Abstract
WRKY transcription factors play important roles in many plant developmental processes and adaptation to the environment. However, little knowledge is available about the WRKY gene family in cultivated strawberry (Fragaria × ananassa Duch.), an important soft fruit worldwide. In this study, a total of 47 WRKY gene members were identified and renamed on the basis of their order on the chromosomes. According to their evolutionary events and conserved structure, the 47 FaWRKYs were divided into three major groups with several subgroups. A cis-element analysis showed that all FaWRKYs possessed at least one stress response-related cis-element. Comprehensive analysis, including phylogenetic analysis and expression profiling, based on real-time qPCR analysis in root, stem, leaf and fruit was performed on group III FaWRKY genes. The phylogenetic tree of the WRKY III genes in cultivated strawberry, wild Strawberry, Arabidopsis, tomato, and rice was divided into five clades. Additionally, the expression profiles of the FaWRKY genes in response to continuous cropping were further investigated based on RNA-seq data. FaWRKY25, FaWRKY32, and FaWRKY45, which are group III FaWRKY genes, were upregulated after continuous cropping. The level of reactive oxygen species (ROS) and the expression levels of PR1 and peroxidase were higher in continuous cropping (CC) than in non-continuous cropping (NCC). The results indicated that group III FaWRKYs might play an important role in continuous cropping. These results provide a foundation for genetic improvements for continuous cropping tolerance in cultivated strawberry.
Similar content being viewed by others
Introduction
Plants are frequently faced with multiple environmental changes, including biotic and abiotic stresses. Biotic stresses include insect pests, fungi, bacteria and viruses, and abiotic stresses include heat, cold, drought, salinity and wounding1,2,3,4. Being sessile organisms, plants sense these stresses through complex signal transduction networks and have evolved specific defensive strategies to adapt to environmental fluctuations during their long evolutionary process5,6. Transcriptional regulation is a major mechanism for organisms to regulate their gene expression in response to physiological or environmental stimuli6. Families of transcription factors regulate gene expression in cells or organisms. They often harbor a DNA-binding region and play an essential role in activating or repressing the rate of transcription of specific target genes7.
The WRKY family of transcription factors was first isolated from plants. Over twenty years ago, Ishiguro and Nakamura identified the first WRKY protein (SPF1) from sweet potato8. WRKY genes form a family of regulatory genes, and the name is derived from the most prominent feature in their WRKY domain, which is composed of a highly conserved region consisting of 60 amino acid residues9. The WRKY amino acid sequence shows high binding affinity to a conserved cognate binding site designated the W box (C/TTGACT/C)9,10.
Numerous WRKY genes have since been identified from many plant species, including more than 70 WRKY members in Arabidopsis thaliana11,12, 81 WRKY members in Solanum lycopersicum5, 102 and 98 WRKY members in Oryza sativa L. ssp. indica and L. ssp. japonica13, 59 WRKY members in Fragaria vesca14, and 54 WRKY members in Ananas comosus15. The WRKY protein family can be classified into three major distinct groups (Groups I, II, and III) depending on the number of WRKY domains and the type of zinc finger motif9. WRKY group I proteins contain two WRKY domains and a C2H2 zinc finger motif; group II proteins encompassing subgroups (II a–e) have one WRKY domain containing the same C2H2 zinc finger motif; and group III proteins have one WRKY domain containing the specific C2HC zinc finger motif9.
Cultivated strawberry (Fragaria × ananassa Duch.) is an octoploid species (2n = 8x = 56) and is an important soft fruit worldwide16. Strawberries are famous for their delicious taste, attractive appearance and abundant phytochemical compounds17. Zhou et al. performed a genome-wide analysis of wild strawberry (Fragaria vesca) WRKY genes, identified 59 FvWRKY genes in wild strawberry, and analyzed their expression in different fruit developmental stages14. However, little knowledge is available about the WRKY gene family in cultivated strawberry.
In this study, we identified 47 cultivated strawberry WRKY gene members and classified them into three major groups with several subgroups. A comprehensive analysis including phylogenetic relationships, multiple sequence alignment, and stress-related cis-element analysis was further performed. The expression patterns of group III FaWRKY genes in different organs, including root, stem, leaf and fruit, were analyzed by real-time quantitative RT-PCR. Global expression analysis of WRKY genes was performed under continuous cropping conditions based on RNA-seq data.
