Abstract
There are strong individual differences in performance during sleep deprivation. We assessed whether baseline features of Psychomotor Vigilance Test (PVT) performance can be used for classifying participants’ relative attentional vulnerability to total sleep deprivation. In a laboratory, healthy adults (n = 160, aged 18–30 years) completed a 10-min PVT every 2 h while being kept awake for ≥24 hours. Participants were categorized as vulnerable (n = 40), intermediate (n = 80), or resilient (n = 40) based on their number of PVT lapses during one night of sleep deprivation. For each baseline PVT (taken 4–14 h after wake-up time), a linear discriminant model with wrapper-based feature selection was used to classify participants’ vulnerability to subsequent sleep deprivation. Across models, classification accuracy was about 70% (range 65–76%) using stratified 5-fold cross validation. The models provided about 78% sensitivity and 86% specificity for classifying resilient participants, and about 70% sensitivity and 89% specificity for classifying vulnerable participants. These results suggest features derived from a single 10-min PVT at baseline can provide substantial, but incomplete information about a person’s relative attentional vulnerability to total sleep deprivation. In the long term, modeling approaches that incorporate baseline performance characteristics can potentially improve personalized predictions of attentional performance when sleep deprivation cannot be avoided.
Similar content being viewed by others
Introduction
Many workers experience insufficient sleep due to long and irregular work hours, including military, public safety, and medical personnel1,2. Sleep deprivation results in a broad range of performance deficits including decreased vigilance, impaired working memory, and reduced processing speed3,4,5,6. There are strong trait-like individual differences in responses to sleep deprivation7,8. For a given performance task, some individuals consistently show severe cognitive impairment during exposure to sleep loss, whereas others are consistently able to maintain relatively high levels of performance. There is currently no simple approach for estimating how a person’s performance will be affected by sleep deprivation. Development of methodologies for predicting individual responses to sleep deprivation may improve fatigue management and lower risk for occupational errors and accidents in safety-sensitive operational settings.
Several studies have shown that groups of participants categorized as either vulnerable or resilient to sleep deprivation exhibit differences in behavior and/or physiology at baseline (i.e., under rested conditions). Group differences at baseline were observed for functional magnetic resonance imaging (fMRI)-based measures9,10,11,12,13, heart rate and its variability14, electroencephalogram (EEG) theta activity14, and performance on the Psychomotor Vigilance Test (PVT)14,15, which is used to assess sustained attention16. We and others have shown that a substantial proportion of variance in PVT performance during sleep deprivation can be explained by individual differences at baseline7,8,17. In participants who were stratified into high-performing and low-performing groups based on their baseline PVT performance, exposure to total sleep deprivation was associated with a much larger between-group difference in attentional lapses during sleep deprivation compared to when the participants were well rested14. These findings suggest that sleep deprivation can amplify individual differences in PVT performance that were already present at baseline. Moreover, these studies suggest that baseline measures of PVT performance can potentially be used to improve predictions of individual differences in attentional responses to sleep deprivation18.
The goal of the present study was to develop models that can predict an individual’s relative performance on an attention task to total sleep deprivation assessed under highly controlled laboratory conditions, using features derived from a single 10-min PVT taken at baseline. Performance on the PVT is highly sensitive to sleep deprivation and circadian timing19,20,21, and the number of PVT lapses during sleep deprivation exhibits trait-like stability7,8 and substantial broad-sense heritability (h2 = 0.83)22 when measured in laboratory studies. In addition, the PVT is suitable for repeated administration with few or no learning effects23, and can be implemented in diverse operational environments24,25,26. For these reasons, we evaluated whether baseline PVT performance characteristics can be used for predicting a person’s relative vulnerability to worsening attention during a night of sleep deprivation. Using a linear discriminant modeling approach with a wrapper-based method for feature subset selection, we tested the hypothesis that PVT features at baseline (i.e., under rested conditions) can be used to classify participants into performance groups (vulnerable, intermediate, or resilient) that differ in their number of attentional lapses during total sleep deprivation.
Methods
Participants and screening procedures
In this retrospective study, we examined data from 185 healthy adults aged 18–30 years who participated in inpatient studies that included an episode of total sleep deprivation (i.e., ≥24 h of continuous wake) in the Intensive Physiologic Monitoring Unit within the Harvard Clinical and Translational Science Center at Brigham and Women’s Hospital (Boston, MA). Data were combined across several previous studies that investigated effects of different types of light stimuli on human circadian rhythms27,28,29,30,31,32. All studies implemented the same screening criteria and laboratory procedures during the sleep deprivation portion of the protocol. Eligibility was assessed by screening questionnaires, a physical examination, blood biochemistry and hematology, an electrocardiogram, and an interview with a clinical psychologist or psychiatrist. Participants who completed the sleep deprivation protocol (n = 185) were considered for inclusion in the present analysis.
Prior to the laboratory study, participants were required to maintain a fixed sleep schedule (8 h of time in bed per night) for at least 1 week. Participants chose a sleep schedule within the range of 2200–0600 (earliest possible schedule) to 0200–1000 (latest possible schedule). Compliance was verified by continuous actigraphy monitoring (Actiwatch-L; Minimitter, Inc., Bend, OR). Participants were ineligible for the laboratory study if their actigraphy-estimated sleep pattern deviated from the prescribed sleep-wake schedule by more than 30 min on any given night. A comprehensive toxicology screen was performed on the day of admission to the laboratory study to ensure that participants had complied with refraining from caffeine, over-the-counter drugs, recreational drugs, alcohol, and nicotine use. Female participants underwent a blood test to verify that they were not pregnant. Informed written consent was obtained from all participants, and research procedures were approved by the Human Research Committee at Partners HealthCare and complied with HIPAA regulations and ethical guidelines in the Declaration of Helsinki.
Protocol overview
Research participants were studied individually in a laboratory environment that was free of time cues. During the first 3 days of the laboratory study, participants were scheduled to sleep and wake at their pre-study sleep-wake times. Participants slept in darkness and were exposed to <200 lux during wake episodes until midway through the third day, after which time the lights were dimmed to <3 lux measured at eye level. Ambient light was provided by ceiling mounted 4100 K lamps (Philips Lighting, Eindhoven, The Netherlands) with illuminance measured using a portable radiometer (International Light Technologies ILT1400) as previously described27,28. Beginning on the fourth morning of the study, participants were kept awake continuously for 30–50 h using constant routine procedures33. During this episode of total sleep deprivation, wakefulness was enforced by a staff member who was present in the participant’s room. The staff member also monitored the participant’s compliance with research procedures, including computer testing. Participants were required to maintain a semi-recumbent position in bed under dim ambient lighting (<3 lux), with small equicaloric snacks given every hour. Following exposure to total sleep deprivation, participants had a recovery sleep episode and an additional 4–5 days of their study (results reported elsewhere)27,28,29,30,31.
