Abstract
The optimum granulocyte colony-stimulating factor (G-CSF) treatment for cancer patients after being treated with cytotoxic chemotherapy remains unknown. Therefore, a systematic review and Bayesian network meta-analysis were performed to assess the efficacy and tolerability of 11 G-CSF drugs on patients after chemotherapy. A total of 73 randomized controlled trials (RCTs) containing 15,124 cancer patients were included for the final network meta-analysis. Compared with pegfilgrastim, there were a higher risk with filgrastim for incidence of febrile neutropenia (FN) (OR [95% CI]: 1.63 [1.07, 2.46]), and a higher risk with short-acting G-CSF (S-G-CSF) biosimilar and lenograstim for incidence of bone pain (BP) (OR [95% CI]: 6.45 [1.10, 65.73], 5.12 [1.14, 26.12], respectively). Mecapegfilgrastim, lipegfilgrastim and balugrastim were best G-CSF drugs in reducing FN (cumulative probabilities: 58%, 15%, 11%, respectively). S-G-CSF biosimilar, empegfilgrastim, and long-acting G-CSF (L-G-CSF) biosimilar were best G-CSF drugs in reducing severe neutropenia (SN) (cumulative probabilities: 21%, 20%, 15%, respectively). Mecapegfilgrastim, balugrastim, lipegfilgrastim and L-G-CSF biosimilar were best G-CSF drugs in reducing BP (cumulative probabilities: 20%, 14%, 8%, 8%, respectively). Mecapegfilgrastim, lipegfilgrastim and balugrastim might be the most appreciate G-CSF drugs with both good efficacy and tolerability when treating cancer patients after cytotoxic chemotherapy.
Similar content being viewed by others
Introduction
Febrile neutropenia (FN) and severe neutropenia (SN) are the most common and serious complications of cancer patients after treatment with cytotoxic chemotherapy1. These complications lead to chemotherapy delay, dose reduction, and increased risk of infection2. Patients with these complications need to be treated with antibiotics and hospitalization3, which indirectly increases the cost for care of these patients4. Furthermore, the condition could deteriorate and lead to death as a result of FN and/or SN after chemotherapy4,5.
Granulocyte colony-stimulating factors (G-CSFs) promote the growth of neutrophils, decrease the incidence of FN and SN, shorten the time of hospital stay, reduce the severity and duration of neutropenia, decrease the risk of infection, and improve the tolerance to cytotoxic chemotherapy6. The guidelines of National Comprehensive Cancer Network (NCCN) recommend primary prophylaxis with G-CSF when the risk of FN associated with chemotherapy regimen is greater than 20%7. Filgrastim was the first short acting G-CSF drug approved for treatment of neutropenia by the United States Food and Drug Administration (FDA) in 1991. Subsequently, a number of new G-CSF drugs have been invented for the treatment of neutropenia worldwide. Long-acting G-CSFs (L-G-CSFs) are PEGylated forms of short-acting G-CSFs (S-G-CSFs) with decreased elimination and increased half-life in serum after subcutaneous injection. Moreover, some of these new G-CSF biosimilar drugs are not as glycosylated as filgrastim8. Since the structure and mechanism of drugs differ, the effect of different G-CSFs remains unclear.
Bone pain (BP) is the most frequent adverse event associated with G-CSF drugs6. Patients might give up treatment due to severe adverse events. The incidence and degree of bone pain after the injection of different G-CSF drugs are diverse9. Although some reviews on the difference of several G-CSF drugs have been reported10,11, these reviews did not include sufficient studies and samples, trials that assessed new G-CSF drugs, or a complete list of G-CSF drugs. The effect of G-CSFs and the optimum choice remains unclear.
Since there is no evidence from head-to-head trials, pairwise meta-analysis for mixed treatment comparisons between multiple medical interventions appears to be impossible. The Bayesian network meta-analysis, which combined direct and indirect evidence to obtain an estimated effect value, has been considered to be a statistical method for mixed multiple trial data comparisons, when a head-to-head trial is not available12. In the present study, a Bayesian network meta-analysis was performed to compare the major 11 G-CSF drugs (balugrastim, empegfilgrastim, filgrastim, S-G-CSF Biosimilar, L-G-CSF Biosimilar, lenograstim, leridistim, lipegfilgrastim, mecapegfilgrastim, pegfilgrastim, and pegteograstim) in terms of efficacy (FN and SN) and tolerability (BP) in the treatment of patients after cytotoxic chemotherapy. This aimed to summarize the direct evidence obtained from the results of randomized controlled trials (RCTs), in order to provide reliable information for guiding clinical treatment decisions.