Results
Identification and analysis of FaWRKY proteins
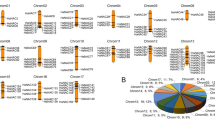
A total of 52 candidate WRKY proteins were obtained from PlantTFDB (http://planttfdb.cbi.pku.edu.cn/) in Fragaria x ananassa Duch. The annotation of these putative genes was further checked using BLASTP analysis in NCBI (https://www.ncbi.nlm.nih.gov). Three erroneously predicted WRKY proteins (FANhyb_rscf00000209.1.g00016.1, FANhyb_rscf00000217.1.g00006.1 and FANhyb_rscf00003540.1.g00001.1) were manually curated, and two redundant sequences (FANhyb_icon00001572_a.1.g00001.1 and FANhyb_icon00043401_a.1.g00001.1) were then removed. Finally, 47 sequences were confirmed and annotated as being cultivated strawberry WRKY genes. To better reflect the appropriate function, we named each FaWRKY based on their order on the chromosomes, and hit them to the similar individual AtWRKYs (Fig. 1, Table 1). Gene names, gene IDs, chromosomal locations, genomic locations, homologous genes, hit IDs and blast E-values are listed in Table 1.
Chromosomal distributions of FaWRKY genes. Chromosome numbers are showed at the top of each chromosome and the approximate size in megabases (Mb) provided at the left side. The names of each FaWRKY gene showed on the right side of each chromosome. Group I members are indicated in black font; group II members are indicated in blue font; group III members are indicated in red font. FaWRKY34 in orange font not belongs to any group. Unmapped WRKY genes (FaWRKY47) are not shown.
Phylogenetic analysis and multiple sequence alignment
To explore the phylogenetic relationship of the FaWRKY proteins, we constructed a phylogenetic tree of 47 FaWRKY proteins using MEGA 7.0. The phylogenetic analysis showed that the cultivated strawberry WRKY proteins could be divided into three major groups (groups I, II and III) with five subgroups (II a, II b, II c, II d, and II e) corresponding to the AtWRKY proteins9 (Fig. 2a). Among the 47 FaWRKY proteins, 10 belong to group I; 31 belong to group II, including 3 to group II a, 6 to group II b, 10 to group II c, 5 to group II d, and 7 to group II e; and 6 belong to group III. However, FaWRKY34 could not be clustered into any group.
The phylogenetic tree, Multiple sequence and domain alignment of FaWRKY proteins. (a) The phylogenetic tree representing relationships among FaWRKY proteins using the neighbor-joining method. The bootstrap values with 1,000 bootstrap replications are shown above the branches. The tree was divided into three major groups (I, II and III) with five subgroups (II a-e). (b) Multiple sequence alignment of FaWRKY domain amino acid sequences. Groups I N and I C indicate the N-terminus and C-terminus of the WRKY domain of a group I WRKY protein. (c) Domains of FaWRKY proteins.
Multiple sequence alignment of the WRKY domains, which span approximately 60 amino acids, revealed that the conserved amino acid sequences were defined by WRKYGQ(K)K at the N-terminal end, together with a zinc finger-like motif at the C-terminal end (Fig. 2b,c). The domain number, conserved heptapeptides and zinc finger types of each group are shown in Table 2. The group I FaWRKY proteins contained two WRKY domains (WRKYGQK) and C2H2-type zinc finger motifs, group IN and group IC. Groups II and III contained one WRKY domain and zinc finger motif. Most members of group II (a, b, c, d, e), except for FaWRKY50, which was defined by WRKYGKK and C2H2-type zinc finger motifs, were defined by WRKYGQK and C2H2-type zinc finger motifs. All six members in group III contain the conserved WRKYGQK and the special C2HC-type zinc fingers.
