Abstract
The biosorption capacities of dried meal and a waste product from the processing for biostimulant extract of Ascophyllum nodosum were evaluated as candidates for low-cost, effective biomaterials for the recovery of indium(III). The use of indium has significantly grown in the last decade, because of its utilization in hi-tech. Two formats were evaluated as biosorbents: waste-biomass, a residue derived from the alkaline extraction of a commercial, biostimulant product, and natural-biomass which was harvested, dried and milled as a commercial, “kelp meal” product. Two systems have been evaluated: ideal system with indium only, and double metal-system with indium and iron, where two different levels of iron were investigated. For both systems, the indium biosorption by the brown algal biomass was found to be pH-dependent, with an optimum at pH3. In the ideal system, indium adsorption was higher (maximum adsorptions of 48 mg/g for the processed, waste biomass and 63 mg/g for the natural biomass), than in the double metal-system where the maximum adsorption was with iron at 0.07 g/L. Good values of indium adsorption were demonstrated in both the ideal and double systems: there was competition between the iron and indium ions for the binding sites available in the A. nodosum-derived materials. Data suggested that the processed, waste biomass of the algae, could be a good biosorbent for its indium absorption properties. This had the double advantages of both recovery of indium (high economic importance), and also definition of a virtuous circular economic innovative strategy, whereby a waste becomes a valuable resource.
Similar content being viewed by others
Introduction
Indium is a metallic element, classified by the European Commission as a critical raw material for Europe based up on both its high supply risk and also economic importance1. Indium prices have mounted to a higher range due to scarce availability of crude indium. Recently, Metal Bulletin evaluated the free market indium price at $250–280 per kg (January 2018). Its utilization over the last decade in particular has increased continuously. This is a result of its characteristics as a semi-conductor and opto-electronic making it suitable for industrial applications2. In particular, indium is involved in the production of high-tech equipment, mainly liquid-crystal displays (LCDs), where it is present, combined with tin, to form indium tin oxide (ITO)3,4. The relevance of this metal is confirmed by the European Substitutability index of 0.82 (in a 0–1 range), that estimates the difficulty in substituting the material, scored and weighted across all applications1. Currently, the entire indium available to the market comes from one primary production site, as a by-product of zinc mining and China is the largest producer1,4,5,6. Considering that the average indium concentration in a LCD, is about 150 ppm2,3,7,8,9,10 which is higher than that occurring in mined ores4, the recycling of electronic waste should be considered an important secondary source2,11,12. Recent articles describe several treatments for the recovery of indium from end-of-life LCDs. Most recovery processes use chemical approaches, including a primary acid leaching2,8,13,14,15,16,17 followed by different treatments such as: cementation, solvent extraction and precipitation5,18,19,20,21. Nevertheless, considering the global requirement for raw materials which in itself leads to the production of a final waste which has to be managed, there are significant environmental impacts associated with the current indium recovery processes22. In this context, an innovative method that uses a biosorption approach and utilizes an industrial waste biomass could have significant, positive economic and environmental impacts23. Biosorption is a physico-chemical process based on the removal of metals from aqueous solutions by passive binding to non-living biomass such as marine macrophytes (e.g. seagrasses and seaweeds)24,25,26,27,28,29. The effectiveness of this approach is independent of metal content which is contrary to other techniques, such as chemical precipitation, evaporation, extraction with solvents, electro-plating, ionic exchange and membrane processes. All of these are relatively ineffective or economically disadvantageous for the recovery of low concentrations (e.g. <100 mg/L Cd)30 and a complete metal removal is limited, especially in large volumes of water31. Furthermore, the use of a non-living dry biomass as an adsorbent material has further advantages, e.g. high performance for the detoxification of diluted effluents for extended periods, minimization of the volume of biological and/or chemical waste, no demand for nutrients, relatively low cost, and generally high availability of the resource32,33. In addition, the use of non-living substrata avoids the limitations of toxicity of cells and the requirement of aseptic conditions which allows for operation in a wider range of operating conditions such as pH and temperature34,35. The complexity of biosorption processes is often increased by the presence of multi-ions in solutions, with three possible outcomes, e.g. (i) increased target metal sorption, (ii) inhibition of target metal sorption, and (iii) no significant change in metal sorption35,36. The technique of biosorption can be also used as an important tool for the recovery of precious metals using waste biomasses as an absorbent, with potentially low economic and environmental costs37,38. Furthermore, once the metal bonding capacity reaches saturation, it is possible to restore the absorptive capacity of the biomasses using acid and/or hydroxyl solutions which releases small volumes of concentrated heavy metals for recovery39,40,41,42. Thereafter, the solutions obtained can be treated with co-precipitation, flocculation or electro-deposition in order to achieve final recovery of the metal. In more detail, the process of biosorption is based on the capacity of biological materials to sequestrate metals by chemical-physical mechanisms43,44,45; ranging from electro-static or Van der Waals’ forces to ionic or co-valent bonding46. Several chemical groups have been suggested as being responsible for biosorption, i.e. carboxylic, hydroxyl, sulfhydryl, sulfate, thioether, amino, iminico, imidazole, phosphate and phosphodiestereric groups47,48,49. The biosorption capability of various groups depends on many factors, e.g. the number of binding sites on the adsorbent material, accessibility, chemical status (availability) and affinity between site and metal scilicet bond strength50. Several types of biomass have been studied to identify the best biosorbents, (instead of synthetic resins), in order to recover dangerous or economically valuable metals (e.g. Pb, As, V, Mo, Cd, Co, Au, Ni, U, Zn, Cu, In, rare