Results
Inclusion studies
A total of 2,551 potentially relevant articles were identified based on the selection criteria (Fig. 1). After the titles and abstracts were examined, 2,451 literatures that did not meet the criteria were excluded. The full texts of 203 eligible articles were further assessed in detail, and 132 of these were further excluded (Fig. 1). Overall, 70 studies13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 of the 73 RCTs from 1991 to 2018 were included for the final network meta-analysis (Table 1). The assessment of risk of bias indicated low risk of bias among the RCTs (Supplementary Figs S1 and S2). These trials were carried out in 19 countries, and almost half of these clinical trials were conducted in Europe. These trials contained a total of 15,124 cancer patients with 12 kinds of tumors. These 12 types of cancers were breast cancer (BC), lung cancer (LC), gastric cancer (GC), ovarian cancer (OC), head and neck cancer (HNC), colorectal cancer (CRC), germ cell malignancy (GCM), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), lymphoma, sarcoma, and neuroblastoma. These patients were randomly assigned to one of the 12 treatments (11 G-CSF drugs and one placebo group). BC (approximately 42%) was the main disease with the most patients among all kinds of tumors. The additional basic characteristics of all the included studies are presented in Table 1.
Eligible comparisons for the multiple-treatments network meta-analysis were shown in Fig. 2. A total of 66 trials containing 13,770 patients were included in the FN analysis, a total of 41 trials containing 9,298 patients were included in the SN analysis, and a total of 45 trials containing 10,021 patients were included in the BP analysis. Furthermore, 72 RCTs were two-arm trials, while only one RCT was a three-arm trial, which compared S-G-CSF biosimilar, filgrastim and placebo. Moreover, 46 trials respectively contained more than 100 participants, and most of the participants were between 45 and 65 years old.
The network of the Bayesian network meta-analysis. Each node represents the treatment, and the size is proportional to the number of patients included. Each line represents the direct comparisons between treatments, and the width of the line is proportional to the number of randomized controlled trials.
Efficacy and tolerability of G-CSF drugs from pair-wise meta-analysis
A traditional direct pair-wise meta-analysis was performed, as shown in Table 2. The result revealed that filgrastim, pegfilgrastim, lenograstim and mecapegfilgrastim could reduce the incidence of FN (OR [95% CI]: 0.49 [0.38, 0.62]; 0.18 [0.06, 0.56]; 0.47 [0.29, 0.76]; 0.05 [0.00, 0.96]) and SN (OR [95% CI]: 0.29 [0.22, 0.38]; 0.16 [0.06, 0.47]; 0.37 [0.19, 0.72]; 0.30 [0.11, 0.81]) compared with placebo. Furthermore, the OR of mecapegfilgrastim compared with placebo was the lowest, but only one trial was included. The incidence of BP was greater in patients treated with filgrastim, pegfilgrastim, or lenograstim, when compared to placebo (OR [95% CI]: 2.07 [1.08, 3.97]; 1.91 [1.27, 2.87]; 8.31 [4.11, 16.80]). Filgrastim was better than leridistim in terms of reducing the incidence of FN (OR [95% CI]: 0.32 [0.11, 0.90]), but was worse than S-G-CSF biosimilar with regard to the incidence of BP (OR [95% CI]: 0.54 [0.30, 0.99]). Filgrastim was worse than pegfilgrastim in terms of reducing the incidence of FN (OR [95% CI]: 1.46 [1.07, 1.99]). The heterogeneity of these meta-analyses was mostly low or moderate. In the meta-analysis of RCTs that compared pegfilgrastim with placebo, a high heterogeneity was observed with FN (I2 = 89%), SN (I2 = 91%), and BP (I2 = 56%). This heterogeneity might have been introduced by the variation that resulted from the multiple types of tumors, since there were approximately five kinds of tumors in these seven trials. Since the sample size for every specific kind of tumor in these trials containing multiple types of tumors that was too small, it was difficult to implement an effective subgroup analysis. In the sensitivity analysis, no significant heterogeneity change was observed after removing studies from the analysis.
Efficacy and tolerability of G-CSF drugs from network meta-analysis
Figure 3 summarizes the results of the random-effects network meta-analysis for the efficacy of G-CSF drugs based on FN and SN and acceptability, in terms of BP. There was no direct comparison trial of pegteograstim (transverse line indicate no comparison in Fig. 3) on SN, or direct comparison trial of empegfilgrastim, leridistim, and pegteograstim on BP. Pegfilgrastim significantly reduced the incidence of FN, when compared with filgrastim (OR [95% CI]: 1.63 [1.07–2.46]). There was no difference among other drugs in reducing the incidence of FN and SN. Compared with placebo, filgrastim, S-G-CSF biosimilar, lipegfilgrastim and pegfilgrastim significantly (P < 0.05) reduced the incidence of FN and SN, while balugrastim and L-G-CSF biosimilar reduced the incidence of SN. Although the difference was not statistically significant (95% CI contains 1), a reduction in the incidence of FN and SN was observed when empegfilgrastim, lenograstim, leridistim, mecapegfilgrastim, and pegteograstim were compared with placebo. The reason may be because the number of trials included was too small. In terms of the incidence of BP, S-G-CSF biosimilar and lenograstim significantly led to more than pegfilgrastim (OR [95% CI]: 6.45 [1.10–65.73]; 5.12 [1.14–26.12]). The incidence of BP by filgrastim, S-G-CSF biosimilar, lenograstim, and pegfilgrastim was significantly higher than placebo. However, there was no difference between other G-CSF drugs in the incidence of BP. By contrasting direct with indirect evidence using the node-split method, the network analysis did not reveal any statistical inconsistency with regards to FN, SN and BP.