Stress-related cis-elements in FaWRKY promoter regions
Plants use complex defense signaling pathways, including stress-related cis-elements, to regulate their adaptation to ever-changing stresses18.The WRKY TFs could regulate gene expression by binding to their cis-elements during stress responses. To further determine if WRKY TFs engage in potential regulatory mechanisms during stress responses, the promoter regions, sequences of approximately 1.5-kb upstream from the translation start sites, were submitted into PlantCARE to identify the cis-elements. In this study, ten stress response elements, including ABRE (cis-acting element involved in abscisic acid responsiveness), AuxRR-core (cis-acting regulatory element involved in auxin responsiveness), CGTCA-motif (cis-acting regulatory element involved in MeJA responsiveness), LTR (cis-acting element involved in low-temperature responsiveness), MBS (MYB transcription factor binding site involved in drought inducibility), P-box (gibberellin-responsive element), TCA-element (cis-acting element involved in salicylic acid responsiveness), TC-rich repeats (cis-acting element involved in defense and stress responsiveness), TGA-element (auxin-responsive element) and W-box (WRKY transcription factor binding site in defense responses), were analyzed, and the data are displayed in Fig. 3.
In this study, all FaWRKY TFs had at least 1 stress response-related cis-element. The elements associated with hormone regulation, including ABRE, AuxRR-core, CGTCA motif, P-box, TCA-element, and TGA-element, were identified in many FaWRKY promoter regions. In total, 38 FaWRKYs (80.8%) had one or more ABRE, suggesting a potential abscisic acid response under stress conditions. One or more CGTCA motifs, which are involved in MeJA responsiveness, existed in 33 FaWRKYs (70.2%). TCA-element, TGA-element, P-box and AuxRR-core were located in 14, 14, 10 and 3 FaWRKYs, respectively.
Cis-elements related to other stresses were found in several FaWRKY promoter regions. For example, 26 FaWRKYs contained one or more W-box elements in their promoter regions. In addition, several LTR, MBS and TC-rich repeats, which are involved in low temperature, drought inducibility and defense responsiveness, were also found in many FaWRKYs.
Evolutionary analysis of WRKY group III TFs in strawberry and several different species
The group III WRKY TFs are thought to play a key role in plant evolution and adaptation19. To further investigate the phylogenetic relationship of the WRKY III genes in cultivated strawberry, wild strawberry, Arabidopsis, tomato, and rice, an unrooted phylogenetic tree of WRKY III complete protein sequences was constructed using MEGA 7.0 (Fig. 4). The phylogenetic tree indicated that the WRKY III proteins were divided into five clades. Clades 5 had the most Group III gene members (19), followed by clade 3 (17), clade 1 (12) and clade 2 (12); clades 4 contained the least WRKY Group III TF members (8) (Fig. 4). Clade 3 and 5 included WRKY Group III TFs in all 5 species, while the members of clades 1 and 2 contained four species. Clade contained other four species except strawberry, while clade 2 only contained all dicots, cultivated strawberry, wild strawberry, Arabidopsis, and tomato. Clade 4 was composed of rice only, which might be a monocot-specific clade. Clade 5 consisted of two sub-branch, one was rice, other belong to dicots. This distribution may be related to the split of monocots and dicots. Among those WRKY Group III TFs, six cultivated strawberry WRKY Group III proteins were classified into clade 2 (FaWRKY31, FaWRKY32 and FaWRKY44), clade 3 (FaWRKY25 and FaWRKY45), and clade 5 (FaWRKY43). Five of the six cultivated strawberry WRKY Group III TFs were clustered together with the wild strawberry members. Based on the phylogenetic analysis, five pairs (FaWRKY25 and FvWRKY27, FaWRKY32 and FvWRKY38, FaWRKY43 and FvWRKY53, FaWRKY44 and SlWRKY80 and FaWRKY45 and FvWRKY56) were identified as orthologous genes.
Phylogenetic relationships of group III WRKY proteins from cultivated strawberry, wild strawberry, Arabidopsis, tomato, and rice. The tree was constructed using MEGA 7.0 by the neighbor-joining method. The phylogenetic tree represents 1,000 bootstrap replications. The tree was clustered into five clades (clades 1–6). The different-colored ranges represent different clades. Clade 1 members are indicated in black color; Clade 2 members are indicated in blue color; Clade 3 members are indicated in red color; Clade 4 members are indicated in lighe blue color; Clade 5 members are indicated in pale green color.