Psychomotor vigilance test
Participants completed a 10-min Psychomotor Vigilance Test (PVT) every 2 h while awake, including the first 3 days of the study and throughout the sleep deprivation protocol. The PVT is a simple reaction time test that is used to assess sustained visual attention16,23. Participants were instructed to maintain their fastest possible reaction time to a visual stimulus that was presented on a computer monitor at random inter-stimulus intervals ranging from 1–9 s. The stimulus comprised a count-up timer that counted up in milliseconds until the participant pressed a button on a dedicated PVT response box with the thumb of their dominant hand. Attentional lapses were defined as reaction times that exceeded 500 milliseconds6,8,34,35.
Of the 185 participants whose data were considered for the present study, 25 participants were excluded from our analyses due to missing or invalid PVT data during the episode of total sleep deprivation (from 16 to 24 h after wake-up time). Participants were excluded from retrospective data selection if they had one or more PVT sessions that were missing or invalid for the 5 sessions that took place during sleep deprivation (i.e., 16, 18, 20, 22, or 24 h after wake-up time) because these data were used to categorize participants into different vulnerability groups (see below). Missing data were defined as PVT sessions that did not take place or were not digitally recorded due to procedural or technical problems. Invalid PVT sessions were defined as those in which the PVT was administered more than an hour later than the scheduled time (e.g., due to procedural or technical problems), the participant used the incorrect button on his/her PVT response box for >10% of trials for a given PVT session, no PVT reaction time was recorded for >1 min during a given PVT session (e.g., due to the participant not responding or a technical problem), or the 10-min PVT session was truncated to <8 min due to a technical problem (e.g., if the computer program crashed during a PVT session). Using these pre-determined criteria for retrospective data selection, there were 8 participants who did not complete all of the PVTs at the scheduled times due to procedural problems; 8 participants who were excluded due to experimental problems that invalidated their data; and 9 participants who were excluded due to incomplete PVT data. Hence, 160 participants were included in the present study (65% male; mean age ± SD = 23.0 ± 2.8 years).
Model for classifying vulnerability to total sleep deprivation
Stratification of participants into different vulnerability groups
To categorize participants as resilient or vulnerable to total sleep deprivation, we ranked them by their performance when they were sleep deprived, as indexed by their average number of PVT lapses (reaction times > 500 ms) during hours 16 to 24 of the sleep deprivation constant routine procedure. The top quartile of participants with the fewest number of lapses was defined as the resilient group (n = 40) and the bottom quartile with the greatest number of lapses was defined as the vulnerable group (n = 40). The middle 50% of participants whose performance fell within the interquartile range were defined as the intermediate performance group (n = 80).
Identification of candidate baseline PVT features
Baseline performance on the PVT was assessed during the first 16 hours of wakefulness of the constant routine procedure. There were 6 PVT sessions during the baseline interval (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time). For each PVT session that a participant completed, 426 summary measures were calculated based on trial-by-trial reaction times. These measures characterized various aspects of PVT performance, including speed and variability of reaction times, response errors (e.g., anticipation errors or failure to respond), and time-on-task effects (Table S1). In addition to standard PVT performance metrics36, we included measures based on those introduced recently by our group and others, including percentile reaction times37 and lapse counts based on reaction time thresholds that reflect different probabilities of the participant’s eyes being closed when lapses occurred38. We also included a series of novel non-parametric measures of reaction time variability, such as the number of times that the difference of consecutive reaction times exceeded a particular threshold, and differences of percentile reaction times.
Baseline PVT features that are consistent over repeated assessments (within and between participants) are more likely to be reliable in predicting responses during sleep deprivation compared to features that are more variable. We therefore pre-selected candidate baseline PVT features for our models that exhibited stable individual differences across multiple days of rested wakefulness (day 2, day 3, and the first 16 h of wakefulness on day 4). PVT measures were chosen as candidate features for the linear discriminant model (see below) if they met the pre-determined criterion of having an intra-class correlation coefficient (ICC) value > 0.60, which corresponds to the threshold for ‘substantial’ or ‘good’ agreement between measurements39,40. For each PVT measure at each time point, the ICC value was calculated as between-participant variance (σ2BS) divided by the sum of between- and within-participant variance (σ2BS + σ2WS), with values ranging from 0 to 1. An ICC value was computed separately for each baseline PVT measure at each time point (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time). A linear mixed model was used with day (day 2, 3, and 4) as a fixed factor to correct for systematic order effects (i.e., to account for day-to-day changes in PVT performance that might be related to the research protocol), and between-participant differences were modeled as a random intercept with a Gaussian distribution41. Across baseline PVT sessions, about one third of PVT measures met the criterion of having an ICC value > 0.60 and were considered for feature subset selection (number of PVT measures with an ICC value > 0.60: 4 h, 165 measures; 6 h, 108 measures; 8 h, 134 measures; 10 h, 135 measures; 12 h, 163 measures; 14 h, 137 measures). Metrics with an ICC value < 0.60 were excluded and were not revisited in the current study.
Three-class linear discriminant model
For each baseline time point (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time on day 4 of the protocol), a different linear discriminant classification model was developed to predict individuals in resilient, intermediate, and vulnerable performance groups. A wrapper-based method was used to determine the optimal feature subset for predicting performance during total sleep deprivation42. In short, a wrapper approach assesses the relative usefulness of candidate feature subsets through its estimated prediction performance, and incorporates downstream prediction performance to obtain an optimized feature subset. We used a forward selection, best-first search algorithm to perform the feature set search42,43.
Within the wrapper best-first selection, for a given candidate feature set we added an additional layer to evaluate model accuracy using cost matrices from 1 to 5 (in 0.5 steps)44. A cost matrix assigns weights to different outcomes, hence penalizing and protecting against specific types of misclassification errors. In the present study, we did not commit to a particular cost matrix because the choice is user-context dependent. For example, in some operational settings it may be more costly (i.e., undesirable) to misclassify a person who is vulnerable to sleep deprivation as resilient, as compared with misclassifying a person who is resilient as vulnerable. Our modeling approach searched for the optimal combination of feature set and cost matrix to optimize prediction accuracy, which was used as the performance metric to select the model for the three-class prediction.
For a given trial feature set, we used repeated 5-fold cross validation to evaluate its performance. Fold assignment was performed for the resilient, intermediate, and vulnerable groups separately, i.e., stratified, to ensure the proportion of participants in each group stayed constant across folds. Prediction performance across 100 repeated cross-validation runs was averaged to reduce the variance expected of 5-fold cross-validation. The best-first search algorithm was terminated upon reaching the condition in which performance did not improve for 5 consecutive iterations. After the best feature subset and cost matrix was determined for a given PVT session, the final classification model was determined by applying the best feature subset on the entire initial dataset.