The pooled odds ratios (ORs) for the efficacy (FN and SN) and tolerability (BP) of the 12 treatments. The ORs are the column treatments compared with the row treatments in efficacy (FN and SN), and the row treatments compared with the column treatments in tolerability (BP). The results of efficacy (FN and SN) are in blue and orange, and the results of tolerability (BP) are in green. The first line of efficacy (FN and SN) in blue is the OR of FN, while the second line in orange is the OR of SN. The numbers in bold indicate the significant results. -, not compared.
Comparison of the possibility of efficacy and tolerability of G-CSF drugs
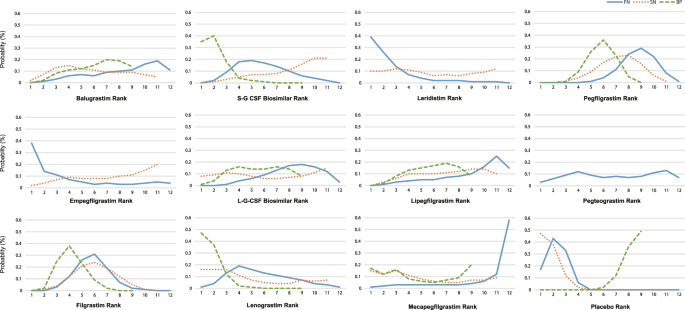
Figure 4 shows the distribution of possibility rank of the 12 treatments in terms of FN, SN, and BP. The higher the probability rank of the 12 treatment, the lower the probability of FN, SN and BP. Mecapegfilgrastim, lipegfilgrastim and balugrastim may be among the three best effective G-CSF drugs that could prevent the incidence of FN (cumulative probabilities: 58%, 15%, and 11%, respectively). S-G-CSF biosimilar, empegfilgrastim, and L-G-CSF biosimilar are possibly among the three more favorable G-CSF drugs that could prevent the occurrence of SN (cumulative probabilities: 21%, 20%, and 15%, respectively). Mecapegfilgrastim, balugrastim, lipegfilgrastim and L-G-CSF biosimilar were ranked as the lowest G-CSF drugs on incidence of BP (cumulative probabilities: 20%, 14%, 8%, and 8%, respectively).
Discussion
In the present network meta-analysis, the efficacy and tolerability of 11 different G-CSF drugs for cancer patients after chemotherapy in 73 RCTs containing 15,124 patients were evaluated using FN, SN and BP as indicators. It was found that pegfilgrastim was better than filgrastim in reducing FN, and more tolerable than S-G-CSF biosimilar and lenograstim in terms of the incidence of BP. In terms of both efficacy and tolerance, mecapegfilgrastim, lipegfilgrastim and balugrastim might be the most efficacious and tolerable among G-CSF drugs.
Since FN is the main and severe adverse event for many chemotherapy regimens, and is intimately associated with chemotherapy-related mortality83, FN was chosen as the primary outcome of the G-CSF drug treatment and a crucial indicator to evaluate the efficacy of G-CSF drugs. In the present study, it was found that compared with placebo, most of the G-CSF drugs could reduce the risk of the incidence of FN, except for empegfilgrastim, leridistim, and pegteograstim. While leridistim might have an opposite effect, although the effect was not statistically significant. The network meta-analysis revealed that there was no difference or inferiority among the tested G-CSF drugs, except for filgrastim and pegfilgrastim in FN (filgrastim vs. pegfilgrastim OR [95% CI]: 1.63 [1.07–2.46]). Filgrastim, pegfilgrastim, lipegfilgrastim and lenograstim reduced the incidence of FN in cancer patients undergoing chemotherapy compared with placebo. Lipegfilgrastim appeared to lead to a greater reduction in the incidence of FN, when compared to pegfilgrastim and filgrastim, although the difference was not statistically significant. These findings were consistent with the previous observations10,84. In accordance with previous reports, pegfilgrastim was more effective than filgrastim in reducing the incidence of FN10,85,86,87,88. SN is also another important evaluation indicator of G-CSF drug efficacy. Filgrastim, pegfilgrastim, lipegfilgrastim, S-G-CSF biosimilar, mecapegfilgrastim, and lenograstim reduced the incidence of SN in patients undergoing myelosuppressive chemotherapy based on direct and indirect evidence. All these results indicate that compared with placebo, most of the tested G-CSF drugs were effective to prevent the incidence of FN and SN.
BP is one of the most common adverse events associated with G-CSF drug treatment89, and is an indicator of G-CSF drug tolerance. Filgrastim (OR [95% CI]: 3.93 [2.07, 8.90]), lenograstim (OR [95% CI]: 11.82 [3.14, 52.88]), pegfilgrastim (OR [95% CI]: 2.32 [1.16, 4.91]) and S-G-CSF biosimilar (OR [95% CI]: 14.84 [2.62, 156.59]) led to a higher incidence of BP, when compared with placebo. Lenograstim (OR [95% CI]: 5.12 [1.14, 26.12]) and S-G-CSF biosimilar (OR [95% CI]: 6.45 [1.10, 65.73)]) led to a much higher incidence of BP than pegfilgrastim. However, the level of incidence of BP widely varied among the RCTs of G-CSF drugs, which might have resulted from the differences in race of patients, stage and type of tumors, chemotherapy regimens, and definition of BP. These results suggest that patients might have different tolerances to different G-CSF drugs.