on earth) from aqueous solutions, such as bacteria, fungi, seaweeds, seagrasses24,27,28,51,52. A major effort has been focused recently on algal biomass as a biosorbent24, as it is considered one of the most promising candidates because of its high uptake capability and, its relatively low-cost. Indeed, marine macroalgae, collected from cultivated plants, are readily available, in relatively large quantities for the development of biosorbent materials. At this point, only a relatively few algal species have been evaluated for their heavy metal biosorption capacity53,54,55,56. Selected red, green and brown algae have been investigated as adsorbent materials57. Specific attention has been placed on some brown algae (e.g. Sargassum spp., Pelvetia canaliculata, Macrocystis spp., Laminaria spp.), because of the characteristics of their cell walls. Carboxylic, and sulfate groups are predominant in brown seaweed walls and they are considered to be useful for the biosorption of metallic ions24,33,43,47,55,58,59. In particular, among the brown algae, members of the Fucales and Laminariales appear to be the best algal groups to be used in biosorption processes60. Their capacities as biosorbents are due, in particular, to the relatively high content of alginic acids (alginates) which are the main polysaccharide present in the cell walls of these brown algae, and they are particularly rich in carboxylic groups32,33. Despite the fact there are numerous published studies related to metal biosorption by algal biomasses30,54,61,62,63, there are none about the recovery of indium by macroalgae. In this study biomass from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (a member of the Fucales) was investigated. This seaweed was chosen to be evaluated as a biosorbent biomass both for its ability to recover metals by adsorption (Table 1) and because abundant waste materials derived from industrial alginate extraction, or extraction of a biostimulant is produced.
The aim of this work was to evaluate the biosorption capacity of dried and milled seaweed meal (Natural biomass) and also a common industrial, Waste biomass, derived from the same seaweed in the process of biostimulants production. Two systems were used, one “ideal” (i.e. one metal present: indium) and the second labelled “non-ideal” (i.e. two metals present: indium + iron). Indeed, the literature identified the iron as a relevant criticality during the indium recovery from electronic waste (mainly end-of-life LCD) for its high concentration in the extraction solution5,8,16. The useful functional groups for bioadsorbent ability of the natural biomass and waste biomass of A. nodosum have been characterized. Moreover, the reports macromolecular area inside of A. nodosum matrix before and after the industrial process have been evaluated. The observed results were very promising for the definition of circular economy innovative strategies, where an industrial waste finds valuable applications as an indium sorbent.
Materials and Methods
Biosorbent materials
The biosorbent materials evaluated in this study were provided by Acadian Seaplants (Canada) which is an industry leader in the processing of seaweed-based products for biochemical, food, agricultural and agri-chemical markets worldwide, and in the cultivation and processing of unique seaweeds for global food markets. The materials were derived from the sustainable harvest of the brown seaweed Ascophyllum nodosum from the coast of Nova Scotia (Canada). The Canadian company uses this biomass for various applications: soil amendment, a source of alginic acid for the commercial production of alginates, and a wide range of animal and human food supplements. The samples were supplied in two formats designated by us as: (1) “Waste biomass” (Fig. 1A) which was a common product of alkaline, open atmosphere extraction. This “waste” would have normally been disposed of by approved field spreading, or as an input to organic soil amendments by third parties; (2) “Natural biomass” (i.e., Granular material; Fig. 1B), which was harvested, air dried and milled.
Preparation and characterization of biosorbents
The Natural biomass derived from the entire dehydrated thalli of Ascophyllum were reduced to small pieces (less than 0.5 cm) to obtain the most similar size to the Waste biomass fragments, washed in deionized water (30 minutes), transferred to HCl (pH 2) solution for 30 minutes and dried at room temperature for 2–3 days and then stored in falcon tubes until use. The samples of Waste biomass were washed and soaked for two hours in a pH 2 HCl solution. After which, they were sieved (pore diameter 125 μm), and the aqueous fraction separated from the solids. The solids were then dried at room temperature for 4–5 days and stored in falcon tubes until use.
Potentiometric titration (i.e., an acid-base titration test) was carried out in order to characterize the functional groups present in the Ascophyllum biosorbent materials (i.e., Waste biomass and Natural biomass), according to the Gran Method64,65. Samples of 5 g of material where rehydrated in 200 mL of deionized water, titrations were performed using standard solutions of NaOH 1 N (basic, Sigma- AldrichH®) and of HCl 1 N (acid, Sigma- AldrichH®). During the experiments, pH measurements were recorded after the addition of each titrant (0.02, 0.04 or 0.05 mL) using an ISteK pH 730p meter. The theoretical prediction of metal speciation in the solutions was carried out using MEDUSA software (Make Equilibrium Diagrams Using Sophisticated Algorithms)66. This software defines the theoretical chemical equilibria of inorganic substances in aqueous solutions. Further characterization of the adsorbents was carried out through Fourier Transform InfraRed (FT-IR) spectroscopy, through which functional groups, bonding types and molecular conformations of the most relevant biological molecules are identified67,68. The samples were analysed by vibrational analysis using the Universal Attenuated Total Reflectance (U-ATR) mode, since it allowed collection of good-quality infra-red spectra from either solid or liquid samples, with almost no sample preparation69,70. Moreover, data were sampled used a Perkin Elmer Spectrum GX1 spectrometer equipped with a U-ATR accessory. The spectral range was 4000–650 cm−1 (resolution 4 cm−1) where each spectrum was the result of 32 scans. Background scans were acquired and ratioed against the sample. The spectra were converted in absorbance, two points baseline linear fitted and vector normalized. In order to unequivocally define the position of all absorption bands, the spectra were also converted by a second derivative mode (DII, 9 points smoothing). For data handling, the Spectrum 10.4 (Perkin Elmer) and Grams AI 7.0 (Galactic) software packages were used.
