Abstract
Following liver transplantation (LT), 10–30% of patients develop recurrent cirrhosis (RC). There is an urgent need for predictive non-invasive markers for improved monitoring of these patients. Here we studied extracellular matrix biomarkers as predictors of RC after LT. Forty-seven LT patients were divided into groups of fast, intermediate or non-progressors towards RC (<1 year, 3–5 years or no advanced fibrosis >5 years after LT), assessed by follow-up liver biopsies. Markers of interstitial matrix type III and V collagen formation (PRO-C3 and PRO-C5), basement membrane type IV collagen formation (PRO-C4) and degradation (C4M) were assessed in serum samples collected 3, 6 and 12 months post-LT using specific ELISAs. PRO-C3, PRO-C4, and C4M were elevated in fast progressors compared to non-progressors 3 months after LT. C4M and PRO-C4 additionally differentiated between intermediate and fast progressors at 3 months. PRO-C3 was best predictor of survival, with LT patients in the highest PRO-C3 tertile having significantly shorter survival time. This shows that interstitial matrix and basement membrane remodeling in RC may be distinguishable. Markers originating from different sites in the extracellular matrix could be valuable tools for a more dynamic monitoring of patients at risk of RC. However, this needs validation in larger cohorts.
Similar content being viewed by others
Introduction
Liver transplantation (LT) is the last therapeutic option for patients with end-stage liver disease. Until recently, before the advent of highly effective antiviral therapies, recurrent hepatitis C infection has been the most common cause of rapid post-LT fibrosis progression1, graft loss and mortality within 1 to 10 years after LT2. Other liver diseases also recur after transplantation with incidence rates ranging from 10% to 50% including alcoholic liver disease (ALD)3, primary sclerosing cholangitis (PSC)4, primary biliary cholangitis (PBC)4, autoimmune hepatitis (AIH)4 and non-alcoholic steatohepatitis (NASH)5. Fibrosis is the result of accelerated accumulation of extracellular matrix (ECM) proteins, in particular interstitial types I, III, and V collagens that increase up to 6-fold in advanced liver fibrosis6. The prominent basement membrane type IV collagen is also prone to substantial remodeling especially during early liver fibrosis and can be increased up to 10-fold7. It has been suggested that basement membrane remodeling, as seen in fibrosis, is largely driven by liver epithelial cells in an attempt to regenerate the ECM as an initial repair response8,9.
By assessing specific fragments of collagens generated by proteases, it should be possible to separate tissue formation vs degradation. Hence, we have developed a panel of serological biomarkers using the Protein Fingerprint Technology™, to quantify the tissue balance. Combining disease relevant proteases and up-regulated proteins of fibrogenesis, results in generation of a fingerprint specific for the affected tissue. The (pro-)collagen fragments are released from the tissue into the circulation, where they can be identified by neo-epitope specific ELISAs to permit evaluation of the ECM remodeling during liver fibrosis, and potentially serve as prognostic biomarkers for progression to cirrhosis10. Measurements of these neo-epitopes have previously proved to be more sensitive and accurate than routinely used diagnostic and prognostic tools11,12,13.
Type III and type V collagens are important components of the reticular fibers generated by (myo-)fibroblasts in the interstitial matrix14, which is the main local area affected by inflammation15. Maturation of type III and V collagen includes cleaving off the N- and C-terminal pro-peptide by specific proteases14. In fibrotic liver diseases, release of these pro-peptides, i.e. PRO-C3 and PRO-C5, can be highly increased13,16,17.
We have previously suggested that the marker of central tetrameric crosslinking domain of type IV collagen, PRO-C4, reflects enhanced basement membrane synthesis and turnover during accelerated ECM remodeling in liver fibrosis8,18,19. Furthermore, a marker of matrix metalloproteinase (MMP)-mediated type IV collagen degradation, C4M, reflects unfavorable basement membrane degradation20. Together these two markers may serve as a tool for monitoring unfavorable basement membrane turnover in liver fibrogenesis19.