Expression patterns of cultivated strawberry WRKY III genes in different tissues
To obtain insight into the potential functions of FaWRKY group III genes during development, the expression patterns of six FaWRKY group III genes were analyzed in roots, stems, leaves and fruits using qRT-PCR. The six FaWRKY group III genes revealed significant tissue-specific expression patterns (Fig. 5). All six FaWRKY group III genes were expressed in roots and stems. Among the six genes, three showed the highest expression in roots (, FaWRKY25, FaWRKY31 and FaWRKY45), two in stems (FaWRKY32 and FaWRKY44), and one in leaves (FaWRKY43). Moreover, FaWRKY25, FaWRKY31 and FaWRKY45 had similar expression patterns and were highly expressed in roots, with little or no expression in the other three tissues.
Expression profiling of FaWRKY genes with RNA-seq in response to continuous cropping
In this study, the expression patterns of the FaWRKY genes in response to continuous cropping were evaluated based on RNA-seq data. Among the 47 predicted FaWRKY genes, 38 showed expression in roots (Fig. 6), and only four FaWRKY genes (FaWRKY25, FaWRKY32, FaWRKY33 and FaWRKY45) changed significantly. The expression of FaWRKY25, FaWRKY32, FaWRKY33 and FaWRKY45 was upregulated in response to continuous cropping (FDR < 0.01 and log2FC ≥ 2). Interestingly, most of the upregulated genes (3/4) belonged to FaWRKY group III. The special expression of group III genes exhibited significant trends in response to continuous cropping. The FPKM of FaWRKY group III genes is shown in Fig. 6b. FaWRKY45 had significantly higher expression (P-value < 0.01) and FaWRKY25 and FaWRKY32 had higher expression (P-value < 0.05) in response to continuous cropping.
RNA-seq data showing FaWRKY gene expression in response to continuous cropping. (a) Heatmap of the expressed FaWRKY genes assigned to continuous cropping. The color (from green to red) indicates gene expression intensity from low to high. (b) Expression profiles of group III FaWRKY genes in response to continuous cropping based on RPKM (reads per kilobase per million mapped reads). *Indicates a significant difference (P < 0.05); **indicates an extremely significant difference (P < 0.01).
Other changes associated with upregulation of FaWRKY genes
Reactive oxygen species (ROS) play a vital role in plant–environment interactions. Massive ROS generation is toxic to plant cells and damages cellular membranes. In this study, the level of ROS in continuous cropping roots was significantly higher than that in non-continuous cropping roots (Fig. 7a). The expression level of PR1 was higher in CC lines than in NCC plants (Fig. 7b). Similarly, the expression levels of peroxidase genes were also significantly higher in CC roots than in NCC roots (Fig. 7c).
Discussion
The WRKY transcription factor genes are involved in the regulation of a large number of processes in plants. Previous studies have revealed some information on the WRKY gene family in many species, such as Arabidopsis, rice, tomato, cotton, pineapple and wild strawberry. However, little is known about WRKY gene families in cultivated strawberry.
In the present study, 47 WRKY gene members were identified in cultivated strawberry, and their evolutionary events, conserved structure, stress-related cis-elements, comprehensive analysis of group III WRKY genes and expression patterns in continuous cropping were also examined. Distinct expansion in group III WRKY genes was detected in cultivated strawberry under continuous cropping. These results showed that three group III WRKY gene members (FaWRKY25, FaWRKY32 and FaWRKY45) were upregulated in response to continuous cropping.
The WRKY gene members were classified into three major groups (I, II and III), and group II was divided into five distinct subgroups (II a–e), as previously described for the model herbaceous plant Arabidopsis9. The phylogenetic analysis of FaWRKY TFs also indicated that the 47 FaWRKY proteins can be divided into the same groups (I, II a-e, and III). However, FaWRKY34 could not be clustered into any group, similar to AtWRKY38 and AtWRKY52, which were found in Arabidopsis9.