Preparation of metal cation test solutions
Two stocks solution were prepared: An indium (III) sulfate solution (In2(SO4)3 Sigma- AldrichH®) at 1 g/L indium was prepared by dissolving 1.1 g indium in 0.5 L of deionized water; a ferric (III) sulphate (Fe2(SO4)3 Sigma- AldrichH®) dihydrate solution (10 g/L Fe) was prepared by dissolving 3.9 g of ferric sulfate in 0.1 L a solution of sulfuric acid 0.1 M. Both stock solutions were assembled at room temperature and stirred.
Biosorption tests
Samples were rehydrated before each test: 0.5 g of dried biomass was suspended in 0.1 L de-ionized water and shaken for 30 minutes using a magnetic stirrer (MICROSTIRRERVELP Scientific). Metal stock solutions (indium or iron at different concentrations) were added successively, as specified in Table 2. During the biosorption test, acid (H2SO4 5 M) and basic (NaOH 10 M) solutions were used to adjust the pH in accordance with the experimental design (Table 2). Measurements of pH were determined by an ISteK 730p meter. Aliquots of solution (1 mL) were then regularly sampled for the determination of metal(s) concentrations. The samples were centrifuged (3000 g for 10 minutes) to remove any suspended materials and successively stocked by adding 9 mL of acid water at pH 2 to stabilize the metal(s) before subsequent analytical measurements. At the same time, control tests were performed without biomass which showed that the concertation of the metal(s) was the same over time. These data confirmed that no metal(s) precipitation took place and that no metal(s) was(were) released by the testing equipment. Indium and iron concentrations in the liquid phase were determined by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry) using respectively the EPA 3050B 1996 + EPA 6010C 2007 methods for indium and EPA 3051A 2007 + 6010C 2007 methods for iron. Metal uptake, q (mg g−1), was calculated as the difference in the metal concentration(s) in the aqueous phase before and after sorption according to29 (Eq. 1):
where, V corresponded to volume of the solution (L), Ci was the initial concentration of metal (indium and iron) in solution (mg L−1), C was the equilibrium concentration of the metal, and W represented the mass analyzed (g). The Langmuir adsorption isotherm71 was adapted to the experimental data through regression analysis (Eq. 2):
where qmax was the maximum adsorption capability (mg/g), Ks was the equilibrium sorption constant and it represented the affinity between the test metal ion(s) and the biosorbent (mg L−1). The model used (suitable for several biological materials) assumed that all binding sites had equal affinity for the adsorbate with the consequent formation of mono-layer of adsorbed molecules36,72.
Experimental design
The ideal (In) and the double metal- (In-Fe) systems were used to assess the indium biosorption capacity of two formats of Ascophyllum nodosum. Table 2 presents the factors and levels investigated. Three constant parameters were maintained in the experimental design: (1) total volume (100 mL), (2) room temperature and (3) biosorbent concentration (5 g/L). Sorption isotherms were evaluated after predictions from the MEDUSA modelling software (see below) at different pH (i.e., 1, 1.5, 2, 2.5, 3) both in the ideal and double metal-systems. For the single metal system, metal (1), type of the samples (2) and pH (3) were the factors investigated in the experiment. In the ideal and double metal-systems, indium was added five times at different concentration: i.e. 20, 40, 80, 160, and 320 mg/L. Before each metal addition, an aliquot of solution was periodically sampled for indium concentration determination. In the double metal-system, two different levels two investigated: i.e. 0.7 and 0.07 g/L of iron.