Development and progression of recurrent liver fibrosis may follow different paths dependent on the underlying etiology. Even though the etiologies are different, the end-stage disease is comparable, i.e. advanced fibrotic and architectural remodeling in the transplanted liver, leading to graft loss and death. Early identification of patients at risk of rapid liver fibrosis progression could enable life-saving interventions with timely intervention, e.g., changes in immunosuppressive regimens or even antifibrotic agents that are in development21,22. Here we investigated the prognostic utility of four Protein Fingerprint serological biomarkers.
Results
Demographics are presented in Table 1. Eleven patients developed cirrhosis within 1 year after LT and 19 within 3–5 years. Another 17 patients showed no or mild fibrosis over the first 5 years post-LT.
Most patients were male with similar mean age (p = 0.218). Mean survival time after transplantation was 5 years in the fast progressor group, whereas patients with no or mild signs of fibrosis after 5 years had a mean survival time of 13 years (p < 0.001); data not shown. At the end of the study period, only 18% of patients in the fast progressor group were alive compared with 32% in the intermediate progressor group, and 88% in the non-progressor group.
At baseline (3 months post-LT), serum levels of PRO-C3, PRO-C4 and C4M were significantly elevated in fast progressors compared to non-progressors (p < 0.05–p < 0.01) (Fig. 1A–C). No difference was observed for PRO-C5 (Fig. 1D). Interestingly, markers of type IV collagen formation and degradation (PRO-C4 and C4M), in contrast to markers of type III and V collagen formation (PRO-C3 and PRO-C5) could additionally distinguishing between the two progressor groups, p < 0.05 and p < 0.01, respectively (Fig. 1A,B).
In addition, serum levels of PRO-C4 and C4M remained elevated at 6 months post-LT, in fast progressors compared to non-progressors (p < 0.001) (Fig. 2A,B) thus continuing the trend of increased biomarker levels. PRO-C5 levels at 6 months post-LT were similarly found to be increased in fast- compared to non-progressors (p < 0.05), whereas no difference was found for PRO-C3 (Fig. 2C,D). When investigating the association between clinical parameters and biomarkers one year after transplantation, tissue inhibitors of metalloproteinase-1 (TIMP-1) was significantly correlated to PRO-C3, PRO-C4, PRO-C5 and C4M. In addition, bilirubin, and ELF, including all three components, were significantly correlated to PRO-C3 (Supplementary Table 2).
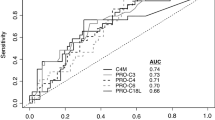
ROC curve for separating fast- from non-progressors further confirmed PRO-C4, C4M, and PRO-C3 as early predictors of fast progressors. No significant ROC curve was found for PRO-C5 (Fig. 3A).
Predictive value of PRO-C4, C4M, PRO-C3 and PRO-C5. (A) ROC curve discriminating non- from fast-progressors towards cirrhosis. N = 11–13 per group. (B) Survival of patients with progression towards cirrhosis after transplantation. All patients were divided into two groups according to serum levels of PRO-C3. Baseline values of PRO-C3 < 20.7 (N = 13), and PRO-C3 ≥ 20.7 (N = 27) were compared as predictors of survival.
Additionally, AUROC analyses found PRO-C4 and C4M to be the best markers for detecting fast progressors towards cirrhosis from non-progressors at baseline with AUCs of 0.90 [95% CI 0.7–0.9] and 0.88 [95% CI 0.6–0.9], and specificities >80% (Table 2). PRO-C3 predicted fast progressors with AUC = 0.79 [95% CI 0.5–0.9], sensitivity of 100%, and with specificity of 54%. PRO-C5 performed only moderate with AUC = 0.67 [95% CI 0.4–0.8], specificity of 69% and sensitivity of 72%. When separating intermediate and fast progressors, PRO-C4 and C4M were superior to PRO-C3 and PRO-C5 with AUCs > 0.85 [95% CI 0.6–0.9] and specificity of 75% and 83% respectively and sensitivity of 91% for both. However, none of the markers could distinguish between intermediate and non-progressors.