For each PVT session completed at baseline (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time), its corresponding model arranged the summary PVT data (which initially consisted of data expressed in different types of units) as a row vector d, and normalized the data for a given PVT measure across subjects by z-scoring them, i.e. z = (d − μ)/σ, where μ and σ are the z-score parameters. Then, for each of the possible outcomes, i.e., resilient, intermediate, or vulnerable, the model computed the posterior probability that the participant belonged to that group, given the set of model parameters and cost matrix (Table S2). Specifically, the discriminant functions for each group, i.e., the natural logarithm of the posterior probabilities, were computed as linear combinations of the summary PVT measures, according to:
In each equation, g is the discriminant function, w is the coefficient, and c is the constant term (R = resilient; I = intermediate; V = vulnerable). Participants were assigned to the group with the highest value for the discriminant function, which is equivalent to assignment based on the highest posterior probability. Depending on the testing time point, a different set of z-score and model parameters was applied.
Assessment of model performance
Performance of the models was assessed using several metrics: (1) Accuracy of the classifier, defined as the proportion of participants who were correctly classified as resilient, intermediate, or vulnerable; (2) Cohen’s kappa, which measures accuracy after discounting agreement by chance, i.e., accounting for the 1:2:1 structure of the outcome assignments (chance-level accuracy was 37.4% for the 3-class classification task); (3) Sensitivity, defined as the proportion of vulnerable participants who were correctly classified as vulnerable (for the vulnerable classifier), or as the proportion of resilient participants who were correctly classified as resilient (for the resilient classifier); (4) Specificity, defined as the proportion of intermediate and resilient participants who were correctly identified as not being vulnerable (for the vulnerable classifier), or as the proportion of intermediate and vulnerable participants who were correctly identified as not being resilient (for the resilient classifier); (5) Positive predictive value, defined as the proportion of participants with a test outcome (i.e., predicted outcome) of vulnerable who were truly vulnerable (for the vulnerable classifier), or as the proportion of participants with a test outcome of resilient who were truly resilient (for the resilient classifier); and (6) Negative predictive value, defined as the proportion of participants with a test outcome of either resilient or intermediate who were truly resilient or intermediate (for the vulnerable classifier), or as the proportion of participants with a test outcome of either intermediate or vulnerable who were truly intermediate or vulnerable (for the resilient classifier). Analyses and model development were implemented by self-written code using MATLAB software (MATLAB 2012a; MathWorks, Inc, Natick, MA).
Results
Characterization of vulnerability to total sleep deprivation
In our sample of 160 young healthy adults, large individual differences in PVT performance were observed during exposure to total sleep deprivation (Fig. 1a). The quartile of participants who were defined as vulnerable had an average of 30.6 to 52.8 lapses per PVT session; the quartile of participants who were defined as resilient had an average of 0.0 to 13.6 lapses per PVT session; and the remaining group of participants with intermediate performance had an average of 14.0 to 30.4 lapses per PVT session. Based on our stratification scheme for defining performance during total sleep deprivation, there was almost no overlap in PVT lapses across individual sessions for participants who were categorized as vulnerable versus resilient (Fig. 1b).
Stratification of participants into resilient, intermediate, and vulnerable performance groups. (a) The average number of lapses (reaction times > 500 ms) on the Psychomotor Vigilance Test (PVT) during total sleep deprivation (TSD; 16 h to 24 h after wake-up time) is shown for 160 participants who were categorized into vulnerable (red, n = 40), intermediate (gray, n = 80), and resilient (blue, n = 40) performance groups. Dashed lines demarcate thresholds (25th percentile and 75th percentile) used to define vulnerable and resilient groups. (b) The time course of PVT lapses is shown on baseline days that preceded TSD (Day 2 and Day 3) and during 24 h of continuous wakefulness (Day 4). (c) The mean ± SEM is shown for PVT lapses in groups of participants categorized as vulnerable, intermediate, or resilient during TSD. (d) Box plots show the increase in the number of lapses from baseline to sleep deprivation in participants whose performance was categorized as Resilient (R), Intermediate (I), or Vulnerable (V) during TSD. Boxes show the median and interquartile range, and whiskers indicate the 95th percentile of the distribution. Asterisks indicate significant between-group differences (P < 0.001).
There was a significant difference between groups in the increase in PVT lapses from baseline to sleep deprivation (Fig. 1c,d; Kruskal-Wallis Test, H = 106.0, P < 0.001). Specifically, the group that was categorized as vulnerable based on lapses during total sleep deprivation exhibited a greater increase in PVT lapses from baseline compared with the intermediate performance group, despite having a greater number of lapses at baseline (Dunn’s post test, Q = 4.7, P < 0.05). Hence, the group we defined as vulnerable was more susceptible to sleep deprivation, even after taking into account baseline differences in PVT performance. Similarly, the intermediate performance group exhibited a greater increase in lapses compared with the resilient group (Dunn’s post test, Q = 7.0, P < 0.05). These results show that our definition for performance vulnerability, which was based on the average number of lapses during total sleep deprivation, also reflects differences in the magnitude of deterioration in performance from baseline to the sleep-deprived state.
Model for predicting performance vulnerability using baseline PVT measures
Separate linear discriminant models were developed using baseline PVT features from 6 different time points (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time; Table S2) to classify participants in resilient, intermediate, and vulnerable performance groups. Across the different models, classification accuracy ranged from 65% to 75%, and Cohen’s kappa ranged from 0.48 to 0.61 (Table 1), indicating ‘moderate’ strength of agreement40 between the classification model and the true status of participants (i.e., vulnerable, intermediate, resilient). Then, we assessed how well each model performed at the binary classification task of classifying resilient participants (top quartile) versus the bottom 75% of performers during total sleep deprivation (Table 1). Models that were developed across the 6 baseline PVT sessions provided about 78% sensitivity (range 63–90%), 86% specificity (range 75–93%), 68% positive predictive value (range 55–78%), and 93% negative predictive value (range 89–96%). We also assessed the performance of each model at classifying vulnerable participants (bottom quartile) versus the top 75% of performers. For this classification task, the models provided about 70% sensitivity (range 57–84%), 89% specificity (range 83–94%), 70% positive predictive value (range 60–79%), and 90% negative predictive value (range 87–94%).
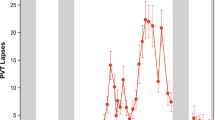
Next, we compared the time course of PVT lapses in groups of participants classified as either vulnerable or resilient to the effects of total sleep deprivation. The goal of this analysis was to assess qualitatively the ability of the models to select for groups of participants enriched for either vulnerability or resilience. For the classification model developed at each baseline time point, we performed 100 runs of 5-fold cross validation, resulting in 100 predictions for a given participant. The most common prediction (i.e., the mode) in each individual was used to determine his/her group assignment (vulnerable, intermediate, or resilient). For classification models developed at each of the 6 baseline PVT sessions, the time course of PVT lapses was markedly different between the predicted vulnerable and resilient groups (Fig. 2; For comparisons between groups at every time point and model, P < 0.001), indicating that baseline features of PVT performance could be used to discriminate groups of participants who differed in their attentional vulnerability during total sleep deprivation.