Results and Discussion
Biosorbent characterization
An acid-base titration of Waste biomass (1) and Natural biomass (2) was made to analyze the number and type of functional groups involved in the binding metal on the biomass64,65,73. This analysis was based on a neutralization reaction, in order to determine an unknown concentration of functional groups that have an acid behaviour in aqueous solution. The titration curves of the samples of Ascophyllum nodosum were made as duplicates with a blank solution as control. The acid groups on the bioabsorbent materials have been neutralized by NaOH titration at pH 7. The titration curves of Waste biomass in Fig. 2A showed the pH profile to be a function of the NaOH added (as milli-equivalents). The curves were typical of weak polyprotic acids, which have more than one proton that may be removed by reaction with a base. The Natural biomass of A. nodosum provided similar profiles (see supplementary Fig. S1). Moreover, the equivalence point (i.e., where the number of protons was enough to completely neutralize the hydroxylic groups) was not easily identifiable, but it appeared in a range of pKa46,74 (Eq. 3):
where, Ka represented the acid dissociation constant of a solution. The lower the pKa value, the stronger the acid. The Gran method was used to linearize the titration curves before and after the equivalence point: in the natural biomass (data not shown), the equivalence volume ranged from 2200–4500 μL, corresponding to a range of 2.2–4.5 milli-equivalents (meq) added. The waste biomass (Fig. 2B) showed an equivalence volume ranging from 8000–12000 μL, with a corresponding range of 8–12 meq added. Through these analyses, it was possible to estimate the pKa range of the functional groups involved, which was in the range of 2.8–4.1 (for the natural biomass) and 3–3.4 (waste biomass), both of which can be associated with -COO− functional groups74. Further characterization of the functional groups within the seaweed biomasses which were able to interact with metal ions was carried out through Fourier transformation analysis (FT-IR). Figure 3 shows the spectra from the waste Ascophyllum biomass in both absorbance and DII modes: typical bands of the C-H bond (3100 cm−1) and C=O bond (1600 cm−1) occurred. The carbonyl group could be attributable to the presence of alginic acid on the cell walls of the brown alga48. This observation supported the notion that the waste biomass still contained some alginic acid after the biostimulant extraction process. The presence of the alginic acid was involved in forming the bond with the metal ions24,27. Moreover, the spectra obtained from samples thallus, nodes (aerocysts) and sodium alginate (Fig. 3). Analysis of the Natural biomass (i.e., separated thallus and nodes) showed typical bands related to alginate component 1602, 1419, 1027 cm−1, together with bands of lipids including the fucoidan (i.e. sulfated polysaccharide) fraction at 1238 cm−1, previously reported in the literature48,75. The spectra of the Natural biomass at different layers of the thallus from the superficial (external zone) to the deepest were reported in Fig. 3. All of the analysed thallus parts provided a similar spectrum but with an increase in the lipid component (1744 cm−1) above all phospholipids with long acyl chains (2924, 2854 and 1077 cm−1) in the external layer76. Indeed, it is primarily the external layer of the cell wall to be involved in the bond with the metal ions24,27,29,31,48,74.
Infrared spectra of: (A) waste biomass, external part of thallus and nodes (aerocysts) of Ascophyllum nodosum, and sodium alginate; (B) thallus of natural Ascophyllum nodosum (i.e., natural biomass) at different depths, from the external (down) to the internal (up) zone. IR spectra were reported in absorbance mode in the spectral range 4000–600 cm−1. For a better understanding, they were shifted along y axis.
Indium and iron speciation
Indium and iron speciation, as a function of pH in aqueous solution, was investigated according to the predictions from the MEDUSA modelling software (Fig. 4). The parameters used were: redox potential 300 mV, pH range from 1–3 and a temperature of 25 °C. This model showed that indium, as well as iron, were present mainly as free cations. Both metals do not seem to form chemical complexes and they are free in solution; hence they theoretically compete for the same binding sites in both natural and waste biomasses of A. nodosum. Moreover, according to this model, precipitation phenomena were not observed, under the operating conditions used. This was also confirmed by control experiments.
Prediction of indium and iron speciation as a function of pH where the y-axis shows the logarithm of the metal concentration66.
Biosorption in the ideal, single metal system
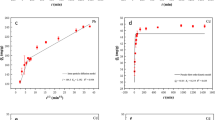
This part of the study presents an assessment of the influence of pH on indium biosorption by Ascophyllum nodosum (both waste and natural biomasses) in the single metal systems. In both samples, the best adsorption performance was at pH 3 (Fig. 5A). In particular, the maximum adsorption was between 48 and 63 mg/g, for the waste biomass and natural biomasses, respectively. Moreover, the waste biomass showed a good adsorption performance at pH = 2.5, with a value of 45 mg/g (Fig. 5A). This could be attributed to the biochemical composition of the external layer constituents of the cell walls. Indeed, de-protonated forms of hydroxyl (i.e., sulfated polysaccharide), carboxylic and carbonyl groups (i.e alginate polysaccharide) of this amorphous substance were probably responsible for the chemical and physical bond created with the metal cations in solution24,27,29,48,77. This hypothesis was confirmed by Fourier transformation infra-red (FT-IR) spectroscopy (Fig. 3), which revealed the presence of sulfate, carboxylic and carbonylic groups in the biomass. It can be observed that the sorption ability of the waste biomass of Ascophyllum increased with pH, in the investigated range, with the highest performance (i.e. 48 mg/g) at pH 3 and the lowest at pH 1.5 (i.e., 18 mg/g) (Fig. 5A). This aspect of biosorption was in line with previous studies dealing with other metals and other biosorbents27,30,78, e.g. when pH is lower than pKa, the carboxyl and carbonyl are more protonated, decreasing their biosorption capacity30. Sorption isotherms in Fig. 5 were mathematically modeled with either linear regression (pH 1, 1.5, 2) or with Langmuir model regression (Eq. 2, pH 2.5 and 3). The Langmuir model parameter qmax was estimated at 43 and 67 mg/g, for pH 2.5 and pH 3, respectively (R2 was 0.98 in both regressions). Comparing the sorption isotherms of the two different substrates in Fig. 5A, the Waste biomass was less effective for indium biosorption than the Natural biomass (dotted line in Fig. 5A). Indeed, the Waste biomass is a residue after the commercial biostimulant extraction process: alginate is rich in active groups (Fig. 3) for biosorption, and its extraction or degradation in the open atmospheric, alkaline process led to a lower sorption performance of that biomass. However, the indium sorption performance observed for the waste biomass (i.e., experimental observation of 48 mg/g, corresponding to 0.42 mmol/g; maximum indium sorption capacity 67 mg/g, corresponding to 0.58 mmol/g, as predicted by Langmuir model) is considered to be significant if compared to other indium biosorbents investigated in the literature, e.g. 6 mg/g for chitosan-coated bentonite79, 0.24–0.57 mmol/g for a chemically modified montmorillonite80, 80 mg/g for a functionalized amino silica81. Consequently, the results presented here support re-classification of the once industrial waste (destined for disposal on agricultural land) into a new resource, to be used as an indium biosorbent, with relevant advantages for the general sustainability of the whole industrial process.