Even though there was no difference in efficacy among the tested G-CSF drugs and tolerability among patients to these G-CSF drugs in the pair-wise meta-analysis, the comparative ranking of these 12 G-CSF drug treatments suggest that mecapegfilgrastim, lipegfilgrastim and balugrastim might be more effective than leridistim, filgrastim and S-G-CSF biosimilar in preventing the incidence of FN, and S-G-CSF biosimilar, empegfilgrastim and L-G-CSF biosimilar might be more effective than filgrastim and pegfilgrastim in preventing the incidence of SN. In terms of BP, mecapegfilgrastim, balugrastim, lipegfilgrastim and L-G-CSF biosimilar might be more tolerable for patients, when compared to other G-CSF drugs. Those results indicate that mecapegfilgrastim, lipegfilgrastim and balugrastim might be the most efficacious and tolerable G-CSF drugs, and might provide a guideline for the selection of G-CSF-drugs for patients after chemotherapy.
Caution should be taken in interpreting the results, since there might be inconsistencies between the direct and indirect comparisons. These inconsistencies might have resulted from the different characteristics of trials, such as the study design, definition of indicators, inclusion criteria of subjects, and method of implementation, as well as the difference in identifying the external effect on the mean effect of the specific comparison between the network meta-analysis and pair-wise meta-analysis methods90. Although no inconsistency was found in FN, SN and BP through the node-split method in the main network analysis, the direct and indirect meta-analyses revealed contradictory results in terms of the comparisons between filgrastim vs. S-G-CSF biosimilar and filgrastim vs. L-G-CSF biosimilar. This mutually exclusive result could be explained as follows90: (1) if the direct evidence of the pair-wise meta-analysis was true, the comparison between other G-CSF drugs in indirect evidence of the network meta-analysis might overstate or understate the efficacy and tolerance; (2) if the indirect evidence was true, significant intrinsic heterogeneity might exist in the comparison among filgrastim, S-G-CSF biosimilar and L-G-CSF biosimilar. A low or moderate heterogeneity was observed in the pair-wise meta-analysis, indicating that the direct pair-wise meta-analysis was true.
Although the present study is the first network meta-analysis to comprehensively assess clinically and commonly used G-CSF drugs, it should be acknowledged that there were some limitations with the present analysis. First, many factors correlated with neutropenia after chemotherapy were not analyzed, such as the duration of neutropenia, duration of SN, depth of the absolute neutrophil count (ANC) nadir, time to recovery of ANC, FN-related hospitalization, and other toxic or side effects of G-CSF drugs. Second, in most of the included trials, the report for FN, SN and BP was incomplete, which caused some of the G-CSF drugs to be ruled out for comparison in terms of SN and BP. Third, trials on some G-CSF drugs were too few to be assessed. For example, merely one trial on mecapegfilgrastim has been reported to date. Fourth, the definition of BP and other indicators varied among these studies. Furthermore, the dose of G-CSF drugs also varied across the studies. These might be the source of heterogeneity and inconsistency. Fifth, the outcomes might only apply to developed countries, since some G-CSF drugs are not available on the market in many developing countries.
In summary, based on the present network meta-analysis, evidence suggests that compared with placebo, most of the tested G-CSF drugs are not different in terms of efficacy and tolerability, except for pegfilgrastim, which is more effective than filgrastim in reducing FN. Furthermore, pegfilgrastim is more tolerable for patients, when compared to S-G-CSF biosimilar and lenograstim, in terms of BP. Mecapegfilgrastim, lipegfilgrastim and balugrastim might be the most appreciate G-CSF drugs, which have both better efficacy and tolerance. It is noteworthy that more large-scale RCTs would be required to further confirm the efficacy and tolerance of the G-CSF drugs observed in the present study. The benefit-risk ratio of these G-CSF drugs still deserves to be further explored.
Methods
Search strategies and selection criteria
A network meta-analysis was performed following the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines84 and PRISMA network meta-analysis extension statement91. RCTs on 11 G-CSF drugs (balugrastim, empegfilgrastim, filgrastim, S-G-CSF Biosimilar, L-G-CSF Biosimilar, lenograstim, leridistim, lipegfilgrastim, mecapegfilgrastim, pegfilgrastim, and pegteograstim) for cancer patients after cytotoxic chemotherapy were searched in PubMed, Embase, Cochrane Library, Cochrane Collaboration Central Register of Controlled Clinical Trials, American Society of Clinical Oncology, and ClinicalTrials.gov up to the 8th of October 2018, without language restrictions. The terms included “balugrastim”, “empegfilgrastim”, “filgrastim”, “Neupogen”, “G-CSF biosimilar”, “lenograstim”, “leridistim”, “lipegfilgrastim”, “mecapegfilgrastim”, “pegfilgrastim”, “Neulasta”, “pegteograstim”, “GCPGC”, “rhG-CSF”, “PEG-rhG-CSF” and “Pegylated Recombinant Human Granulocyte Colony Stimulating Factor” (Detailed terms can be found in supplementary appendix. S3). The reference lists of the relevant retrieved articles and reviews were also manually searched.