To evaluate the prognostic utility of the markers, the patients were stratified into tertiles in accordance to the baseline level of each marker. Baseline levels of C4M and PRO-C4 were able to predict progression towards cirrhosis after transplantation. Patients in the highest C4M tertile (T3) had a 21-fold [95% CI 1.5–293.2] higher risk of recurrent cirrhosis compared with the patients in the lowest tertile (T1) (p < 0.05). Similarly, baseline PRO-C4 in patients of T3 predicted progression with an OR of 85 [95% CI 3–2417] compared with T1 (p < 0.01) (Table 3).
In multivariate Cox regression analysis of baseline measurements, PRO-C3 was independently associated with survival. Kaplan-Meier analysis, using a cut-off value = 20.7 ng/mL defined from the Youden index that was derived from ROC curve analysis, was used to investigate the association of PRO-C3 and survival. Mean survival was significantly shorter in patients with high PRO-C3 levels compared to patients with low PRO-C3 levels (Mean age 9.3 vs 13.1 years, respectively, p < 0.05) (Fig. 3B).
Discussion
Rapid recurrent cirrhosis after LT occurs in subjects with viral and non-viral etiologies of end-stage liver disease1,3,4,5. Despite advances in surgical techniques and periprocedural medical therapy, including effective therapies to eliminate HCV infection, there remains a high risk due to immune suppression and/or recurrent non-HCV liver disease1,5. Thus, the need to noninvasively monitor patients with risk of progression after LT is an urgent need as repeated biopsies is associated with patient discomfort and complications, and do not reflect the molecular dynamics of fibrosis progression21,22,23. Here we investigated the prognostic utility of four defined ECM remodeling markers as non-invasive tools to identify patients at risk of rapid liver fibrosis progression. Irrespective of progressor group, the cause of initial fibrosis and cirrhosis was either viral or non-viral, supporting that RC occurs in non-viral LT patients. The main findings of the study were: (1) Three months post-LT, PRO-C4, C4M and PRO-C3 differentiated between patients with fast progression towards RC vs non-progression; (2) PRO-C4 and C4M could discriminate intermediate progressors from fast progressors towards RC; and (3) PRO-C3 was independent predictor of post-LT survival.
Elevated serum levels three months post-LT in fast progressors indicate that ECM turnover can be detected earlier by the serological biomarkers before histological evaluation show advanced fibrosis or cirrhosis, thus enabling identification of a fast fibrosis progression endotype and early recognition of patients in most need of regular monitoring and possibly urgent treatment.
Some patients are more prone to fibrosis progression than others after LT, and apart from some early clinical indicators such as repetitive severe rejection episodes, predictive markers are lacking24. Interestingly, markers associated with formation and degradation of basement membrane collagen (PRO-C4 and C4M, respectively) and interstitial collagen formation (PRO-C3) were elevated already 3 months after transplantation, whereas a marker of type V collagen formation, PRO-C5, related to interstitial collagen fibril formation seemed to increase only with a delay in the fast progressors. This could indicate that unfavorable liver collagen turnover and unbalanced remodeling are operative immediately after transplantation due to factors related to the host, donor, or surgical procedure25, whereas other specialized ECM structures (e.g. type V procollagen) are established later, possible also due to co-morbidities associated with end-stage liver disease, regardless of primary cause of chronic liver disease.
PRO-C3 was not only independent predictor of RC, but patients with high baseline PRO-C3 levels had significantly shorter survival time after transplantation than patients with lower PRO-C3 levels, indicating that PRO-C3 reflects both higher risk of RC and survival. This has important clinical implications since it has potential to provide early diagnostic and prognostic information and thus help guide clinical decision-making and early institution of potential antifibrotic therapies1,21,22. Moreover, by using serum markers, the efficacy of antifibrotic intervention with possible positive effects in the immunosuppressive regimen, may be detectable after only a few days or weeks. However, these results need to be validated in larger intervention cohorts.