The loss of the WRKY domain usually occurs in many monocotyledon species, such as rice and maize13,20,21,22. Group I WRKY TFs in cultivated strawberry all contain two WRKY domains, and no domain loss events were found. The conserved structural domains of their encoded proteins were assessed in this study. Multiple sequence alignments showed that FaWRKY37 in group II c had unique sequences in the WRKY domain (WRKYGKK). Based on previous studies, sequence variation in the WRKY domain might influence the normal interactions and binding specificities with downstream target genes15,23,24.
Stress-related cis-elements were identified in the promoter regions of 47 FaWRKYs involved in different functions, such as hormone regulation (ABRE, AuxRR-core, CGTCA motif, P-box, TCA-element, and TGA-element), abiotic stress (LTR and MBS), and disease resistance (TC-rich repeats and W-box elements). The WRKY transcription factors could be regulated by binding different cis-elements in their own promoters25,26,27. The WRKY TF promoters could be regulated by autoregulation or cross-regulation by interaction with each other28,29. In total, 26 FaWRKYs had one or more W-boxes, suggesting that those WRKY TFs might be regulated by autoregulation or cross-regulation.
The group III WRKY gene members might be the most dynamic group with regard to gene family evolution15,19. There are 13 group III WRKY genes in Arabidopsis30, 6 in grape19, 28 in rice19, 10 in Populus19, and 10 in wild strawberry14. However, the number of group III WRKY gene members was related to the diversity of the WRKY gene family size19. In this study, group III FaWRKY gene members totaled six, which was fewer than the corresponding number in most other plants; this is a potential cause of the smaller number of cultivated strawberry WRKY family members.
The group III gene members play an important role in plant evolution, and their evolutionary history would provide more clues to the origin and evolution of the WRKY gene family19. In the current study, the plant WRKY III members were clustered into five clades, and the closely related species might tend to be clustered together. Differences in the orthologous gene pairs between cultivated strawberry/wild strawberry (5) and cultivated strawberry/tomato (1) showed a closer relationship in different strawberries. FaWRKY44, which was clustered together with SlWRKY80, is not found in wild strawberry.
The expression patterns of WRKY group III genes in different tissues have been evaluated in many species, and there is no uniform gene expression profile for plant WRKY group III genes15,30. According to the qRT-PCR expression patterns of all FaWRKY group III gene members in different cultivated strawberry tissues, different FaWRKY group III proteins may have diverse functions. In this study, the expression analysis revealed that FaWRKY25, FaWRKY31, and FaWRKY45 were highly expressed in the roots, suggesting a putative role in the development of roots. Moreover, those three genes showed similar expression patterns in different tissues, suggesting that these three genes might have retained redundant functions in regulating the same functions19.
In plants, WRKY gene members play significant roles in regulating defense gene expression in response to adverse conditions19, including biotic and abiotic stresses. Among those WRKY members, the Group III WRKY proteins have been considered to play important roles in regulating plant immunity, resistance and development. For example, the majority of group III members in Arabidopsis are involved in pathogen attack and salicylic acid (SA) treatment30. The WRKY Group III transcription factors in tomato were identified to participate in the TYLCV defense signaling pathway25. Group III GhWRKY genes are involved in fiber development and leaf senescence and can be induced by plant hormones, such as jasmonic acid (JA), abscisic acid (ABA), ethylene, and salicylic acid (SA)6. Therefore, certain WRKY Group III proteins have significant functions in defense regulation against abiotic and/or biotic stresses.