Biosorption in the double (multi)-metal system
In this part of the study, the influence of iron on indium biosorption by the waste biomass of Ascophyllum nodosum, under different pH conditions, was estimated. Iron was added in order to evaluate the possible competition with the indium ions for the active sites involved in biosorption on the waste biomass. Two experiments in multi-metal system were performed. Iron was added at a concentration of either 0.7 or 0.07 g/L, at the beginning of the experiments; Fig. S2 (see Supplementary Materials) shows all of the sorption isotherms obtained. The analysis under different pH conditions (Fig. 5B), showed that, for a Fe concentration of 0.07 g/L, the general behavior was similar to that observed in the absence of iron (Fig. 5A). The adsorption of indium had a general positive trend with increasing pH, and the isotherms obtained at pH 2.5 and 3 in the In-Fe system with Fe at 0.07 g/L could be successfully modeled by the Langmuir equation. Similar considerations for a pH effect can be done in the presence of a ten-fold iron concentration. In this case, the mathematical modeling followed a linear correlation rather than a Langmuir trend.
Concerning the possible effects of the presence of iron ions on indium adsorption, the results suggested an inhibition of indium sorption in the presence of iron. Indeed, the maximum indium adsorption observed was 41 and 31 mg/g in the presence of 0.07 and 0.7 g/L of Fe respectively (Fig. 6). Iron ions were probably competing with indium for the active sorbent sites, due to its similarity in valence and molecular weight. However, even with a relatively high concentration of iron (i.e. 700 mg/L Fe vs. a range of indium initial concentrations of 20–200 mg/L In), indium sorption was still significant as compared to published data on sorbents (see above). Figure 7 shows the profile of the maximum observed indium sorption (at pH 3), expressed as mmol/g, as a function of the initial iron concentration, also in this case expressed as mmol/L. It can be observed that the decrease in sorption performance was not linear, as hypothesized; indeed, it was successfully modeled (as a continuous line in Fig. 7) by the empirical equation (Eq. 4):
This aspect was considered very important for the potential application of the algal waste residue as a potential, potent indium biosorbent, since real-world, indium-bearing solutions, coming from indium recovery processes20,22,82,83,84 also contain other metal ions such as iron, at higher concentrations than the indium. Consequently, our results support the notion to successfully re-use the residual biomass of biostimulant production from Ascophyllum nodosum as an indium biosorbent.
Conclusions
Through this work has been demonstrated that the waste biomass from Ascophyllum could be utilized as an effective biosorption resource in the recovery of Indium (III) (one man’s ceiling is another man’s floor? Paul Simon). The biosorption capacity of the industrial waste biomass of Ascophyllum was of interest, both in the single (In) and the two-metal ion systems (In-Fe) proving so that the it had potential commercial applications. This work demonstrated the suitability of both natural and waste biomasses as indium sorbents, with a maximum sorption ability estimated at 63 and 46 mg/g respectively. Sorption of indium was explained by chemico-physical interactions, mainly based on natural ion-exchange, with carboxylic and carbonyl groups present in many macromolecules on the brown algal cell walls such as alginic acid polysaccharides. Considering that in real systems, indium is often accompanied by the presence of iron, in the In-Fe simulation we observed competition between these two metals. In particular, a comparative analysis in a pH-constant system with varying iron ion concentrations established that the adsorption of the indium was slowed down by the presence of iron, although yet still maintained a relatively high performance. Future work will be directed to improve the rendering of the In-Fe real system, in order to find a suitable process configuration for the application of Ascophyllum nodosum waste biomass at an industrial scale: this will be very important to boost circular economy approaches.
References
European Commission. Communication on the 2017 list of Critical Raw Materials for the EU. (2017).
Lee, C. H. et al. Recovery of indium from used LCD panel by a time efficient and environmentally sound method assisted HEBM. Waste Manag. 33, 730–734 (2013).
Ma, E. & Xu, Z. Technological process and optimum design of organic materials vacuum pyrolysis and indium chlorinated separation from waste liquid crystal display panels. J. Hazard. Mater. 263, 610–617 (2013).
Alfantazi, A. M. & Moskalyk, R. R. Processing of indium: A review. Miner. Eng. 16, 687–694 (2003).
Yang, J., Retegan, T. & Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 137, 68–77 (2013).
Virolainen, S., Ibana, D. & Paatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometallurgy 107, 56–61 (2011).