With this study, we showed that patients with high type IV collagen turnover after LT had a up to 85-fold increased risk of RC compared to patients with low levels. The type IV collagen formation marker PRO-C4 was best at separating non- from fast-progressors towards RC with an AUC = 0.90 with sensitivity and specificity of 72% and 92%, respectively. PRO-C4 has previously been shown to be associated with liver fibrosis18 but this is the first study showing its prognostic potential. The robustness of PRO-C4 and C4M as prognostic markers of clinical outcome needs to be investigated in larger cohorts.
PRO-C3 has previously been shown to be a useful prognostic marker of fibrosis progression12. In a cohort of chronic hepatitis C patients, we found that high PRO-C3 baseline levels were associated with higher risk of fibrosis progression within 52 weeks. This further strengthens the clinical utility of PRO-C3 as a robust prognostic marker for liver fibrosis progression in both non-transplanted and transplanted patients.
It is well established that the collagen content is up to 10-fold higher in a cirrhotic than in a healthy liver6,7. Local tissue inflammation and damage to the epithelium result in remodeling of the underlying basement membrane19, monitored by type IV collagen biomarkers PRO-C4 and C4M. In the event of chronic inflammation, damage to the interstitial matrix occurs with subsequent remodeling. To replace the damaged tissue, excessive amount of interstitial types I, III and V collagens are produced by fibroblasts. The lower prognostic value of the interstitial collagen synthesis markers PRO-C3 and PRO-C5 at baseline, may reflect the fact that remodeling and excessive deposition of interstitial matrix occurs at later stages of liver fibrosis. In addition, it is notably that levels of ALT did not predict RC in this study, suggesting that the nature of RC might be repair more than inflammation. This confirms previous findings that fibrosis is not just fibrosis19.
The use of non-invasive diagnostic techniques, like transient elastography (Fibroscan®), has been widely accepted as alternative to biopsy to assess liver fibrosis in non-transplant patients26,27,28. TE might also be useful for monitoring post-LT patients as imaging techniques are unaffected by post-operative biochemical alterations. However, in post-LT settings TE may be affected by postoperative inflammatory reactions, rejection episodes, both of which are associated with swelling and edema that increase stiffness, hematoma, and bile duct or vascular complications29. Performance of TE might furthermore be comprised by the presence of ascites or obesity30.
It is recognized that the sample size is relatively small, which is due to the limitation of the availability of follow-up samples in this unique patient cohort. Development of advanced fibrosis and cirrhosis within 1 year is a rare event, thus limiting number of patients in the study. Nonetheless, the data provides clear results as to prediction of rapid vs intermediate vs no fibrosis progression in these patients. In this sense, this study is intended to provide the first proof-of-concept that these markers are indeed reflecting the dynamics of fibrogenesis in a unique cohort, where rapid progression to cirrhosis can occur. To our knowledge this is the first such study. It needs to be investigated whether the prognostic capacity of the markers can be repeated in larger cohorts of different etiologies of liver fibrosis given etiology, to create refined profiles of those patients likely to progress cirrhosis.
In conclusion, we have shown that remodeling of interstitial matrix is linked to clinical outcome (survival), whereas remodeling of basement membrane is rather associated with the rate of fibrosis progression. The major limitation of the study is the relatively low number of patients due to the rare event of patients that progressed to advanced fibrosis and cirrhosis within 1 year after liver transplantation. Thus, PRO-C4, C4M and PRO-C3 are proposed as clinically useful tools for prognosis, surveillance, and possibly therapy control and adjustment, in patients with progressive liver disease.
Patients and Methods
Patients
Caucasian patients conducted at the University of Mainz Medical Center between 1996 and 2010 were identified from a prospective transplant database.