Long-term continuous cropping often brings a complex change in the structure of soil, and strawberry is vulnerable to this problem. The complex stresses to which strawberry responds include biotic stresses, such as the accumulation of soil-borne pathogens and plant-feeding nematodes, and abiotic stresses, such as nutrient availability imbalance, soil physicochemical property deterioration, and autotoxic substance accumulation31,32,33,34,35. The ROS network plays a vital role in the signal transduction of resistance to environmental stresses. In plants, WRKYs played essential roles in the ROS scavenging system in plant–environment interactions. During continuous cropping, three FaWRKY Group III gene members (FaWRKY25, FaWRKY32 and FaWRKY45) may respond to adverse stress by participating in plant immune ROS bursts by several signaling pathways, such as plant hormone signal transduction and plant-pathogen interactions (Fig. 8). FaWRKY25, FaWRKY32 and FaWRKY45 have high sequence similarity with AtWRKY53, AtWRKY70 and AtWRKY41, respectively. Previous studies showed that AtWRKY41, AtWRKY53 and AtWRKY70 play important roles in plant basal defense36,37. The expression of WRKY is activated by MAPKs and induces the expression of a series of defense-related proteins, such as PR1 protein, peroxidase and plant hormone signaling, similar to salicylic acid (SA) and jasmonic acid (JA)38,39. AtWRKY70 was demonstrated to be involved in regulating SA-JA-mediated signaling pathways, and AtWRKY41, AtWRKY53 and AtWRKY70 are induced by SA37,39,40. The hormone signaling pathway is important in plant immunity and can induce related protein expression to regulate secondary metabolism25,41. The extensive cross-regulation mechanism of FaWRKY25, FaWRKY32 and FaWRKY45, which belong to FaWRKY Group III, might play an important role in cultivated strawberry continuous cropping defense.
Materials and Methods
Data collection
The whole Fragaria x ananassa annotated genome sequences were obtained from Strawberry GARDEN (http://strawberry-garden.kazusa.or.jp)42. The family assignment rules in PlantTFDB (http://planttfdb.cbi.pku.edu.cn/)43 were used to identify strawberry WRKY TFs. Finally, the sequences containing WRKY DNA-binding domain (PF03106) were identified as candidates. The annotation of these candidate genes was further checked using BLASTP analysis in NCBI (https://www.ncbi.nlm.nih.gov). The Arabidopsis thaliana and Fragaria vesca WRKY transcription factor (TF) Group III members were downloaded from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The Oryza sativa and Solanum lycopersicum WRKY TFs were also taken from PlantTFDB.
Phylogenetic analysis and sequence alignment
The FaWRKY genes in cultivated strawberry were classified into different groups based on the AtWRKY classification9. Multiple alignments of amino acid sequences of FaWRKY proteins were performed by DNAMAN and Clustal X. Multiple sequence alignment for the domain was constructed using the Hidden Markov Model-guided method. MEGA 7.0 was utilized to construct the phylogenetic trees by the neighbor-joining (NJ) method with a bootstrap test of 1000-fold44,45.
Analysis of cis-acting elements in FaWRKY promoter regions
The upstream sequences (1.5 kb) of the FaWRKY-coding sequences were downloaded from Strawberry GARDEN (http://strawberry-garden.kazusa.or.jp)42. PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was utilized to identify seven regulatory elements of FaWRKYs46.
Plant materials and stress treatments
Fragaria × ananassa Duch. Benihoppe, a typical cultivated variety, were planted in Beijing Academy of Forestry and Pomology Sciences, Haidian District, Beijing, China (40°1′21″N, 116°16′32″E). All plants were planted in greenhouses at 22 ± 1 °C in a 16 h light/8 h dark photoperiod. The plant materials included two groups: Non-continuous cropping (NCC) and continuous cropping (CC). For NCC strawberry, the plants were cultivated in NCC soil, which cultivated with strawberry for the first time. For CC strawberry, the plants were cultivated in CC soil, which were annually mono-cultivated with strawberry for more than 12 years. The root, stem, leaf and fruit were collected separately for qRT-PCR analysis. Root samples of noncontinuous cropping and continuous cropping treatments were collected at harvest stage and used for further RNA-seq analysis. Each sample contains 3 replicates, and each replicate including 3 plants. All of the collected samples were snap-frozen in liquid nitrogen and kept at −80 °C until further use.