RCTs that compared at least two different G-CSF drugs (placebo-controlled included) in all kinds of cancer after chemotherapy were included. These trials should report the data on FN, SN, and/or BP in cancer patients after the use of G-CSF drugs. Non-randomized controlled trials, non-interventional studies, retrospective studies, or trials that contained only one treatment (single-arm) were excluded. Furthermore, studies that included healthy volunteers, but not cancer patients who received chemotherapy, were also excluded.
Study selection and data extraction
Study selection, data extraction and review, and quality assessment were independently performed by two authors (Y. Wang and L. Chen), according to the predefined criteria from eligible studies. The Cochrane Collaboration’s tool for assessing risk of bias92 was independently used for the quality assessment and evaluation of risk of bias by the same authors. The key characteristics of each study were recorded, which included: the first author’s name and year of publication, country, study design, patient characteristics, chemotherapy regimens, dose and protocol of treatment, and outcomes (FN, SN and BP). All data for the study characteristics and clinical responses were summarized in a structured table to ensure consistency. All the disagreements were resolved by discussion and consensus with a third author (Y. Li).
Outcome measurements
The incidence of FN after cytotoxic chemotherapy within two weeks was taken as the primary indicator of efficacy of G-CSF drugs, the incidence of SN was taken as the secondary indicator of G-CSF drug efficacy, and BP was taken as the primary indicator for the tolerability of G-CSF drugs. FN was defined as an absolute neutrophil count (ANC) of <0.5 or 1.0 × 109/L, with an oral temperature of ≥38.0 °C. SN was defined as ANC < 0.5 or 1.0 × 109/L. If both data of both grade 3 and 4 bone marrow suppression (ANC < 0.5 and 1.0 × 109/L) were reported in a study, the data of the ANC < 0.5 × 109/L was used with priority for analysis, because grade 3 had lesser clinical significance, and was not always reported in the included studies.
Statistical analyses
Pair-wise meta-analysis was carried out for FN, SN and BP to compare the corresponding interventions. The random effects model for pair-wise meta-analysis was used to account for the heterogeneity. The heterogeneity among different trials was estimated by Cochran’s Q-test (P < 0.05 indicated significant heterogeneity) and I2 statistic. If I2 = 0–25%, it is designated as low heterogeneity, if I2 = 25–50%, this was designated as moderate heterogeneity, if I2 = 50–75%, this was designated as high heterogeneity, and if I2 = 75–100%, this was designated as extremely high heterogeneity. According to the Cochrane handbook, heterogeneity can be accepted when I2 ≤ 50%93. Pair-wise meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and STATA 12.0 (Stata Corporation, TX, USA) statistical software.
Random-effects models were applied for the network meta-analysis. Bayesian network meta-analysis was used to combine the collected data. The Bayesian network meta-analysis was performed with WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). Random effects models were used to incorporate the effects from different studies, while heterogeneity within the comparison was evaluated in a relatively conservative and appropriate manner94. The models were performed using the Markov Chain Monte Carlo simulation. The initial values were set for three different chains, 150,000 interactions with 5,000 burn-in samples were produced to obtain the model parameters from the posterior distributions, and 50 thinning rates were adopted for each chain. The odds ratios (ORs) were collected or calculated from combing the direct evidence, and the significance was assessed by P < 0.05, or the 95% confidence interval (CI) did not contain 1. The best efficacious and tolerant regimen was confirmed by ranking the included G-CSF drugs according to the OR for each G-CSF drug compared with placebo, and assessing the probability. Inconsistencies in the present study were assessed by comparing the direct evidence with indirect evidence from the network meta-analysis using the node-split method95.
A sensitivity analysis was performed by determining whether there was statistically significant heterogeneity in the meta-analysis after studies were randomly removed from the others.
References
Crawford, J. et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 6, 109–118 (2008).
Rossi, L. et al. Efficacy and safety analysis of once per cycle pegfilgrastim and daily lenograstim in patients with breast cancer receiving adjuvant myelosuppressive chemotherapy FEC 100: a pilot study. Ther Clin Risk Manag. 9, 457–462 (2013).
Pettengell, R., Schwenkglenks, M. & Bosly, A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 87, 429–430 (2008).
Kuderer, N. M., Dale, D. C., Crawford, J., Cosler, L. E. & Lyman, G. H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 106, 2258–2266 (2006).
Smith, T. J. et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 24, 3187–3205 (2006).
Kuderer, N. M., Dale, D. C., Crawford, J. & Lyman, G. H. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 25, 3158–3167 (2007).
Network NCC. NCCN® Clinical Practice Guidelines in Oncology:Myeloid Growth Factors, version 1.2016. Fort Washington, PA: National Comprehensive Cancer Network https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed 25.07.16 (2016).
Gascon, P. et al. Development of a new G-CSF product based on biosimilarity assessment. Ann Oncol. 21, 1419–1429 (2010).
Kuan, J. W., Su, A. T. & Leong, C. F. Pegylated granulocyte-colony stimulating factor versus non-pegylated granulocyte-colony stimulating factor for peripheral blood stem cell mobilization: A systematic review and meta-analysis. J Clin Apher. 32, 517–542 (2017).