Patients were selected based on rapidity of fibrosis progression and retrospectively divided into three groups. Those who developed histologically proven cirrhosis within 1 year (fast progressors) or within 3–5 years after LT (intermediate progressors) were compared with those who showed no or only mild fibrosis (F0/F1) in protocol biopsies taken at 5 years post-LT (non-progressors). A selection criterion was the availability of three follow up sera in the first year after LT. All patients showed no significant fibrosis of the donor organ before implantation (F0/F1). Per protocol biopsies were taken at 1- and 5-year post-LT. Hepatitis C infection (HCV) positive patients were from the era before the availability of highly effective antivirals. HCV re-infection was diagnosed by positive HCV-RNA post-LT and characteristic laboratory findings. Patients with ischemic type biliary lesions or ischemic damage of the biliary tract are summarized under “biliary” in Table 1. For execution of this study, no biological material (serum, tissue) from prisoners was used. All biological material (serum, plasma) was obtained exclusively from patients being treated and followed at Mainz University Medical Center, samples were handled in an anonymized way, and ethical approval was obtained for diagnostic and exploratory serum/plasma sampling. The study followed the ethical guidelines of the Declaration of Helsinki with signed informed consent from all patients and was approved by the local Ethics Committee of the State of Rhineland-Palatinate [no. 837.533.11(8075)].
Biochemical analysis was performed using standard routine laboratory protocols for tests including assessment of bilirubin and alanine aminotransferase (ALT). In addition, enhanced liver fibrosis (ELF) test was assessed in year 1 samples.
Histological assessment
Liver specimens were evaluated by two experienced pathologists. The second was blinded to the results of the first pathologist. The degree of liver fibrosis was staged according to the scoring system of Desmet31 using a scale of 0–4 (F0: Absent; F1: mild fibrosis with periportal fibrous expansion; F2: moderate fibrosis with portal-portal septa (≥1 septum); F3: severe fibrosis with porto-central septa (≥1 septum); F4: cirrhosis).
Immunosuppression
Patients were prescribed different immunosuppressive regiments according to individual risk factors and co-morbidities. The standard immunosuppressive regimen was the combination of a calcineurin inhibitor, either tacrolimus or cyclosporine, with mycophenolate mofetil. For tacrolimus levels were 5–7 ng/ml during the first year and 3–5 ng/ml thereafter. For cyclosporine, target trough levels were 70–90 ng/ml the first year and 50–70 ng/ml thereafter. No patients received mTOR-inhibitors, known to have potential anti-fibrotic activity5. All patients received steroids (methylprednisolone) until 3 months post-LT. Methylprednisolone was reduced from 1.5 mg/kg on day 1 and 2 post-LT to 1.0 mg/kg on days 3 and 4, to 0.5 mg/kg on days 5 to 14, and to 0.2 mg/kg on day 15 until 3 months post-LT. Acute rejection episodes were treated with methylprednisolone 500 mg/d i.v. for 3 days whenever a biopsy proven acute rejection occurred with a rejection activity index (RAI) ≥5.
Collagen biomarker assessment
In this study biomarkers of interstitial type III and V collagen formation (PRO-C332 and PRO-C516), basement membrane type IV collagen formation (PRO-C48) and MMP-2 and MMP-9 degradation (C4M20) were assessed by ELISAs developed by Nordic Bioscience, Herlev, Denmark. Briefly, the assays were performed as follows: 96-well pre-coated streptavidin plates (Roche Diagnostics, Mannheim, Germany) were coated with the appropriate biotinylated synthetic peptides and incubated for 30 minutes at 20 °C. Twenty µL of standard peptide or pre-diluted serum sample were added to appropriate wells, followed by peroxidase-conjugated specific monoclonal antibodies and incubated for 1 hour or overnight at 20 °C or 4 °C. Finally, tetramethylbenzidine (TMB) (cat.438OH, Kem-En-Tec Diagnostics, Taastrup, Denmark) was added, and the plates were incubated for 15 minutes at 20 °C in darkness. All the above incubation steps included shaking at 300 rpm. After each incubation step, the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB reaction was stopped by adding 0.18 M H2SO4 as stopping solution and measured at 450 nm with 650 nm as reference. A calibration curve was plotted using a 4-parametric mathematical fit model. All samples were measured within the measurement range of each assay. Technical performance data for PRO-C3, PRO-C4, PRO-C5 and C4M can be found in Supplementary Table 1.