PVT lapses (reaction times > 500 ms) in groups of participants predicted to be resilient or vulnerable to total sleep deprivation. Models were developed using baseline PVT performance features to classify individuals as resilient (blue, open circles) or vulnerable (red, open circles) to the effects of total sleep deprivation. Separate models were developed using a 10-min PVT performed at 6 different time points (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after habitual wake time). In all models and at every time point, P < 0.001 for pairwise comparisons between predicted resilient and vulnerable groups. For comparison, the time course of PVT lapses is shown for participants categorized as resilient or vulnerable based on their average number of lapses during total sleep deprivation (resilient, blue circles; vulnerable, red circles). The mean ± SEM is shown in each panel.
Predicting performance vulnerability in participants with high performance at baseline
Our modeling approach was based on the premise that baseline individual differences in performance carry information about attentional responses during total sleep deprivation. Given that there were substantial individual differences in PVT performance at baseline in the full sample (n = 160), we sought to establish whether our modeling approach could be used to predict vulnerability to total sleep deprivation in a group of participants with less heterogeneity in PVT performance (i.e., high-performing subjects). We therefore performed a post-hoc stratification and analysis in which we applied our modeling approach to the subset of participants who exhibited ≤ 2 lapses per PVT session at baseline (n = 60). In this subgroup of high-performing individuals, we stratified participants according to the same criteria as those used in the full sample, i.e. the quartile of participants with the greatest number of PVT lapses during sleep deprivation was defined as vulnerable (n = 15), and the quartile with the fewest number of lapses was defined as resilient (n = 15).
Using the same modeling approach described for the full dataset, we developed a set of new models in the subgroup with ≤ 2 lapses at baseline (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time). In these models, classification accuracy ranged from 57% to 64%, and Cohen’s kappa ranged from 0.38 to 0.46, indicating ‘fair-to-moderate’ strength of agreement40 between the classification model and participants’ actual vulnerability status (Table 2). For classification of resilient participants (top quartile) versus the bottom 75% of performers during sleep deprivation, our models provided about 69% sensitivity (range 49–90%), 80% specificity (range 75–89%), 56% positive predictive value (range 45–76%), and 90% negative predictive value (range 83–97%). For classification of vulnerable participants (bottom quartile) versus the top 75% of performers, the models provided about 80% sensitivity (range 67–87%), 76% specificity (range 68–81%), 56% positive predictive value (range 47–62%), and 93% negative predictive value (range 89–95%).
In the subgroup of participants with ≤2 lapses at baseline, we evaluated the average time course of PVT lapses in groups of participants who were classified as either resilient or vulnerable to total sleep deprivation. This was assessed for models developed for each baseline time point. Similar to results in the full sample, these models were able to classify participants who were high performers at baseline into groups that differed in their attentional responses to total sleep deprivation (Fig. 3). Irrespective of the time point of PVT testing that was used to develop each model, the group of participants that was predicted to be more vulnerable to sleep deprivation exhibited more lapses than the group that was predicted to be resilient to the effects of sleep deprivation.
PVT lapses (reaction times > 500 ms) in groups of participants with high performance at baseline (≤2 lapses; n = 60) who were classified as resilient or vulnerable to total sleep deprivation. Participants were classified as resilient (blue, open circles) or vulnerable (red, open circles) to the effects of total sleep deprivation using models developed on baseline PVT measures. Separate models were developed using a 10-min PVT performed at 6 different time points (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after habitual wake time). Asterisks (*) indicate significant pairwise differences in PVT lapses between predicted resilient and vulnerable groups during the sleep deprivation period (P < 0.05). For comparison, the time course of PVT lapses is shown for participants categorized as resilient or vulnerable based on their average number of lapses during total sleep deprivation (resilient, blue circles; vulnerable, red circles). The mean ± SEM is shown in each panel.
Discussion
In the present study, we developed and tested a series of models for predicting individual differences in attentional vulnerability to total sleep deprivation. Using features derived from a single 10-min PVT taken under rested conditions, participants in the full dataset (n = 160) were classified in vulnerable, intermediate, and resilient performance groups with about 70% accuracy across models developed at different baseline time points (range, 65–76%). These results demonstrate that baseline individual differences in PVT performance metrics associate with relative vulnerability to total sleep deprivation. The robustness of our modeling approach was assessed in a subgroup of high-performing persons (≤2 lapses at baseline), in whom baseline individual differences were much smaller compared with the full sample. As expected, performance of the classifier dropped in this subgroup, but the model was still able to classify participants in different performance groups (vulnerable, intermediate, or resilient) with about 60% accuracy for models developed across different baseline PVT sessions (range, 57–64%), i.e., above a chance-level prediction (37.4%). Hence, even in top-performing participants, individual differences in baseline performance associated with responses to total sleep deprivation.
In our prior work based on data collected from a different group of participants14, we showed that individuals who were categorized as vulnerable to the effects of total sleep deprivation on PVT lapses exhibited slower and more variable reaction times at baseline. This led us to hypothesize that exposure to sleep deprivation may contribute to enhancement of individual differences in PVT performance that were already present at baseline14. In a follow-up analysis of participants in that study who were exposed to total sleep deprivation on 2 separate occasions, we showed that individual differences in baseline PVT performance contributed significantly to between-participant variance in performance during sleep deprivation17. Based on variance components analysis of PVT performance, nearly 50% of the variance in performance during exposure to total sleep deprivation was explained by individual differences at baseline. Consequently, the intra-class correlation coefficient value dropped substantially when adjusting for baseline performance as a covariate. Although baseline individual differences did not fully account for individual differences in PVT performance during prolonged wakefulness7,8, we hypothesized that baseline differences in PVT performance could nonetheless be used to estimate a person’s relative vulnerability to total sleep deprivation. The present study addressed this question, demonstrating that various measures of baseline PVT performance could be used to classify participants’ vulnerability to total sleep deprivation with nearly double the accuracy compared with chance-level prediction accuracy (i.e., much greater than 37.4% chance-level prediction for the 3-class classification task).