RNA extraction and gene expression analysis
Total RNA was extracted using the plant RNA Kit (BioTeke, Beijing, China). First-strand cDNA was reverse transcribed using a FastQuant RT Kit (TIANGEN, Beijing, China). Gene-specific primers for qRT-PCR were obtained from qPrimerDB (https://biodb.swu.edu.cn/qprimerdb/) (Table S1)47. Real-time PCR was performed using a QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR® Select Master Mix (Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 10 μl of 2 × SYBR® Select Master Mix, 1 μl of diluted cDNA product from reverse-transcription PCR, 0.8 μl of each of two primers, and 7 μl of DNase/RNase-free water, and each reaction was repeated using three independent biological and technical replicates. The housekeeping strawberry DNA binding protein (BDP, EU727547) gene was used as an internal control48. Gene expression was presented as relative units after standardization using the 2−ΔΔCT method49. RNA-seq analysis was performed using an Illumina platform at Beijing BioMarker Corporation. The FaWRKY gene expression levels were estimated by fragments per kilobase of transcript per million fragments mapped (FPKM). The resulting FDR (false discovery rate) was adjusted by the PPDE (posterior probability of being DE). The FDR < 0.01 & |log2(foldchange)| ≥ 2 was set as the threshold for significant differential expression. The heat maps were created by OmicShare tools, a free online platform for data analysis (http://www.omicshare.com/tools).
References
Kunkel, B. N. & Brooks, D. M. Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology 5, 325–331 (2002).
Singh, K., Foley, R. C. & Oñatesánchez, L. Transcription factors in plant defense and stress responses. Current Opinion in Plant Biology 5, 430–436 (2002).
Ramamurthy, M. et al. Characterizing the stress/defense transcriptome of Arabidopsis. Genome Biology 4, R20 (2003).
Katagiri, F. A global view of defense gene expression regulation a highly interconnected signaling network. Current Opinion in Plant Biology 7, 506–511 (2004).
Huang, S. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Molecular Genetics & Genomics Mgg 287, 495–513 (2012).
Dou, L. L. et al. Identification and expression analysis of group III WRKY transcription factors in cotton. Journal of Integrative Agriculture 15, 2469–2480 (2016).
Ulker, B. & Somssich, I. E. WRKY transcription factors: from DNA binding towards biological function. Current Opinion in Plant Biology 7, 491–498 (2004).
Ishiguro, S. & Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Molecular Genetics and Genomics 244, 563–571 (1994).
Eulgem, T., Rushton, P. J., Robatzek, S. & Somssich, I. E. The WRKY superfamily of plant transcription factors. Trends in Plant Science 5, 199–206 (2000).
Ciolkowski, I., Wanke, D., Birkenbihl, R. P. & Somssich, I. E. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Molecular Biology 68, 81–92 (2008).
Wang, Q. et al. WRKY gene family evolution in Arabidopsis thaliana. Genetica 139, 973 (2011).
Rushton, P. J., Somssich, I. E., Ringler, P. & Shen, Q. J. WRKY transcription factors. Trends in Plant Science 15, 247–258 (2010).
Ross, C. A., Liu, Y. & Shen, Q. J. The WRKY Gene Family in Rice (Oryza sativa). Journal of Integrative Plant Biology 49, 827–842 (2007).
Zhou, H. et al. Genome-Wide Analysis of the Expression of WRKY Family Genes in Different Developmental Stages of Wild Strawberry (Fragaria vesca) Fruit. PLOS ONE 11, e154312 (2016).
Xie, T. et al. Genome-wide investigation of WRKY gene family in pineapple: evolution and expression profiles during development and stress. BMC genomics 19, 490 (2018).
Hancock, J. F. Ecological genetics of natural strawberry species. HortScience 25, 869–871 (1990).
Gerdakaneh, M., Mozafari, A. A., Khalighi, A. & Siosehmardah, A. The effects of carbohydrate source and concentration on somatic embryogenesis of strawberry (Fragaria × ananassa Duch.). American-Eurasian Journal of Agricultural and Environmental Science 50, 377–385 (2009).
Walley, J. W. & Dehesh, K. Molecular Mechanisms Regulating Rapid Stress Signaling Networks in Arabidopsis. Journal of Integrative Plant Biology 52, 354–359 (2010).
Wang, Y. et al. Comparative genomic analysis of the WRKY III gene family in populus, grape, arabidopsis and rice. Biology Direct 10, 48 (2015).
Brand, L. H., Fischer, N. M., Harter, K., Kohlbacher, O. & Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Research 41, 9764–9778 (2013).
Wei, K. F., Chen, J., Chen, Y. F., Wu, L. J. & Xie, D. X. Molecular Phylogenetic and Expression Analysis of the Complete WRKY Transcription Factor Family in Maize. DNA Research: An International Journal for Rapid Publication of Reports on Genes and Genomes 19, 153 (2012).