Bond, T. C. et al. Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events. J Oncol Pharm Pract. 24, 412–423 (2018).
Lyman, G. H. et al. The effectiveness and safety of same-day versus next-day administration of long-acting granulocyte colony-stimulating factors for the prophylaxis of chemotherapy-induced neutropenia: a systematic review. Support Care Cancer. 25, 2619–2629 (2017).
Caldwell, D. M., Ades, A. E. & Higgins, J. P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj. 331, 897–900 (2005).
Crawford, J. et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther. 3, 36–46 (2005).
Fossa, S. D. et al. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy. European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J Clin Oncol. 16, 716–724 (1998).
Dunlop, D. J. et al. Randomized multicentre trial of filgrastim as an adjunct to combination chemotherapy for Hodgkin’s disease. West of Scotland Lymphoma Group. Clin Oncol (R Coll Radiol). 10, 107–114 (1998).
Geissler, K. et al. Granulocyte colony-stimulating factor as an adjunct to induction chemotherapy for adult acute lymphoblastic leukemia–a randomized phase-III study. Blood. 90, 590–596 (1997).
Pinter, T. et al. A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Pegfilgrastim in Patients Receiving First-Line FOLFOX/Bevacizumab or FOLFIRI/Bevacizumab for Locally Advanced or Metastatic Colorectal Cancer: Final Results of the Pegfilgrastim and Anti-VEGF Evaluation Study (PAVES). Clin Colorectal Cancer. 16, 103–114 (2017).
Kubo, K. et al. A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol. 174, 563–570 (2016).
Zhang, W., Jiang, Z., Wang, L., Li, C. & Xia, J. An open-label, randomized, multicenter dose-finding study of once-per-cycle pegfilgrastim versus daily filgrastim in Chinese breast cancer patients receiving TAC chemotherapy. Med Oncol. 32, 147 (2015).
Kosaka, Y. et al. Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer. 23, 1137–1143 (2015).
Shi, Y. K. et al. Pegylated filgrastim is comparable with filgrastim as support for commonly used chemotherapy regimens: a multicenter, randomized, crossover phase 3 study. Anticancer Drugs. 24, 641–647 (2013).
Hecht, J. R. et al. A randomized, placebo-controlled phase ii study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer. 9, 95–101 (2010).
Fox, E. et al. Randomized trial and pharmacokinetic study of pegfilgrastim versus filgrastim after dose-intensive chemotherapy in young adults and children with sarcomas. Clin Cancer Res. 15, 7361–7367 (2009).
Sierra, J. et al. A single dose of pegfilgrastim compared with daily filgrastim for supporting neutrophil recovery in patients treated for low-to-intermediate risk acute myeloid leukemia: results from a randomized, double-blind, phase 2 trial. BMC Cancer. 8, 195 (2008).
Vogel, C. L. et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 23, 1178–1184 (2005).
Grigg, A. et al. Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 44, 1503–1508 (2003).
Vose, J. M. et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol. 21, 514–519 (2003).
Green, M. D. et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 14, 29–35 (2003).
Holmes, F. A. et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 20, 727–731 (2002).
Holmes, F. A. et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 13, 903–909 (2002).
Zhou, C. et al. A Randomized Multicenter Phase III Study of Single Administration of Mecapegfilgrastim (HHPG-19K), a Pegfilgrastim Biosimilar, for Prophylaxis of Chemotherapy-Induced Neutropenia in Patients With Advanced Non-Small-Cell Lung Cancer (NSCLC). Clin Lung Cancer. 17, 119–127 (2016).
Volovat, C. et al. Phase III, randomized, double-blind, placebo-controlled, multicenter study of lipegfilgrastim in patients with non-small cell lung cancer receiving myelosuppressive therapy. Springerplus. 4, 316 (2015).
Buchner, A., Elsasser, R. & Bias, P. A randomized, double-blind, active control, multicenter, dose-finding study of lipegfilgrastim (XM22) in breast cancer patients receiving myelosuppressive therapy. Breast Cancer Res Treat. 148, 107–116 (2014).
Bondarenko, I., Gladkov, O. A., Elsaesser, R., Buchner, A. & Bias, P. Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer. 13, 386 (2013).
Gladkov, O. et al. A Phase III Study of Balugrastim Versus Pegfilgrastim in Breast Cancer Patients Receiving Chemotherapy With Doxorubicin and Docetaxel. Oncologist. 21, 7–15 (2016).
Volovat, C. et al. Efficacy and safety of balugrastim compared with pegfilgrastim in patients with breast cancer receiving chemotherapy. Clin Breast Cancer. 14, 101–108 (2014).
Lee, K. H. et al. A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy-induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10-09. Support Care Cancer. 24, 1709–1717 (2016).
Xu, B. et al. A multicenter, randomized, controlled, phase clinical study of PEG-rhG-CSF for preventing chemotherapy-induced neutropenia in patients with breast cancer and non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 38, 23–27 (2016).