Most predictive performance models that have been developed have been used to identify critical times of performance vulnerability in an average individual (i.e., based on group data)45,46,47,48, rather than providing individualized predictions of performance under conditions of sleep loss or circadian misalignment. Adaptive individual-specific performance models address this issue49,50,51,52, but such models rely on repeated assessments of PVT performance over time under sleep loss conditions. They approach the problem by assessing a person’s responses to sleep deprivation periodically and updating the model to predict his/her responses to subsequent sleep deprivation, rendering it an impractical approach for many situations. More recently, drift diffusion model53 parameters derived from baseline PVT data have been used to classify participants in resilient and vulnerable groups with 77% accuracy using a support vector machine (SVM) modeling approach18. In its current form, however, that model requires data to be combined across consecutive PVTs for reliable estimates of drift diffusion parameters, whereas our classifier relies on data derived from a single 10-min PVT. Additionally, participants in that study were categorized as vulnerable or resilient to sleep deprivation based on a median split of PVT lapses after exposure to sleep deprivation. Using such a definition, there is little separation between higher-performing vulnerable participants and lower-performing resilient participants. By comparison, our modeling approach included an intermediate performance group, hence ensuring that participants who were categorized as vulnerable or resilient to the effects of total sleep deprivation on PVT performance were phenotypically distinct. The slightly lower classification accuracy in our study might be explained in part by the more challenging 3-class classification task (37.4% versus 50.0% chance-level prediction) and the use of a single PVT session for building the classifier.
The performance and generalizability of our modeling approach are likely influenced by the definitions used for vulnerability to sleep deprivation. PVT lapses have been widely used in sleep and circadian rhythms research to assess sustained attention performance and to model group-level or individual responses to different types of sleep deprivation15,18,49,50,51,52,54,55. There are, however, other PVT-based metrics that have been proposed for quantifying performance impairment that may better capture the influence of homeostatic and circadian processes56. Recent work has shown that PVT metrics derived from diffusion modeling (e.g., the log transformation of the signal-to-noise ratio) have better psychometric properties than PVT lapses, including higher sensitivity, stability, degree of normality, and absence of floor and ceiling effects57. Hence, future modeling efforts should consider including other metrics of PVT performance for measuring and predicting changes in vigilant attention.
Consistent with previous studies, we observed strong individual differences in PVT performance during total sleep deprivation2,8. Prior work has demonstrated that these individual differences are partially explained by genetic make-up22,58, but are likely modulated by other physiologic and behavioral factors. The earlier deterioration in PVT performance during sleep deprivation in participants categorized as vulnerable could be explained by a faster buildup of homeostatic sleep pressure, or an earlier circadian increase in sleep propensity. The present study did not, however, examine effects of individual differences in sleep pressure, circadian phase, or chronotype on the time-course of PVT performance. We also cannot determine the degree to which individual differences in performance were affected by participants’ motivation or effort to perform their best. Effort allocation on the PVT is sensitive to sleep deprivation and reward59,60, and is also likely influenced by an individual’s level of intrinsic motivation.
We must also emphasize that individual differences in PVT lapses do not necessarily correspond with individual differences in performance on other cognitive tasks, e.g. those that assess working memory or processing speed8,61. As such, our modeling approach may only be relevant for estimating performance deterioration on tasks in which sustained attention is the most important determinant of task performance. It is also well-established that performance on the PVT does not associate with individual differences in self-reported sleepiness8,14,62, indicating a dissociation between how sleepy participants feel and how well they can perform during exposure to sleep deprivation. Nonetheless, it has been shown that PVT lapses correlate with physiologic indicators of sleepiness, including percentage eyelid closure over the pupil over time (PERCLOS), low-frequency EEG activity, and some measures of heart rate variability14. Thus, PVT performance reflects overall vigilance, with longer reaction times and lapses associated with increased drowsiness and intermittent intrusion of sleep23,38.
The performance of our models may be influenced by our definition of baseline PVT performance. During the pre-study screening process, we attempted to minimize the possibility that participants were sleep-deficient before enrolling in the laboratory study. Specifically, participants were instructed to spend 8 h of time in bed for sleep each night for at least one week, and the same sleep schedule was enforced during the first 3 nights of the laboratory study. This amount of time in bed for sleep falls in the middle of the range recommended by the National Sleep Foundation for young adults (7–9 h)63, but the duration of sleep required for optimal performance likely varies across individuals. In our previous work we showed that participants who were categorized as vulnerable to the effects of total sleep deprivation on PVT performance exhibited longer sleep durations and more regular sleep patterns under free-living conditions, as compared with participants categorized as resilient14. Therefore, we cannot exclude the possibility that participants in the current study who were categorized as vulnerable to total sleep deprivation needed more than 8 h of time in bed for sleep each night for optimal performance54,64, and were getting insufficient sleep during the 10+ days immediately prior to undergoing sleep deprivation in the laboratory. Relatedly, the duration of time participants maintained their fixed pre-study sleep-wake schedule ranged from 1 to 3 weeks and was not factored into our analyses. Additionally, the fixed sleep-wake schedule that participants chose did not necessarily reflect their preferred sleep timing and may have been influenced by factors such as work hours, school schedule (for participants who were college students), and family responsibilities and/or home environment. It is also possible that baseline PVT performance was influenced by carry-over effects from sleep history before screening. In future studies, these issues can potentially be addressed by having participants undergo a sleep-extension protocol in which they are given extended opportunities for sleep to minimize any sleep debt20, prior to being exposed to sleep deprivation.
There are several sources of selection bias in our study related to participant recruitment. Our participants were selected to be young, very healthy, and willing to undergo rigorous screening and laboratory procedures. The latter included staying in a highly-controlled laboratory setting for 9–10 days, during which participants were exposed to total sleep deprivation, frequent computer testing, and frequent intravenous blood draws (data reported elsewhere) without access to caffeine. Hence, our findings are based on a non-random sample of individuals who may differ in health, personality, lifestyle, and socioeconomic status from the general population. Our participant selection and laboratory procedures likely served to minimize sources of variance in PVT performance, and hence the model performance metrics in the present study may reflect the best-case scenario for predicting individual differences in attentional lapses during total sleep deprivation. Looking forward, it will be important to test our modeling approach prospectively in more diverse groups of participants who differ in age, health, caffeine intake, and sleep history.
Our analyses were limited to a single overnight period of exposure to total sleep deprivation. While pulling an all-nighter is common among students and shift workers, longer episodes of sleep deprivation can occur, e.g. in medical and military personnel. Future modeling efforts should therefore consider both overnight and extended periods of sleep deprivation. While it has been demonstrated that individual differences in PVT performance are preserved across different types of sleep deprivation (total sleep deprivation versus chronic sleep restriction)7, it will also be important to test whether our modeling approach can be used to classify performance vulnerability in individuals exposed to chronic sleep restriction and/or irregular sleep schedules26,55.