Gotze, R. & Rotter, S. V. Challenges for the Recovery of Critical Metals from Waste Electronic Equipment - A Case Study of Indium in LCD Panels. 2012 Electron. Goes Green 2012+ (2012).
Rocchetti, L. et al. Cross-current leaching of indium from end-of-life LCD panels. Waste Manag. 42, 180–187 (2015).
Hasegawa, H. et al. Recovery of indium from end-of-life liquid-crystal display panels using aminopolycarboxylate chelants with the aid of mechanochemical treatment. Microchem. J. 106, 289–294 (2013).
Her-Yung, W. A study of the engineering properties of waste LCD glass applied to controlled low strength materials concrete. Constr. Build. Mater. 23, 2127–2131 (2009).
Amato, A. & Beolchini, F. End of life liquid crystal displays recycling: A patent review. J. Environ. Manage. 225, 1–9 (2018).
Cui, J. & Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 158, 228–256 (2008).
Li, L. et al. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 176, 288–293 (2010).
Li, Y., Liu, Z., Li, Q., Liu, Z. & Zeng, L. Recovery of indium from used indium-tin oxide (ITO) targets. Hydrometallurgy 105, 207–212 (2011).
Dodbiba, G., Nagai, H., Wang, L. P., Okaya, K. & Fujita, T. Leaching of indium from obsolete liquid crystal displays: Comparing grinding with electrical disintegration in context of LCA. Waste Manag. 32, 1937–1944 (2012).
Ruan, J., Guo, Y. & Qiao, Q. Recovery of Indium from Scrap TFT-LCDs by Solvent Extraction. Procedia Environ. Sci. 16, 545–551 (2012).
Wang, X., Lu, X. & Zhang, S. Study on the waste liquid crystal display treatment: Focus on the resource recovery. J. Hazard. Mater. 244–245, 342–347 (2013).
Jiang, J., Liang, D. & Zhong, Q. Precipitation of indium using sodium tripolyphosphate. Hydrometallurgy 106, 165–169 (2011).
Silveira, A. V. M., Fuchs, M. S., Pinheiro, D. K., Tanabe, E. H. & Bertuol, D. A. Recovery of indium from LCD screens of discarded cell phones. Waste Manag. 45, 334–342 (2015).
Rocchetti, L., Amato, A. & Beolchini, F. Recovery of indium from liquid crystal displays. J. Clean. Prod. 116 (2016).
Chen, L. et al. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108, 80–86 (2011).
Amato, A., Rocchetti, L. & Beolchini, F. Environmental impact assessment of different end-of-life LCD management strategies. Waste Manag. 59 (2017).
Morbee, J. & Tzimas, E. Strategic Energy Technology Plan: Scientific Assessment in support of the Materials Roadmap enabling Low Carbon Energy Technologies- Fossil Fuel Energies Sector, including Carbon Capture and Storage, https://doi.org/10.2790/3946 (2011).
Davis, T. A., Volesky, B. & Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 37, 4311–4330 (2003).
Topcuoǧlu, S., Güven, K. C., Balkis, N. & Kirbaşoǧlu, Ç. Heavy metal monitoring of marine algae from the Turkish Coast of the Black Sea, 1998–2000. Chemosphere 52, 1683–1688 (2003).
Seki, H. & Suzuki, A. Kinetic study of metal biosorption to a brown alga, Kjellmaniella crassiforia. J. Colloid Interface Sci. 246, 259–62 (2002).
Pennesi, C. et al. Marine Macrophytes as Effective Lead Biosorbents. Water Environ. Res. 84, 9–16 (2012).
Pennesi, C., Vegliò, F., Totti, C., Romagnoli, T. & Beolchini, F. Nonliving biomass of marine macrophytes as arsenic(V) biosorbents. J. Appl. Phycol. 24, 1495–1502 (2012).
Pennesi, C., Totti, C. & Beolchini, F. Removal of Vanadium(III) and Molybdenum(V) from Wastewater Using Posidonia oceanica (Tracheophyta) Biomass. PLoS One 8 (2013).
Lodeiro, P., Cordero, B., Barriada, J. L., Herrero, R. & Sastre De Vicente, M. E. Biosorption of cadmium by biomass of brown marine macroalgae. Bioresour. Technol. 96, 1796–1803 (2005).
Karthikeyan, S., Balasubramanian, R. & Iyer, C. S. P. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresource Technology 98 (2007).
Bailey, S. E., Olin, T. J., Bricka, R. M. & Adrian, D. D. A review of potentially low-cost sorbents for heavy metals. Water Res. 33, 2469–2479 (1999).
Bhatnagar, A., Vilar, V. J. P., Ferreira, C., Botelho, C. M. S. & Boaventura, R. A. R. Optimization of nickel biosorption by chemically modified brown macroalgae (Pelvetia canaliculata). Chem. Eng. J. 193–194, 256–266 (2012).
Ahluwalia, S. S. & Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 98, 2243–2257 (2007).
Zeraatkar, A. K., Ahmadzadeh, H., Talebi, A. F., Moheimani, N. R. & McHenry, M. P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manage. 181, 817–831 (2016).
Kumar, D., Pandey, L. K. & Gaur, J. P. Metal sorption by algal biomass: From batch to continuous system. Algal Res. 18, 95–109 (2016).