Statistical analyses
Data were analyzed using MedCalc® version 14 (MedCalc Software, Ostend, Belgium). Distribution of data was found to be skewed. All correlations were calculated by Spearman rank correlation. Non-parametric continuous variables were compared between multiple groups by the Kruskal-Wallis test, p-values were adjusted for multiple comparisons. Receiver operating characteristics (ROC) curves with 95% confidence interval (CI) were calculated to determine the performance of the biomarkers. Area under the ROC (AUROC) curves were compared using the method of DeLong et al.33. Sensitivity, specificity, positive and negative predictive values and likelihood ratios were calculated at thresholds derived from ROC curves. Serum levels of the biomarkers were divided into tertiles, to calculate the odds ratios (OR) for progression towards recurrent cirrhosis (RC). Tertiles were chosen due to the small number of patients. The lowest tertile was used as reference. Univariate analyses on patient’s characteristics as predictors of mortality were calculated by Logistic regression. Patient survival was calculated by Kaplan-Meier analysis, and survival curves were compared using Log-rank test.
Graphs were designed using GraphPad Prism® version 7 (GraphPad Software, Inc., La Jolla, CA, USA). Data are shown as Tukey box plots. Dotted lines represent reference level from normal population. P-values less than 0.05 were considered statistically significant, given as *p < 0.05; **p < 0.01 and ***p < 0.001.
References
Berenguer, M. & Schuppan, D. Progression of liver fibrosis in post-transplant hepatitis C: Mechanisms, assessment and treatment. J. Hepatol. 58, 1028–1041 (2013).
Charlton, M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transplant. 9, S58–S62 (2003).
Rice, J. P. et al. Abusive drinking after liver transplantation is associated with allograft loss and advanced allograft fibrosis. Liver Transplant. 19, 1377–1386 (2013).
Gautam, M., Cheruvattath, R. & Balan, V. Recurrence of autoimmune liver disease after liver transplantation: A systematic review. Liver Transplant. 12, 1813–1824 (2006).
El-Masry, M., Gilbert, C. P. & Saab, S. Recurrence of non-viral liver disease after orthotopic liver transplantation. Liver Int. 31, 291–302 (2011).
Schuppan, D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin. Liver Dis. 10, 1–10 (1990).
Gressner, A. M. & Weiskirchen, R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-β as major players and therapeutic targets. J. Cell. Mol. Med. 10, 76–99 (2006).
Leeming, D. J. et al. Enzyme-linked immunosorbent serum assay specific for the 7S domain of Collagen Type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol. Res. 42, 482–493 (2012).
Schierwagen, R. et al. Serum markers of the extracellular matrix remodeling reflect antifibrotic therapy in bile-duct ligated rats. Front. Physiol. 4, 195 (2013).
Karsdal, M. A. et al. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin. Biochem. 43, 793–804 (2010).
Daniels, S. J. et al. ADAPT: An algorithm incoporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology In press (2018).
Nielsen, M. J. et al. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 35, 429–437 (2015).
Nielsen, M. J. et al. Serological markers of extracellular matrix remodeling predict transplant-free survival in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. (2018).
Gelse, K., Pöschl, E. & Aigner, T. Collagens–structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55, 1531–46 (2003).
Karsdal, M. A. et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G807–30 (2015).