Prateek, T. et al. The WRKY transcription factor family in Brachypodium distachyon. BMC Genomics 13, 270 (2012).
Verk, M. C. V., Pappaioannou, D., Neeleman, L., Bol, J. F. & Linthorst, H. J. M. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiology 146, 1983–1995 (2008).
Zhou, Q. Y. et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnology Journal 6, 486–503 (2008).
Ying, H. et al. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genomics 17, 788 (2016).
Xiao, J. et al. Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiology 163, 1868–1882 (2013).
Robatzek, S. & Somssich, I. E. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes & Development 16, 1139–1149 (2002).
Eulgem, T. & Somssich, I. E. Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology 10, 366–371 (2007).
Zeng, T. et al. OsWRKY45 alleles play different roles in abscisic acid signalling and salt stress tolerance but similar roles in drought and cold tolerance in rice. Journal of Experimental Botany 62, 4863–4874 (2011).
Kalde, M., Barth, M., Somssich, I. E. & Lippok, B. Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Mol Plant Microbe Interact 16, 295–305 (2003).
Li, H. Q., Zhang, L. L., Jiang, X. W. & Liu, Q. Z. Allelopathic effects of phenolic acids on the growth and physiological characteristics of strawberry plants. Allelopathy Journal 35, 61–75 (2015).
Wei-hua, L., Qi-zhi, L. & Peng, C. Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity. Journal of Integrative Agriculture, 60345–60347 (2018).
Li, X. et al. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Scientific Reports 6, 30466 (2016).
Wei-hua, L. & Qi-zhi, L. Changes in fungal community and diversity in strawberry rhizosphere soil after twelve years in the greenhouse. Journal of Integrative Agriculture, https://doi.org/10.1016/S2095-3119(18)62003-9 (2018).
Liu, Y. B., Li, X. Y. & Liu, Q. Z. Soil nematode communities in jujube (Ziziphus jujuba Mill.) rhizosphere soil under monoculture and jujube/wheat (Triticum aestivum Linn.) intercropping systems, a case study in Xinjiang arid region, northwest of China. European Journal of Soil Biology 74, 52–59 (2016).
Hu, Y., Dong, Q. & Yu, D. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Science 185–186, 288–297 (2012).
Besseau, S., Li, J. & Palva, E. T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. Journal of Experimental Botany 63, 2667–2679 (2012).
Park, C. J. et al. A hot pepper gene encoding WRKY transcription factor is induced during hypersensitive response to Tobacco mosaic virus and Xanthomonas campestris. Planta 223, 168–179 (2006).
Yu, D. & Chen, Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13, 1527–1540 (2001).
Li, J., Brader, G. & Palva, E. T. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16, 319–331 (2004).
Nakashima, K. & Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology 149, 88–95 (2009).
Hideki, H. et al. Dissection of the octoploid strawberry genome by deep sequencing of the genomes of fragaria species. DNA Research 21, 169–181 (2014).
Jin, J. et al. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Research 45, D1040–D1045 (2017).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology & Evolution 33, 1870 (2016).
Saitou, N. The neighbor-joining method: a new method for reconstructing phylogenetic tree. Molecular Biology and Evolution 4, 406 (1987).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research 30, 325–327 (2002).
Lu, K. et al. qPrimerDB: a thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Research 46, D1229–D1236 (2018).
Sang, J. et al. ICG: a wiki-driven knowledgebase of internal control genes for RT-qPCR normalization. Nucleic Acids Research 46, D121 (2017).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3, 1101–1108 (2008).
Acknowledgements
This work was supported by the National Science and Technology Support Program (2014BAD16B07-2).
Author information
Authors and Affiliations
Contributions
Q.L. conceived and designed the research. P.C. performed the experiments, analyzed the data, prepared the figures and wrote the manuscript. Q.L. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, P., Liu, Qz. Genome-wide characterization of the WRKY gene family in cultivated strawberry (Fragaria × ananassa Duch.) and the importance of several group III members in continuous cropping. Sci Rep 9, 8423 (2019). https://doi.org/10.1038/s41598-019-44479-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44479-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.