Xie, J. et al. Advantages with prophylactic PEG-rhG-CSF versus rhG-CSF in breast cancer patients receiving multiple cycles of myelosuppressive chemotherapy: an open-label, randomized, multicenter phase III study. Breast Cancer Res Treat. 168, 389–399 (2018).
Blackwell, K. et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 26, 1948–1953 (2015).
Park, K. H. et al. A randomized, multi-center, open-label, phase III study of once-per-cycle DA-3031, a pegylated G-CSF, in comparison with daily filgrastim in patients receiving TAC chemotherapy for breast cancer. Support Care Cancer. 25, 505–511 (2017).
Park, K. H. et al. A randomized, multi-center, open-label, phase II study of once-per-cycle DA-3031, a biosimilar pegylated G-CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early-stage breast cancer. Invest New Drugs. 31, 1300–1306 (2013).
Hegg, R. et al. A phase III, randomized, non-inferiority study comparing the efficacy and safety of biosimilar filgrastim versus originator filgrastim for chemotherapy-induced neutropenia in breast cancer patients. Clinics (Sao Paulo). 71, 586–592 (2016).
Blackwell, K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist. 21, 789–794 (2016).
Harbeck, N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol. 12, 1359–1367 (2016).
Waller, C. F. et al. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 33, 504–511 (2010).
Gatzemeier, U. et al. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 4, 736–740 (2009).
Engert, A., Griskevicius, L., Zyuzgin, Y., Lubenau, H. & del Giglio, A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 50, 374–379 (2009).
del Giglio, A., Eniu, A., Ganea-Motan, D., Topuzov, E. & Lubenau, H. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 8, 332 (2008).
Gisselbrecht, C. et al. Placebo-controlled phase III study of lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin’s lymphoma: factors influencing chemotherapy administration. Groupe d’Etude des Lymphomes de l’Adulte. Leuk Lymphoma. 25, 289–300 (1997).
Bui, B. N. et al. Efficacy of lenograstim on hematologic tolerance to MAID chemotherapy in patients with advanced soft tissue sarcoma and consequences on treatment dose-intensity. J Clin Oncol. 13, 2629–2636 (1995).
Nabholtz, J. M. et al. Phase III trial comparing granulocyte colony-stimulating factor to leridistim in the prevention of neutropenic complications in breast cancer patients treated with docetaxel/doxorubicin/cyclophosphamide: results of the BCIRG 004 trial. Clin Breast Cancer. 3, 268–275 (2002).
Welte, K. et al. A randomized phase-III study of the efficacy of granulocyte colony-stimulating factor in children with high-risk acute lymphoblastic leukemia. Berlin-Frankfurt-Munster Study Group. Blood. 87, 3143–3150 (1996).
Pettengell, R. et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trial. Blood. 80, 1430–1436 (1992).
Johnston, E. et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol. 18, 2522–2528 (2000).
Timmer-Bonte, J. N. et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch Randomized Phase III Study. J Clin Oncol. 23, 7974–7984 (2005).
Crawford, J. et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 325, 164–170 (1991).
Osby, E. et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood. 101, 3840–3848 (2003).
Trillet-Lenoir, V. et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 29, 319–324 (1993).
Zinzani, P. L. et al. Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin’s lymphoma. Blood. 89, 3974–3979 (1997).
von Minckwitz, G. et al. Pegfilgrastim +/− ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol. 19, 292–298 (2008).
Balducci, L. et al. Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist. 12, 1416–1424 (2007).
Doorduijn, J. K. et al. CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 21, 3041–3050 (2003).
Chevallier, B. et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. J Clin Oncol. 13, 1564–1571 (1995).
Gebbia, V. et al. A prospective evaluation of the activity of human granulocyte-colony stimulating factor on the prevention of chemotherapy-related neutropenia in patients with advanced carcinoma. J Chemother. 5, 186–190 (1993).
Romieu, G. et al. Pegfilgrastim supports delivery of FEC-100 chemotherapy in elderly patients with high risk breast cancer: a randomized phase 2 trial. Crit Rev Oncol Hematol 64, 64–72 (2007).
Bozzoli, V. et al. Four doses of unpegylated versus one dose of pegylated filgrastim as supportive therapy in R-CHOP-14 for elderly patients with diffuse large B-cell lymphoma. Br J Haematol. 169, 787–794 (2015).
Filon, O. et al. Efficacy and safety of empegfilgrastim, a novel pegylated G-CSF: results of complete analysis after 4 cycles of myelosuppressive chemotherapy in phase III double-dummy randomized clinical study. J Clin Oncol. 33, e20735 (2015).
Salafet, O. V. et al. Efficacy and safety of BCD-017, a novel pegylated filgrastim: Results of open-label controlled phase II study in patients with breast cancer receiving myelosuppressive chemotherapy. Journal of Clinical Oncology. 31, e20593 (2013).
Satheesh, C. T. et al. To analyze efficacy and safety of pegfilgrastim versus filgrastim in patients with breast cancer. J Clin Oncol. 27, e20587 (2009).
Glaspy, J., Tang, T. & Rutty, D. A Phase II, Randomized, Multi-Centre, Open-Label, ActiveControlled, Dose-Finding Trial of F-627 (benefilgrastim) in Women with Breast Cancer Receiving Myelotoxic Chemotherapy. Presented at: 56th Annual Meeting of American Society of Hematology. poster 1584 (2014).