The present study used a linear discriminant model that incorporated a best-first search algorithm with wrapper-based heuristics, which represents an iterative fitting and testing process. An advantage of wrapper methods for feature selection is that they can provide for high classification accuracy42. Such methods can, however, result in overfitting if the training data are modeled too well (i.e., modeling peculiarities in the data) resulting in a feature set with poor prediction performance in other datasets. Given that we used an internal cross-validation scheme and did not test the generalizability of our modeling approach using data derived from other experiments or in a hold-out set, our results for accuracy are likely optimistically biased, representing the upper limit of prediction performance that can be achieved. This limitation implies that further improvement will require the discovery and implementation of new metrics (e.g., from physiologic data). There are many other modeling approaches that can be used to select predictor variables and classify participant performance. A comparison of our model with other classifiers was beyond the scope of the present study, but prior attempts to classify vulnerability to sleep deprivation based on PVT performance have included SVM models18 and pattern classification based on a k-nearest neighbor algorithm and a Naïve Bayes classifier55. Additionally, there are alternative approaches for feature selection that may perform better at finding all relevant features, while discarding those that are redundant, e.g. the Boruta algorithm which uses a wrapper approach built around a random forest classifier65. While our approach was successful in discriminating groups of individuals who differed in their attentional vulnerability to total sleep deprivation, future work should investigate the performance of alternative models that may be more robust (e.g., more generalizable and resistant to noise) and amenable to protection against over-fitting. It is also possible that combining outputs from multiple models (e.g., model fusion or ensemble classifiers) may improve classification performance66.
In the present study, it is likely that some PVT measures included in each candidate feature set carried redundant or overlapping information. It is therefore unsurprising that related but non-identical PVT features were selected by models developed at each time point (4 h, 6 h, 8 h, 10 h, 12 h, and 14 h after wake-up time). In all models, PVT features were selected that measured the difference in reaction times across different percentiles of the reaction time distribution. In particular, differences in reaction times from the faster-to-middle part of the distribution (e.g., difference in 20th to 55th percentile reaction times) were selected most often across models, with a narrower distribution of reaction times associated with greater resilience to the effects of total sleep deprivation. Measures of reaction time variability (e.g., standard deviation of reciprocal reaction time) and differences in reaction times between fast and slow tails of the distribution (e.g., difference in 15th to 85th percentile reaction times) were also selected in some models, suggesting that distribution-based measures of PVT performance may be especially useful for estimating individual responses to total sleep deprivation.
Recently, there has been progress in understanding mechanisms that underlie individual differences in performance-related responses to total sleep deprivation. Studies that have used fMRI have shown that individual differences in patterns of task-dependent brain activation are reproducible across multiple exposures to sleep deprivation67. Participants who are categorized as vulnerable to the effects of sleep deprivation on performance show reduced global brain activation at baseline when they are well rested9,10,11, as well as poorer separation of cortical networks that are functionally segregated12. These studies demonstrate that brain imaging markers can potentially be used to explain and predict attentional responses to total sleep deprivation. Additionally, polymorphisms in genes implicated in sleep and circadian physiology have been shown to associate with individual differences in neurobehavioral performance, either during baseline or in response to sleep deprivation58,68,69,70,71,72,73. It is possible that our models for predicting attentional vulnerability to total sleep deprivation would perform better if additional factors were included as predictor variables, including brain imaging markers, genotype, or physiological variables associated with attentional vulnerability to sleep deprivation14.
In conclusion, our analyses of laboratory data suggest that it is possible to classify participants into groups that are either more vulnerable or more resilient to the effects of total sleep deprivation on attentional lapses, using data derived from a 10-min PVT taken under rested daytime conditions. Under ideal experimental conditions, we show that the upper limit of classification accuracy is about 75%. Further improvement in predicting attentional lapses during sleep deprivation will require the discovery and implementation of new metrics. In the long term, our modeling approach and related methods can potentially be used toward developing personalized fatigue management strategies when exposure to long work hours and sleep deprivation cannot be avoided. With further development of such algorithms and testing of their predictive ability in other populations and in real-world operational environments, it might be possible to minimize the risk of attentional failures by screening for personnel resilient to the effects of sleep loss and/or deploying appropriate individualized fatigue countermeasures in those persons who are predicted to be more vulnerable to sleep deprivation.
Data Availability
The authors will make relevant anonymized data available on reasonable request. Execution of a Materials Transfer Agreement is required if the data will be used in research supported by a for-profit company, per Partners Healthcare Institutional Review Board policy.
References
Lockley, S. W. et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med 351, 1829–1837, https://doi.org/10.1056/NEJMoa041404 (2004).
Van Dongen, H. P., Caldwell, J. A. Jr. & Caldwell, J. L. Individual differences in cognitive vulnerability to fatigue in the laboratory and in the workplace. Prog Brain Res 190, 145–153, https://doi.org/10.1016/B978-0-444-53817-8.00009-8 (2011).
Belenky, G. et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12, 1–12 (2003).
Lim, J. & Dinges, D. F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 136, 375–389, https://doi.org/10.1037/a0018883 (2010).
Lo, J. C., Ong, J. L., Leong, R. L., Gooley, J. J. & Chee, M. W. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep 39, 687–698, https://doi.org/10.5665/sleep.5552 (2016).
Van Dongen, H. P., Maislin, G., Mullington, J. M. & Dinges, D. F. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26, 117–126 (2003).
Rupp, T. L., Wesensten, N. J. & Balkin, T. J. Trait-like vulnerability to total and partial sleep loss. Sleep 35, 1163–1172, https://doi.org/10.5665/sleep.2010 (2012).
Van Dongen, H. P., Baynard, M. D., Maislin, G. & Dinges, D. F. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 27, 423–433 (2004).
Caldwell, J. A. et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci 119, 694–707, https://doi.org/10.1037/0735-7044.119.3.694 (2005).
Chee, M. W. et al. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage 31, 419–428, https://doi.org/10.1016/j.neuroimage.2005.12.001 (2006).
Mu, Q. et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep 28, 433–446 (2005).
Yeo, B. T., Tandi, J. & Chee, M. W. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 111, 147–158, https://doi.org/10.1016/j.neuroimage.2015.02.018 (2015).
Cui, J. et al. Microstructure of frontoparietal connections predicts individual resistance to sleep deprivation. Neuroimage 106, 123–133, https://doi.org/10.1016/j.neuroimage.2014.11.035 (2015).
Chua, E. C. et al. Sustained attention performance during sleep deprivation associates with instability in behavior and physiologic measures at baseline. Sleep 37, 27–39, https://doi.org/10.5665/sleep.3302 (2014).
Patanaik, A., Zagorodnov, V., Kwoh, C. K. & Chee, M. W. Predicting vulnerability to sleep deprivation using diffusion model parameters. J Sleep Res 23, 576–584, https://doi.org/10.1111/jsr.12166 (2014).
Dinges, D. F. & Powell, J. W. Microcomputer analyses of performance on a portable simple visual RT task during sustained operations. Behav Res Meth Instr Comp 17, 652–655 (1985).
Chua, E. C. et al. Individual differences in physiologic measures are stable across repeated exposures to total sleep deprivation. Physiol Rep 2, https://doi.org/10.14814/phy2.12129 (2014).
Patanaik, A., Kwoh, C. K., Chua, E. C., Gooley, J. J. & Chee, M. W. Classifying vulnerability to sleep deprivation using baseline measures of psychomotor vigilance. Sleep 38, 723–734, https://doi.org/10.5665/sleep.4664 (2015).