Leeming, D. J. et al. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand. J. Gastroenterol. 50, 584–592 (2015).
Karsdal, M. A. et al. Assessment of Liver Fibrosis Progression and Regression by a Serological Collagen Turnover Profile. Am. J. Physiol. - Gastrointest. Liver Physiol. (2018).
Nielsen, M. J. et al. Markers of Collagen Remodeling Detect Clinically Significant Fibrosis in Chronic Hepatitis C Patients. PLoS One 10, e0137302 (2015).
Nielsen, M. J. et al. Fibrosis is not just fibrosis - basement membrane modelling and collagen metabolism differs between hepatitis B- and C-induced injury. Aliment. Pharmacol. Ther. 44, 1242–1252 (2016).
Sand, J. M. et al. MMP Mediated Degradation of Type IV Collagen Alpha 1 and Alpha 3 Chains Reflects Basement Membrane Remodeling in Experimental and Clinical Fibrosis – Validation of Two Novel Biomarker Assays. PLoS One 8, e84934 (2013).
Schuppan, D. & Kim, Y. O. Evolving therapies for liver fibrosis. J. Clin. Invest. 123, 1887–901 (2013).
Schuppan, D., Ashfaq-Khan, M., Yang, A. T. & Kim, Y. O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 68–69, 435–451 (2018).
Bedossa, P., Dargere, D. & Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457 (2003).
Faria, L. C. et al. Hepatocellular Carcinoma Is Associated With an Increased Risk of Hepatitis B Virus Recurrence After Liver Transplantation. Gastroenterology 134, 1890–1899 (2008).
Roche, B. & Samuel, D. Risk factors for hepatitis C recurrence after liver transplantation. J. Viral Hepat. 14, 89–96 (2007).
Castera, L., Forns, X. & Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 48, 835–847 (2008).
Deffieux, T. et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 62, 317–324 (2015).
Barrault, C. et al. Non-invasive assessment of liver graft fibrosis by transient elastography after liver transplantation. Clin. Res. Hepatol. Gastroenterol. 37, 347–352 (2013).
Kochhar, G., Parungao, J. M., Hanouneh, I. A. & Parsi, M. A. Biliary complications following liver transplantation. World J. Gastroenterol. 19, 2841–6 (2013).
Sánchez Antolin, G. et al. FibroScan Evaluation of Liver Fibrosis in Liver Transplantation. Transplant. Proc. 41, 1044–1046 (2009).
Desmet, V. J., Gerber, M., Hoofnagle, J. H., Manns, M. & Scheuer, P. J. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology 19, 1513–1520 (1994).
Nielsen, M. J. et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 5, 303–15 (2013).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 44, 837 (1988).
Acknowledgements
We acknowledge the funding from the Danish Science Foundation, the Danish Ministry of Science and Innovation, EU H2020 under Grant No. 634413 for the EPOS project and EU IMI under Grant No. 777377 for the Litmus project.
Author information
Authors and Affiliations
Contributions
D.S. conceived the study; T.Z. collected the patient sera; M.J.N., T.Z. and M.A.K. contributed to study design; M.J.N. and I.F.V. performed the statistical analyses and manuscript writing; all authors contributed to writing the manuscript; M.J.N., M.A.K., I.F.V., T.Z. and D.S. did the final manuscript editing.
Corresponding author
Ethics declarations
Competing interests
M.J. Nielsen, I.F. Villesen, N.S. Gudmann, D.J. Leeming and M.A. Karsdal are all full-time employed at Nordic Bioscience. D.J. Leeming and M.A. Karsdal hold stock in Nordic Bioscience. T. Zimmermann, A. Krag and D. Schuppan declare no potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nielsen, M.J., Villesen, I.F., Gudmann, N.S. et al. Serum markers of type III and IV procollagen processing predict recurrence of fibrosis in liver transplanted patients. Sci Rep 9, 14857 (2019). https://doi.org/10.1038/s41598-019-51394-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51394-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.