Das, N. Recovery of precious metals through biosorption - A review. Hydrometallurgy 103, 180–189 (2010).
Volesky, B. & Holan, Z. R. Biosorption of heavy metals. Biotechnol. Prog. 11, 235–250 (1995).
Veglio’, F. & Beolchini, F. Removal of metals by biosorption: a review. Hydrometallurgy 44, 301–316 (1997).
Schneider, I. A. H. & Rubio, J. Sorption of heavy metal ions by the nonliving biomass of freshwater macrophytes. Environ. Sci. Technol. 33, 2213–2217 (1999).
Schneider, I. A. H., Rubio, J. & Smith, R. W. Biosorption of metals onto plant biomass: exchange adsorption or surface precipitation? Int. J. Miner. Process. 62, 111–120 (2001).
Elifantz, H. & Tel-Or, E. Heavy Metal Biosorption by Plant Biomass of the Macrophyte Ludwigia Stolonifera. Water. Air. Soil Pollut. 141, 207–218 (2002).
Hashim, M. A. & Chu, K. H. Biosorption of cadmium by brown. green, and red seaweeds. Chem. Eng. J. 97, 249–255 (2004).
Volesky, B. Biosorption of heavy metals. (CRC Press, 1990).
John Wase, D. & Christopher Forster, D. Biosorbents for Metal Ions. (1997).
Lodeiro, P., Rey-Castro, C., Barriada, J. L., Sastre De Vicente, M. E. & Herrero, R. Biosorption of cadmium by the protonated macroalga Sargassum muticum: Binding analysis with a nonideal, competitive, and thermodynamically consistent adsorption (NICCA) model. J. Colloid Interface Sci. 289, 352–358 (2005).
Dodson, J. R., Hunt, A. J., Parker, H. L., Yang, Y. & Clark, J. H. Elemental sustainability: Towards the total recovery of scarce metals. Chem. Eng. Process. Process Intensif. 51, 69–78 (2012).
Pennesi, C., Rindi, F., Totti, C. & Beolchini, F. Marine Macrophytes: Biosorbents. In Hb25_Springer Handbook of Marine Biotechnology 597–610, https://doi.org/10.1007/978-3-642-53971-8_24 (Springer Berlin Heidelberg, 2015).
Crist, R. H., Oberholser, K., Shank, N. & Ming, N. Nature of bonding between metallic ions and algal cell walls. Environ. Sci. Technol. 15, 1212–1217 (1981).
Vieira, R. H. S. F. & Volesky, B. Biosorption: a solution to pollution? Int. Microbiol. 3, 17–24 (2010).
Cerino-Córdova, F. J. et al. Experimental design for the optimization of copper biosorption from aqueous solution by Aspergillus terreus. J. Environ. Manage. 95, S77–S82 (2012).
Ogi, T., Tamaoki, K., Saitoh, N., Higashi, A. & Konishi, Y. Recovery of indium from aqueous solutions by the Gram-negative bacterium Shewanella algae. Biochem. Eng. J. 63, 129–133 (2012).
Volesky, B. & Prasetyo, I. Cadmium removal in a biosorption column. Biotechnol. Bioeng. 43, 1010–1015 (1994).
Holan, Z. R., Volesky, B. & Prasetyo, I. Biosorption of cadmium by biomass of marine algae. Biotechnol. Bioeng. 41, 819–825 (1993).
Fourest, E. & Volesky, B. Contribution of Sulfonate Groups and Alginate to Heavy Metal Biosorption by the Dry Biomass of Sargassum fluitans. Environ. Sci. Technol. 30, 277–282 (1996).
Chong, K. H. & Volesky, B. Description of two-metal biosorption equilibria by Langmuir-type models. Biotechnol. Bioeng. 47, 451–460 (1995).
Vilar, V. J. P., Botelho, C. M. S. & Boaventura, R. A. R. Copper removal by algae Gelidium, agar extraction algal waste and granulated algal waste: Kinetics and equilibrium. Bioresour. Technol. 99, 750–762 (2008).
Ballester, A. et al. Design of remediation pilot plants for the treatment of industrial metal-bearing effluents (BIOMETAL DEMO project): Lab tests. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2016.10.010 (2016).
Vijayaraghavan, K. & Jegan, J. Entrapment of brown seaweeds (Turbinaria conoides and Sargassum wightii) in polysulfone matrices for the removal of praseodymium ions from aqueous solutions. J. Rare Earths 33, 1196–1203 (2015).
Freitas, O., Delerue-Matos, C. & Boaventura, R. Optimization of Cu(II) biosorption onto Ascophyllum nodosum by factorial design methodology. J. Hazard. Mater. 167, 449–454 (2009).
Areco, M. M., Hanela, S., Duran, J. & dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 213–214, 123–132 (2012).
Zhou, J. L., Huang, P. L. & Lin, R. G. Sorption and desorption of Cu and Cd by macroalgae and microalgae. Environ. Pollut. 101, 67–75 (1998).
Abdel -Aty, A. M., Ammar, N. S., Abdel Ghafar, H. H. & Ali, R. K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 4, 367–374 (2013).
Michałowski, T., Toporek, M. & Rymanowski, M. Overview on the Gran and other linearisation methods applied in titrimetric analyses. Talanta 65, 1241–1253 (2005).
