scholz, R. & Zacharias, M. Enhanced surface-excitonic emission in ZnO/Al2O3 core–shell nanowires. Nanotechnology 19, 305202 (2008).
Ning, X., Zhen, W., Wu, Y. & Lu, G. Inhibition of CdS photocorrosion by Al2O3 shell for highly stable photocatalytic overall water splitting under visible light irradiation. Applied Catalysis B: Environmental 226, 373–383 (2018).
Acknowledgements
This work was supported by National Natural Science Foundation of China (21671092, 21401093), LiaoNing Revitalization Talents Program (XLYC1802057), basic research projects of Liaoning Provincial Education Department (L2017LQN004, L2017LQN006) and talent scientific research fund of LSHU (No. 2016XJJ-080).
Author information
Authors and Affiliations
Contributions
Ying Zhang prepared photocatalysts and measured performance. Lei Shi wrote the main manuscript text. Zhongxing Geng and Tieqiang Ren measured the chemical structure of catalyst. Zhanxu Yang designed catalyst.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Shi, L., Geng, Z. et al. The improvement of photocatalysis O2 production over BiVO4 with amorphous FeOOH shell modification. Sci Rep 9, 19090 (2019). https://doi.org/10.1038/s41598-019-54940-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54940-2
This article is cited by
-
Enhancement of photoelectrochemical performance of ZnIn2S4 films for water oxidation by cation exchange and hydroxy iron oxide decoration
Journal of Materials Science: Materials in Electronics (2023)
-
Preparation of a Core–Shell-Like TiO2/Bi2O3Heterostructure and Qualitative Analysis of the Function of the Heterojunction Region in the Photocatalytic Process
Water, Air, & Soil Pollution (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.