Cohen, D. A. et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med 2, 14ra13, https://doi.org/10.1126/scitranslmed.3000458 (2010).
Silva, E. J., Wang, W., Ronda, J. M., Wyatt, J. K. & Duffy, J. F. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep 33, 481–490 (2010).
McHill, A. W., Hull, J. T., Wang, W., Czeisler, C. A. & Klerman, E. B. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci USA 115, 6070–6075, https://doi.org/10.1073/pnas.1706694115 (2018).
Kuna, S. T. et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep 35, 1223–1233, https://doi.org/10.5665/sleep.2074 (2012).
Dorrian, J., Rogers, N. L. & Dinges, D. F. In Sleep deprivation: clinical issues, pharmacology and sleep loss effects (ed. Kushida, C. A.) Ch. 4, 39–70 (Marcel Dekker, Inc, 2005).
Dijk, D. J. et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol Regul Integr Comp Physiol 281, R1647–1664 (2001).
Zhang, C. et al. Psychomotor vigilance testing of professional drivers in the occupational health clinic: a potential objective screen for daytime sleepiness. J Occup Environ Med 54, 296–302, https://doi.org/10.1097/JOM.0b013e318223d3d6 (2012).
Anderson, C. et al. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. Sleep 35, 1137–1146, https://doi.org/10.5665/sleep.2004 (2012).
Gooley, J. J. et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab 96, E463–472, https://doi.org/10.1210/jc.2010-2098 (2011).
Gooley, J. J. et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2, 31ra33, https://doi.org/10.1126/scitranslmed.3000741 (2010).
Lockley, S. W., Brainard, G. C. & Czeisler, C. A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88, 4502–4505, https://doi.org/10.1210/jc.2003-030570 (2003).
St Hilaire, M. A. et al. Human phase response curve to a 1 h pulse of bright white light. J Physiol 590, 3035–3045, https://doi.org/10.1113/jphysiol.2012.227892 (2012).
Rahman, S. A. et al. Circadian phase resetting by a single short-duration light exposure. JCI Insight 2, e89494, https://doi.org/10.1172/jci.insight.89494 (2017).
Rahman, S. A. et al. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol 596, 2147–2157, https://doi.org/10.1113/JP275501 (2018).
Duffy, J. F. & Dijk, D. J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 17, 4–13, https://doi.org/10.1177/074873002129002294 (2002).
Dinges, D. F. et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep 20, 267–277 (1997).
Jewett, M. E., Dijk, D. J., Kronauer, R. E. & Dinges, D. F. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep 22, 171–179, https://doi.org/10.1093/sleep/22.2.171 (1999).
Basner, M. & Dinges, D. F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 34, 581–591 (2011).
Santhi, N., Aeschbach, D., Horowitz, T. S. & Czeisler, C. A. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms 23, 341–352, https://doi.org/10.1177/0748730408319863 (2008).
Anderson, C., Wales, A. W. & Horne, J. A. PVT lapses differ according to eyes open, closed, or looking away. Sleep 33, 197–204 (2010).
Cicchetti, D. V. & Sparrow, S. A. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86, 127–137 (1981).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Van Dongen, H. P., Olofsen, E., Dinges, D. F. & Maislin, G. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol 384, 139–171, https://doi.org/10.1016/S0076-6879(04)84010-2 (2004).
Kohavi, R. & Joahn, G. H. Wrappers for feature subset selection. Artificial Intelligence 97, 273–324 (1997).
Russell, S. & Norvig, P. Artificial Intelligence: a modern approach. (Prentice-Hall, Inc, 1995).
Elkan, C. In Proceedings of the Seventeenth International Joint Conference on Artificial Intelligence Vol. 2, 973–978 (2001).
Akerstedt, T., Folkard, S. & Portin, C. Predictions from the three-process model of alertness. Aviat Space Environ Med 75, A75–83 (2004).
Hursh, S. R. et al. Fatigue models for applied research in warfighting. Aviat Space Environ Med 75, A44–53, discussion A54–60 (2004).
Jewett, M. E. & Kronauer, R. E. Interactive mathematical models of subjective alertness and cognitive throughput in humans. J Biol Rhythms 14, 588–597, https://doi.org/10.1177/074873099129000920 (1999).
Mallis, M. M., Mejdal, S., Nguyen, T. T. & Dinges, D. F. Summary of the key features of seven biomathematical models of human fatigue and performance. Aviat Space Environ Med 75, A4–14 (2004).
Liu, J., Ramakrishnan, S., Laxminarayan, S., Balkin, T. J. & Reifman, J. Real-time individualization of the unified model of performance. J Sleep Res. https://doi.org/10.1111/jsr.12535 (2017).
Rajaraman, S., Gribok, A. V., Wesensten, N. J., Balkin, T. J. & Reifman, J. Individualized performance prediction of sleep-deprived individuals with the two-process model. J Appl Physiol (1985) 104, 459–468, https://doi.org/10.1152/japplphysiol.00877.2007 (2008).
Rajaraman, S., Gribok, A. V., Wesensten, N. J., Balkin, T. J. & Reifman, J. An improved methodology for individualized performance prediction of sleep-deprived individuals with the two-process model. Sleep 32, 1377–1392 (2009).
Van Dongen, H. P. et al. Optimization of biomathematical model predictions for cognitive performance impairment in individuals: accounting for unknown traits and uncertain states in homeostatic and circadian processes. Sleep 30, 1129–1143 (2007).
Ratcliff, R. & Van Dongen, H. P. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proc Natl Acad Sci USA 108, 11285–11290, https://doi.org/10.1073/pnas.1100483108 (2011).
St Hilaire, M. A. et al. Modeling Neurocognitive Decline and Recovery During Repeated Cycles of Extended Sleep and Chronic Sleep Deficiency. Sleep 40, https://doi.org/10.1093/sleep/zsw009 (2017).
St Hilaire, M. A. et al. Classifying performance impairment in response to sleep loss using pattern recognition algorithms on single session testing. Accid Anal Prev 50, 992–1002, https://doi.org/10.1016/j.aap.2012.08.003 (2013).
Rajaraman, S. et al. A new metric for quantifying performance impairment on the psychomotor vigilance test. J Sleep Res 21, 659–674, https://doi.org/10.1111/j.1365-2869.2012.01008.x (2012).
Chavali, V. P., Riedy, S. M. & Van Dongen, H. P. Signal-to-Noise Ratio in PVT Performance as a Cognitive Measure of the Effect of Sleep Deprivation on the Fidelity of Information Processing. Sleep 40, https://doi.org/10.1093/sleep/zsx016 (2017).