Michałowski, T., Kupiec, K. & Rymanowski, M. Numerical analysis of the Gran methods A comparative study. Anal. Chim. Acta 606, 172–183 (2008).
Puigdomenech, I. Program MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) (2009).
Movasaghi, Z., Rehman, S. & ur Rehman, D. I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 43, 134–179 (2008).
Stuart, B. Stuart & Barbara. Infrared Spectroscopy. in Kirk-Othmer Encyclopedia of Chemical Technology, https://doi.org/10.1002/0471238961.0914061810151405.a01.pub2 (John Wiley & Sons, Inc., 2005).
Hashimoto, A., Nakanishi, K., Motonaga, Y. & Kameoka, T. Sugar Metabolic Analysis of Suspensions of Plant Cells Using an FT-IR/ATR Method. Biotechnol. Prog. 17, 560–564 (2001).
Hashimoto, A. & Kameoka, T. Mid-Infrared Spectroscopic Determination of Sugar Contents in Plant-Cell Culture Media Using an ATR Method. Appl. Spectrosc. Vol. 54, Issue 7, pp. 1005-1011 54, 1005–1011 (2000).
Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918).
Anastopoulos, I., Bhatnagar, A. & Lima, E. C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 221, 954–962 (2016).
Szabadváry, F. History of analytical chemistry. (Gordon and Breach Science Publishers, 1992).
Sheng, P. X., Ting, Y.-P., Chen, J. P. & Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 275, 131–141 (2004).
Marais, M.-F. & Joseleau, J.-P. A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res. 336, 155–159 (2001).
Silverstein, R., Webster, F. & Kiemle, D. Spectrometric identification of organic compounds (2005).
Sánchez, A. et al. Biosorption of copper and zinc by Cymodocea nodosa. FEMS Microbiol. Rev. 23, 527–536 (1999).
Lodeiro, P., Gudiña, Á., Herrero, L., Herrero, R. & Sastre de Vicente, M. E. Aluminium removal from wastewater by refused beach cast seaweed. Equilibrium and dynamic studies. J. Hazard. Mater. 178, 861–866 (2010).
Calagui, M. J. C. et al. Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J. Hazard. Mater. 277, 120–126 (2014).
Timofeev, K. L., Maltsev, G. I., Usol’tsev, A. V. & Naboichenko, S. S. Sorption technology of recovery of indium from solutions of zinc production. Russ. J. Non-Ferrous Met. 58, 225–230 (2017).
Hassanien, M. M., Mortada, W. I., Kenawy, I. M. & El-Daly, H. Solid Phase Extraction and Preconcentration of Trace Gallium, Indium, and Thallium Using New Modified Amino Silica. Appl. Spectrosc. 71, 288–299 (2017).
Amato, A., Rocchetti, L., Fonti, V., Ruello, M. L. & Beolchini, F. Secondary indium production from end-of-life liquid crystal displays. Phys. Status Solidi Curr. Top. Solid State Phys. 13 (2016).
Rocchetti, L., Amato, A., Fonti, V., Vegliò, F. & Beolchini, F. Innovative method to extract indium from LCD panels. Chemical Engineering Transactions 43 (2015).
Amato, A. et al. Recovery of critical metals from LCDs and Li-ion batteries. in 2016 Electronics Goes Green 2016+, EGG 2016, https://doi.org/10.1109/EGG.2016.7829832 (2017).
Alhakawati, M. S. & Banks, C. J. Removal of copper from aqueous solution by Ascophyllum nodosum immobilised in hydrophilic polyurethane foam. J. Environ. Manage. 72, 195–204 (2004).
Çetinkaya Dönmez, G., Aksu, Z., Öztürk, A. & Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem. 34, 885–892 (1999).
Matheickal, J. T. & Yu, Q. Biosorption of lead(II) and copper(II) from aqueous solutions by pre-treated biomass of Australian marine algae. Bioresour. Technol. 69, 223–229 (1999).
Cechinel, M. A. P. et al. Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chem. Eng. J. 286, 1–15 (2016).
Kiliç, M., Keskin, M. E., Mazlum, S. & Mazlum, N. Effect of conditioning for Pb(II) and Hg(II) biosorption on waste activated sludge. Chem. Eng. Process. Process Intensif. 47, 31–40 (2008).
Pozdniakova, T. A., Mazur, L. P., Boaventura, R. A. R. & Vilar, V. J. P. Brown macro-algae as natural cation exchangers for the treatment of zinc containing wastewaters generated in the galvanizing process. J. Clean. Prod. 119, 38–49 (2015).
Acknowledgements
Acadian Seaplants is thanked for kindly providing samples for evaluation.
Author information
Authors and Affiliations
Contributions
F.B. and C.P. designed research; S.O., C.T., A.T.C., E.G. and C.C., performed research; F.B., C.P., A.A., C.C., E.G. and S.O. analyzed data; and C.P., A.A., F.B. and S.O. wrote the main manuscript text and the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pennesi, C., Amato, A., Occhialini, S. et al. Adsorption of indium by waste biomass of brown alga Ascophyllum nodosum. Sci Rep 9, 16763 (2019). https://doi.org/10.1038/s41598-019-53172-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53172-8
This article is cited by
-
Saved by seaweeds: phyconomic contributions in times of crises
Journal of Applied Phycology (2021)
-
A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis
Journal of Applied Phycology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.