Usuki, K. et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol. 116, 103–112 (2002).
Desai, K., Misra, P., Kher, S. & Shah, N. Clinical confirmation to demonstrate similarity for a biosimilar pegfilgrastim: a 3-way randomized equivalence study for a proposed biosimilar pegfilgrastim versus US-licensed and EU-approved reference products in breast cancer patients receiving myelosuppressive chemotherapy. Exp Hematol Oncol. 7, 22 (2018).
Ottmann, O. G. et al. Concomitant granulocyte colony-stimulating factor and induction chemoradiotherapy in adult acute lymphoblastic leukemia: a randomized phase III trial. Blood. 86, 444–450 (1995).
Bondarenko, I. M., Bias, P. & Buchner, A. Incidence of bone pain in patients with breast cancer treated with lipegfilgrastim or pegfilgrastim: an integrated analysis from phase II and III studies. Support Care Cancer. 24, 267–273 (2016).
Godwin, J. E. et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood. 91, 3607–3615 (1998).
Gladkov, O. et al. Phase II dose-finding study of balugrastim in breast cancer patients receiving myelosuppressive chemotherapy. Med Oncol. 32, 623 (2015).
Michon, J. M. et al. An open-label, multicentre, randomised phase 2 study of recombinant human granulocyte colony-stimulating factor (filgrastim) as an adjunct to combination chemotherapy in paediatric patients with metastatic neuroblastoma. Eur J Cancer. 34, 1063–1069 (1998).
Maher, D. W. et al. Filgrastim in patients with chemotherapy-induced febrile neutropenia. A double-blind, placebo-controlled trial. Ann Intern Med. 121, 492–501 (1994).
Gatzemeier, U. et al. Lenograstim as support for ACE chemotherapy of small-cell lung cancer: a phase III, multicenter, randomized study. Am J Clin Oncol. 23, 393–400 (2000).
Seymour, A. M. et al. A single-blind, randomised, vehicle-controlled dose-finding study of recombinant human granulocyte colony-stimulating factor (lenograstim) in patients undergoing chemotherapy for solid cancers and lymphoma. Eur J Cancer. 31, 2157–2163 (1995).
Muhonen, T. et al. Prophylactic filgrastim (G-CSF) during mitomycin-C, mitoxantrone, and methotrexate (MMM) treatment for metastatic breast cancer. A randomized study. Am J Clin Oncol. 19, 232–234 (1996).
Freifeld, A. G. et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 52, 56–93 (2011).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 339, 2535 (2009).
Madan, J. et al. Consistency between direct and indirect trial evidence: is direct evidence always more reliable? Value Health. 14, 953–960 (2011).
Pinto, L. et al. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 23, 2283–2295 (2007).
Pfeil, A. M. et al. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer. 23, 525–545 (2015).
Cooper, K. L., Madan, J., Whyte, S., Stevenson, M. D. & Akehurst, R. L. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 11, 404 (2011).
Abboud, C. N. et al. Real-world safety experience of tevagrastim/ratiograstim/biograstim and tbo-filgrastim, short-acting recombinant human granulocyte colony-stimulating factors. Support Care Cancer. 27, 2569–2577 (2019).
Giacoppo, D. et al. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. Bmj. 351, 5392 (2015).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 162, 777–784 (2015).
Higgins, J. P. & Altman, D. G. Assessing risk of bias in included studies. In Higgins, J. P. & Green, S., eds Cochrane handbook for systematic reviews of interventions: Cochrane book series. Chichester: John Wiley & Sons. 187–241 (2008).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj. 327, 557–560 (2003).
Mills, E. J., Thorlund, K. & Ioannidis, J. P. Demystifying trial networks and network meta-analysis. Bmj. 346, 2914 (2013).
Dias, S., Welton, N. J., Caldwell, D. M. & Ades, A. E. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 29, 932–944 (2010).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81560379, 81460292), Jiangxi Natural Science Foundation (20181BAB205046). The funders did not participate in the study design, data collection, analysis and decision to publish.
Author information
Authors and Affiliations
Contributions
Y.W., L.C. and Y.L. contributed to the design of the study, data collection, statistical analysis, and literature evaluation. Y. Wang wrote the main manuscript text. Y. Wang, L.C., F.L., N.Z., L.X., B.F. prepared figures and tables. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Chen, L., Liu, F. et al. Efficacy and tolerability of granulocyte colony-stimulating factors in cancer patients after chemotherapy: A systematic review and Bayesian network meta-analysis. Sci Rep 9, 15374 (2019). https://doi.org/10.1038/s41598-019-51982-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51982-4
This article is cited by
-
A comparative assessment of neutropenia events, healthcare resource use, and costs among cancer patients treated with lipegfilgrastim compared with pegfilgrastim in Germany
Supportive Care in Cancer (2022)
-
Phase I/II study to assess the clinical pharmacology and safety of single ascending and multiple subcutaneous doses of PF-06881894 in women with non-distantly metastatic breast cancer
Cancer Chemotherapy and Pharmacology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.