Satterfield, B. C., Wisor, J. P., Field, S. A., Schmidt, M. A. & Van Dongen, H. P. TNFalpha G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behav Immun 47, 66–74, https://doi.org/10.1016/j.bbi.2014.12.009 (2015).
Massar, S. A. A., Lim, J., Sasmita, K. & Chee, M. W. L. Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia 123, 169–177, https://doi.org/10.1016/j.neuropsychologia.2018.03.032 (2019).
Sasmita, K., Massar, S. A. A., Lim, J. & Chee, M. W. L. Reward motivation normalises temporal attention after sleep deprivation. J Sleep Res, e12796, https://doi.org/10.1111/jsr.12796 (2018).
Frey, D. J., Badia, P. & Wright, K. P. Jr. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res 13, 305–315, https://doi.org/10.1111/j.1365-2869.2004.00429.x (2004).
Bermudez, E. B. et al. Prediction of Vigilant Attention and Cognitive Performance Using Self-Reported Alertness, Circadian Phase, Hours since Awakening, and Accumulated Sleep Loss. PLoS One 11, e0151770, https://doi.org/10.1371/journal.pone.0151770 (2016).
Hirshkowitz, M. et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 1, 40–43 (2015).
Rajaratnam, S. M., Middleton, B., Stone, B. M., Arendt, J. & Dijk, D. J. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol 561, 339–351, https://doi.org/10.1113/jphysiol.2004.073742 (2004).
Kursa, M. B. & Rudnicki, W. R. Feature selection with the Boruta Package. J Statistical Software 36, 1–13 (2010).
Chowdhury, A. K., Tjondronegoro, D., Chandran, V. & Trost, S. G. Ensemble Methods for Classification of Physical Activities from Wrist Accelerometry. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0000000000001291 (2017).
Lim, J., Choo, W. C. & Chee, M. W. Reproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory task. Sleep 30, 61–70 (2007).
Bachmann, V. et al. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex 22, 962–970, https://doi.org/10.1093/cercor/bhr173 (2012).
Bachmann, V. et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 35, 335–344, https://doi.org/10.5665/sleep.1690 (2012).
Bodenmann, S. et al. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br J Pharmacol 165, 1904–1913, https://doi.org/10.1111/j.1476-5381.2011.01689.x (2012).
Goel, N., Banks, S., Mignot, E. & Dinges, D. F. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology 75, 1509–1519, https://doi.org/10.1212/WNL.0b013e3181f9615d (2010).
Groeger, J. A. et al. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep 31, 1159–1167 (2008).
Viola, A. U. et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol 17, 613–618, https://doi.org/10.1016/j.cub.2007.01.073 (2007).
Acknowledgements
We thank the research volunteers, subject recruiters, and research staff at the Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital. We also thank the technicians and nursing staff of the Center for Clinical Investigation and the Harvard Clinical and Translational Science Center for their assistance in carrying out the inpatient protocols; Drs. Shantha Rajaratnam, Eliza van Reen, Shadab Rahman, and Melissa St. Hilaire for managing and performing experiments that were included in the present study; R. Todesco (BWH) for administrative support; and J.M. Ronda, M.S. (BWH) for technical support. This work was supported by the Defense Advanced Research Projects Agency, USA (N66001–13-C-4035); the Office of Naval Research, USA (N00014-15-1-2408); and the Duke-NUS Signature Research Program funded by the Agency for Science, Technology and Research, Singapore, and the Ministry of Health, Singapore. Studies used in the analysis were supported by the National Institutes of Health (NIH), USA (NIMH R01 MH45130; NCCAM R01 AT002129; NHLBI K24HL105664); the National Space Biomedical Research Institute through NASA NCC 9-58; and the Brigham and Women’s General Clinical Research Center (M01 RR02635). J.J.G. was supported by an NIH Institutional Training Grant (T32-HL07901).
Author information
Authors and Affiliations
Contributions
J.J.G. and S.W.L. performed the experiments. E.C.-P.C., J.P.S., and J.J.G. analyzed the data. E.C.-P.C. and J.J.G. wrote the paper. E.C.-P.C., J.P.S., J.F.D., E.B.K., S.W.L., B.S.K., C.A.C. and J.J.G. designed the research, interpreted the findings, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
E.C.-P.C., J.J.G., J.P.S., and J.F.D. report no conflicts of interest. E.B.K. received travel support from the Sleep Research Society and the National Sleep Foundation and has consulted for Pfizer Pharmaceuticals. S.W.L. has received consulting fees from BHP Billiton, Delos Living LLC, Noble Insights, Pegasus Capital Advisors LP, and Team C Racing; and has current consulting contracts with Akili Interactive, Apex2100 Ltd., Consumer Sleep Solutions, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, PlanLED, Six Senses, Stantec, and Wyle Integrated Science and Engineering. S.W.L. has received unrestricted equipment gifts from Bionetics Corporation and F.Lux Software LLC; has equity in iSLEEP, Pty; receives royalties from Oxford University Press; support for travel, accommodation and/or meals for invited seminars, conference presentations or teaching with honoraria from BHP Billiton, Ineos, and Teague; and without honoraria from DIN, Emory University, IES, SLTBR and Solemma. S.W.L has an ongoing investigator initiated grant from F. Lux Software LLC. S.W.L. holds a process patent for ‘Systems and methods for determining and/or controlling sleep quality’, which is assigned to the Brigham and Women’s Hospital per Hospital policy. S.W.L. has also served as a paid expert for legal proceedings related to light, sleep and health. S.W.L. was a Program Leader for the CRC for Alertness, Safety and Productivity, Australia on behalf of Monash University. C.A.C. reports grants from Cephalon Inc., Jazz Pharmaceuticals Plc., Inc., National Football League Charities, Optum, Philips Respironics, Inc., Regeneron Pharmaceuticals, ResMed Foundation, San Francisco Bar Pilots, Sanofi S.A., Sanofi-Aventis, Inc, Schneider Inc., Sepracor, Inc, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Sysco, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., and Wake Up Narcolepsy; and personal fees from Bose Corporation, Boston Celtics, Boston Red Sox, Cephalon, Inc., Columbia River Bar Pilots, Ganésco Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics, Inc, Teva Pharma Australia, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, Zurich Insurance Company, Ltd. In addition, C.A.C. holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker), and holds an equity interest in Vanda Pharmaceuticals, Inc. Since 1985, C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Casper Sleep Inc., Comair/Delta Airlines, Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, Purdue Pharma, LP, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC and United Parcel Service (UPS). C.A.C. receives royalties from the New England Journal of Medicine; McGraw Hill; Houghton Mifflin Harcourt/Penguin; and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chua, E.CP., Sullivan, J.P., Duffy, J.F. et al. Classifying attentional vulnerability to total sleep deprivation using baseline features of Psychomotor Vigilance Test performance. Sci Rep 9, 12102 (2019). https://doi.org/10.1038/s41598-019-48280-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48280-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.